Abstract
The parasite Trypanosoma cruzi causes Chagas disease, which remains a serious public health concern and continues to victimize thousands of people, primarily in the poorest regions of Latin America. In the search for new therapeutic drugs against T. cruzi, here we have evaluated both the in vitro and the in vivo activity of 5-hydroxy-3-methyl-5-phenyl-pyrazoline-1-(S-benzyl dithiocarbazate) (H2bdtc) as a free compound or encapsulated into solid lipid nanoparticles (SLN); we compared the results with those achieved by using the currently employed drug, benznidazole. H2bdtc encapsulated into solid lipid nanoparticles (a) effectively reduced parasitemia in mice at concentrations 100 times lower than that normally employed for benznidazole (clinically applied at a concentration of 400 µmol kg−1 day−1); (b) diminished inflammation and lesions of the liver and heart; and (c) resulted in 100% survival of mice infected with T. cruzi. Therefore, H2bdtc is a potent trypanocidal agent.
Author Summary
The protozoan parasite Trypanosoma cruzi causes Chagas disease, a condition that affects the poorest regions of Latin America mainly. The chronic phase of this disease disables thousands of patients, constituting an important public health issue. The pharmacotherapy that is currently applied to treat the disease emerged many decades ago, is ineffective in most patients, mainly during the chronic phase, and has serious side effects. In a recent study, we showed that the compound 5-hydroxy-3-methyl-5-phenyl-pyrazoline-1-(S-benzyldithiocarbazate) (H2bdtc) is a potential drug candidate against the in vitro trypomastigote form of Tulahuen strains of T. cruzi. Here we report that H2bdtc loaded into solid lipid nanoparticles (H2bdtc-SLNs) displays good trypanocidal activity against the trypomastigote form of the Y strain of T. cruzi both in vitro and in vivo. Our in vivo experiments revealed that H2bdtc-SLN is 100 times more active than benznidazole (BZN), the drug that is commercially available to treat Chagas disease. Surprisingly, this compound has no side effects on the T. cruzi acute phase. Hence, we propose that H2bdtc-SLNs possesses interesting anti-Trypanosoma properties.
Introduction
T. cruzi parasites are transmitted by insect vectors (triatomine bugs). T.cruzi is the causative agent of Chagas disease, which is silent and can remain asymptomatic for years [1], [2], [3]. A century after its discovery, this disease remains a serious public health issue—it is closely associated with human poverty and political instability as well as with little investment in drug development. According to the World Health Organization (WHO), between seven and eight million people are infected with T. cruzi worldwide, primarily in Latin America [4], [5], [6]. One in every four Chagas patients develops a fatal symptom of the disease due to lack of adequate diagnosis and treatment.
Nifurtimox and benznidazole (BZN) are currently available to treat the disease [7], [8], [9], [10]. However, neurological side effects have led commercial nifurtimox production to be discontinued [11]. As for BZN, although it is mainly effective during the acute phase of the infection, it presents undesirable side effects such as rash and gastrointestinal symptoms [12], so patients often fail to comply with the treatment [8]. Long treatment periods (30, 60, or 90 days) and appropriate pediatric formulations not available (administration of the medication to children often requires tablet fractionation) also limit BZN use [9], [11], [13]. A further concern is that no effective treatment for the symptomatic chronic phase of Chagas disease exists, so the patients usually receive palliative drugs at this stage [14], [15]. Therefore, a number of researchers are making considerable efforts to find new drugs to combat this disease.
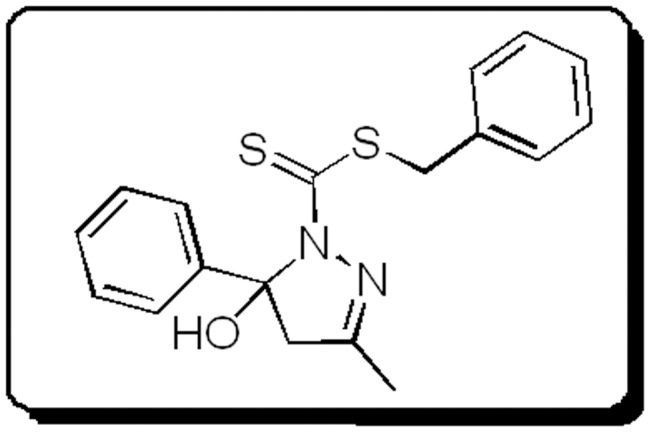
Dithiocarbazates display notable biological and pharmacological properties, including anticancer [16], [17], antimicrobial [17], [18], [19], and insecticidal activities [20]. A recent study has shown that cyclic compounds derived from S-dithiocarbazate and 1,3-diketones exhibit significant trypanocidal activity [21]: in particular, 5-hydroxy-3-methyl-5-phenyl-pyrazoline-1-(S-benzyldithiocarbazate) (previously referred to as H2L2a [21]) which was renamed H2bdtc in this reference in this work (Figure 1) constitutes a potential drug lead to develop a new agent against the trypomastigote form of Tulahuen strains of T. cruzi [21]. Nevertheless, the lipophilic character of H2bdtc may limit its administration and result in low oral bioavailability [22].
