Abstract
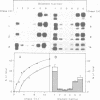
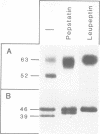
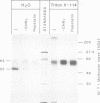
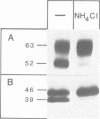
BHK cells expressing human lysosomal acid phosphatase (LAP) transport LAP to lysosomes as an integral membrane protein. In lysosomes LAP is released from the membrane by proteolytic processing, which involves at least two cleavages at the C terminus of LAP. The first cleavage is catalysed by a thiol proteinase at the outside of the lysosomal membrane and removes the bulk of the cytoplasmic tail of LAP. The second cleavage is catalysed by an aspartyl proteinase inside the lysosomes and releases the luminal part of LAP from the membrane-spanning domain. The first cleavage at the cytoplasmic side of the lysosomal membrane depends on acidification of lysosomes and the second cleavage inside the lysosomes depends on prior processing of the cytoplasmic tail. These results suggest that the cytoplasmic tail controls the conformation of the luminal portion of LAP and vice versa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gieselmann V., Hasilik A., von Figura K. Tartrate-inhibitable acid phosphatase. Purification from placenta, characterization and subcellular distribution in fibroblasts. Hoppe Seylers Z Physiol Chem. 1984 Jun;365(6):651–660. doi: 10.1515/bchm2.1984.365.1.651. [DOI] [PubMed] [Google Scholar]
- Gottschalk S., Waheed A., von Figura K. Targeting of lysosomal acid phosphatase with altered carbohydrate. Biol Chem Hoppe Seyler. 1989 Jan;370(1):75–80. doi: 10.1515/bchm3.1989.370.1.75. [DOI] [PubMed] [Google Scholar]
- Green G. D., Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem. 1981 Feb 25;256(4):1923–1928. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Pohlmann R., Krentler C., Schmidt B., Schröder W., Lorkowski G., Culley J., Mersmann G., Geier C., Waheed A., Gottschalk S. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J. 1988 Aug;7(8):2343–2350. doi: 10.1002/j.1460-2075.1988.tb03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini M. S., Van Etten R. L. A homogeneous isoenzyme of human liver acid phosphatase. Arch Biochem Biophys. 1978 Dec;191(2):613–624. doi: 10.1016/0003-9861(78)90399-5. [DOI] [PubMed] [Google Scholar]
- Waheed A., Gottschalk S., Hille A., Krentler C., Pohlmann R., Braulke T., Hauser H., Geuze H., von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J. 1988 Aug;7(8):2351–2358. doi: 10.1002/j.1460-2075.1988.tb03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]