Abstract
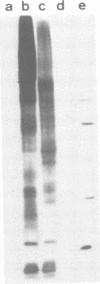
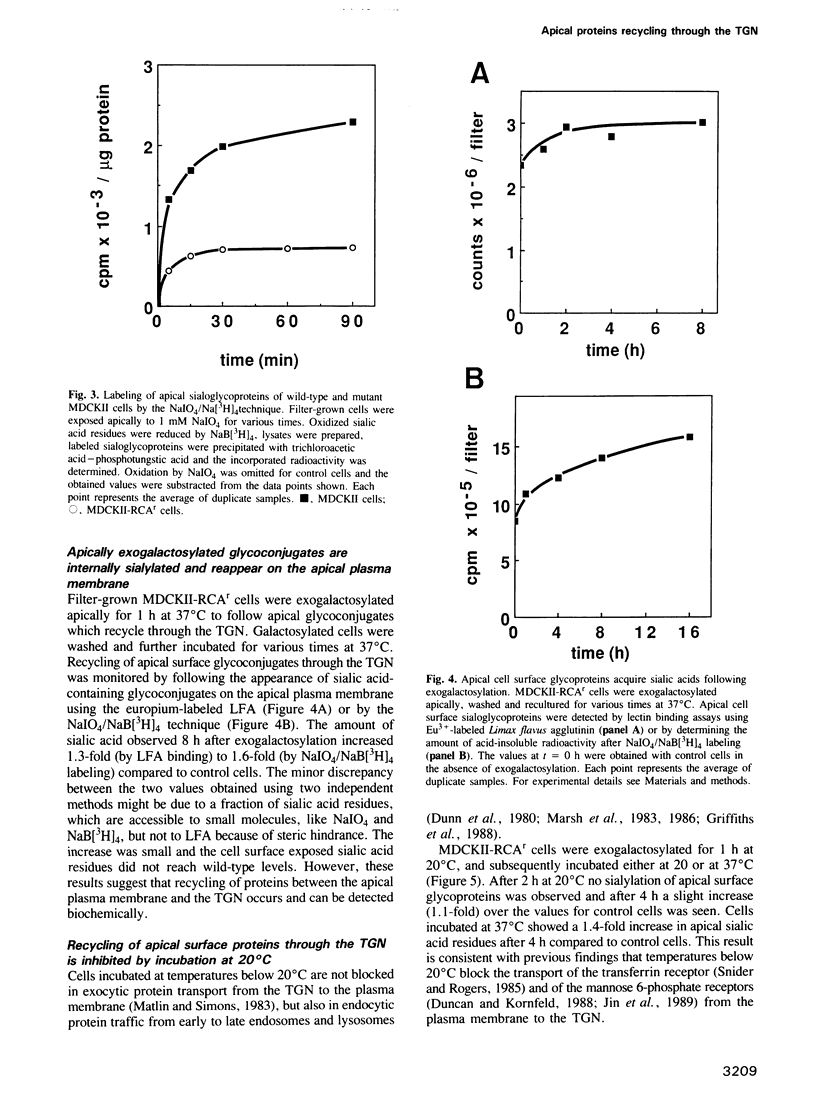
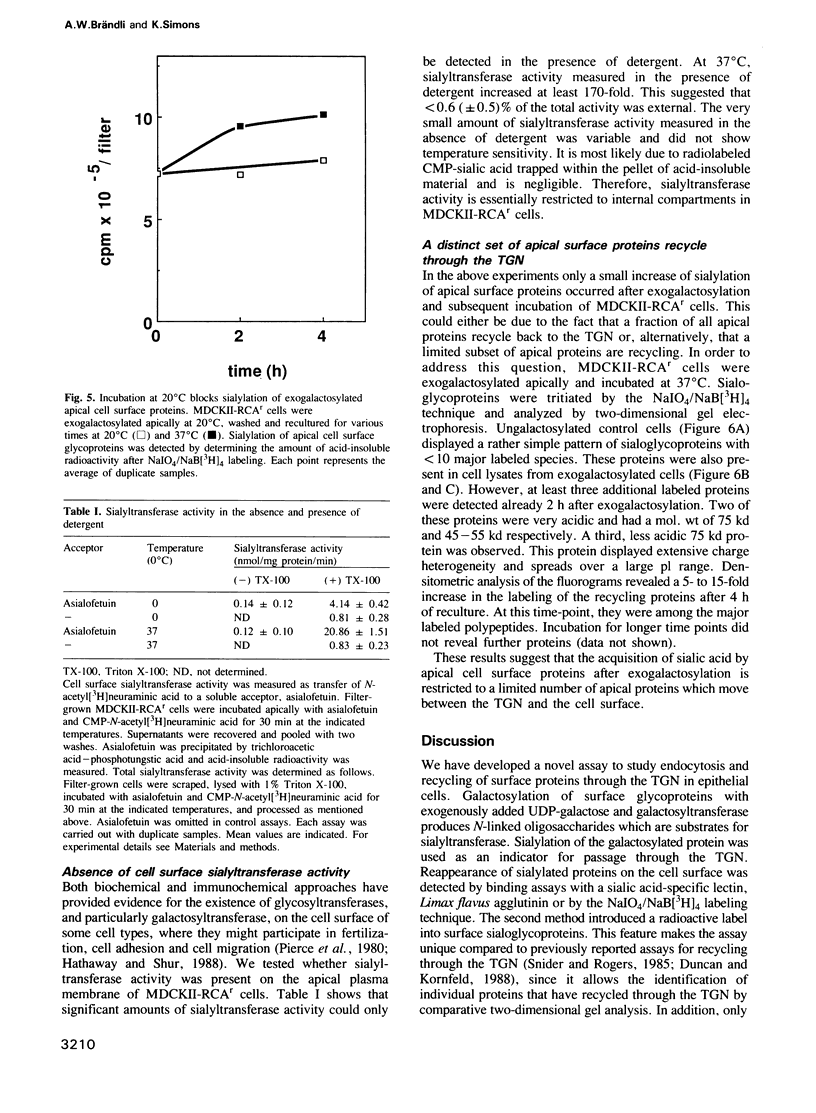
Sorting of newly synthesized proteins destined for the apical plasma membrane takes place in the trans-Golgi network (TGN) in MDCK cells. This process is most likely receptor mediated and requires components that recycle between both compartments. We have developed an assay to detect apical proteins that recycle through the sialyltransferase-containing TGN. Cell surface glycoproteins were exogalactosylated apically using a mutant cell line derived from MDCK, MDCKII-RCAr. The mutant exhibits impaired galactosylation of glycoconjugates and thereby allows maximal incorporation of exogenously added galactose in the presence of galactosyltransferase. Upon reculture at 37 degrees C, a time-dependent increase of sialylated apical surface glycoproteins was observed by lectin binding as well as by the sialic acid-specific NaIO4/NaB[3H]4 labeling technique. This indicates that some galactosylated surface molecules had returned to the TGN. Recycling through the TGN was blocked, if exogalactosylated cells were incubated at 20 degrees C. Two-dimensional gel electrophoresis identified three apical proteins which recycle through the TGN, suggesting that this pathway is selective for a subset of the apical surface proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. K., Wandinger-Ness A., Simons K. Release of putative exocytic transport vesicles from perforated MDCK cells. EMBO J. 1988 Dec 20;7(13):4075–4085. doi: 10.1002/j.1460-2075.1988.tb03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. G., Thurnher M., Müller U. Galactosyltransferase and sialyltransferase are located in different subcellular compartments in HeLa cells. Exp Cell Res. 1987 Nov;173(1):267–273. doi: 10.1016/0014-4827(87)90352-1. [DOI] [PubMed] [Google Scholar]
- Bravo R. Epidermal growth factor inhibits the synthesis of the nuclear protein cyclin in A431 human carcinoma cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4848–4850. doi: 10.1073/pnas.81.15.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändli A. W., Hansson G. C., Rodriguez-Boulan E., Simons K. A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J Biol Chem. 1988 Nov 5;263(31):16283–16290. [PubMed] [Google Scholar]
- Duncan J. R., Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988 Mar;106(3):617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hathaway H. J., Shur B. D. Novel cell surface receptors during mammalian fertilization and development. Bioessays. 1988 Nov;9(5):153–158. doi: 10.1002/bies.950090504. [DOI] [PubMed] [Google Scholar]
- Hemmilä I., Dakubu S., Mukkala V. M., Siitari H., Lövgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984 Mar;137(2):335–343. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Jin M., Sahagian G. G., Jr, Snider M. D. Transport of surface mannose 6-phosphate receptor to the Golgi complex in cultured human cells. J Biol Chem. 1989 May 5;264(13):7675–7680. [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Griffiths G., Dean G. E., Mellman I., Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Green R. F., Rodriguez-Boulan E. J. Lectin-resistant mutants of polarized epithelial cells. Mol Cell Biol. 1982 Oct;2(10):1287–1294. doi: 10.1128/mcb.2.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Verkerk J. M., Broxterman H. J., van der Marel G. A., van Boom J. H., Ploegh H. L. Recycling glycoproteins do not return to the cis-Golgi. J Cell Biol. 1988 Jul;107(1):79–87. doi: 10.1083/jcb.107.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pierce M., Turley E. A., Roth S. Cell surface glycosyltransferase activities. Int Rev Cytol. 1980;65:1–47. doi: 10.1016/s0074-7696(08)61958-0. [DOI] [PubMed] [Google Scholar]
- Reichner J. S., Whiteheart S. W., Hart G. W. Intracellular trafficking of cell surface sialoglycoconjugates. J Biol Chem. 1988 Nov 5;263(31):16316–16326. [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Rodriguez-Boulan E., Sabatini D. D. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J Cell Biol. 1984 Apr;98(4):1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Salas P. J. External and internal signals for epithelial cell surface polarization. Annu Rev Physiol. 1989;51:741–754. doi: 10.1146/annurev.ph.51.030189.003521. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Sasavage N. L., Smith M., Gillam S., Woychik R. P., Rottman F. M. Variation in the polyadenylylation site of bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1982 Jan;79(2):223–227. doi: 10.1073/pnas.79.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985 Mar;100(3):826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Colombatti M. Hybridoma cells containing intracellular anti-ricin antibodies show ricin meets secretory antibody before entering the cytosol. J Biol Chem. 1987 Apr 5;262(10):4676–4682. [PubMed] [Google Scholar]
- van Deurs B., Sandvig K., Petersen O. W., Olsnes S., Simons K., Griffiths G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol. 1988 Feb;106(2):253–267. doi: 10.1083/jcb.106.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]