Figure 1. General molecular structure of the new cyclic S-dithiocarbazate derivatives.
Drug delivery systems can help to circumvent this problem. Because lipids have excellent physiological acceptability and can promote drug absorption as well as selective lymphatic uptake, researchers have focused on lipid-based drug release systems [23]. In particular, solid lipids constitute solid lipid nanoparticles (SLNs) at room and body temperature. Since SLNs consist of biocompatible and biodegradable lipids with low or no human toxicity, they can function as drug delivery systems [24], [25]. SLNs offer many advantages: they protect the drug against degradation, enable controlled drug release, and dismiss the use of organic solvents. Moreover, SLNs can be produced on a large scale, meeting industrial requirements [24], [26].
Our group has used H2bdtc in vitro experiments involving the Tulahuen strain (group I) [21]. The resistances of this group and of the Y strain (group II) have been reported to be different, based on phosphatase activities in T. cruzi homogenates [27]. Tulahuen had an optimum phosphatase activity at pH 4.0 and the Y strain at pH 7.0 [27]. Also in chronic phase has been associated with T. cruzi II-restricted infections [28]. In this sense, evaluating the trypanocidal activity of H2bdtc against the Y strain could support the use of this compound as a new drug against T. cruzi. In addition, so far little attention has been paid to the use of SLNs to treat Chagas disease [29], [30]. Therefore, this work investigates the in vitro and in vivo trypanocidal activity of free H2bdtc and H2bdtc encapsulated into SLNs (H2bdtc-SLNs) against the Y strain of T. cruzi and compares results with data obtained for the currently available drug BZN.
Materials and Methods
General
BZN, a product manufactured by Lafepe, Brazil, was used as a reference drug. The synthesis of H2bdtc has been described previously [21]. RPMI Medium 1640 supplemented with 5% bovine fetal serum (GIBCO, Grand Island, NY, USA), 100 IU mL−1 penicillin G, and 100 mg mL−1 streptomycin (Gibco-BRL, Grand Island, NY, USA) was employed. Dimethyl sulfoxide (DMSO) and propidium iodide (PI) were obtained from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). Stearic acid and sodium taurodeoxycholate were purchased from Sigma-Aldrich (St. Louis, MO, USA), Lipoid S 100 (soya lecithin) was acquired from Lipoid (Ludwigshafen, KOLN, Germany), and Amicon Ultra 15, MWCO 100 K, was provided by Millipore (Billerica, MA, USA).
Preparation of H2bdtc-loaded solid lipid nanoparticles (H2bdtc-SLNs)
SLNs were prepared using a microemulsion method [31]. Briefly, the desired amount of sodium taurodeoxycholate (0.12% w/v) was dissolved in hot aqueous phase, which was added to melted stearic acid (0.95% w/v) containing soya lecithin (0.48% w/v) and H2bdtc (0.02% w/v). The mixture was emulsified via magnetic stirring at 90.0±2.0°C, until a thermodynamically stable microemulsion formed. The SLNs dispersion was obtained by cooling the hot microemulsion in cold water (2–5°C) under vigorous stirring at 20,000 rpm for 10 min (IKA-T25 Ultra-turrax, Germany) at 1∶20 ratio (microemulsion/cold water). Next, the SLNs aqueous dispersion was subjected to high-pressure homogenization (EmulsiFlex – C3, Germany) at 500 bars for 10 min.
Particle size, dispersity index (D-stroke), and zeta potential determination
The particle size and dispersity of the H2bdtc-SLNs dispersion were measured via photon correlation spectroscopy (PCS) [32]; the zeta potential was determined on the basis of the electrophoresis mobility of the nanoparticles using the Zetasizer ZS Nano 90 (Malvern Instruments, UK.). The samples were diluted (1∶10) with distilled water at 25.0°C.
Atomic Force Microscopy (AFM)
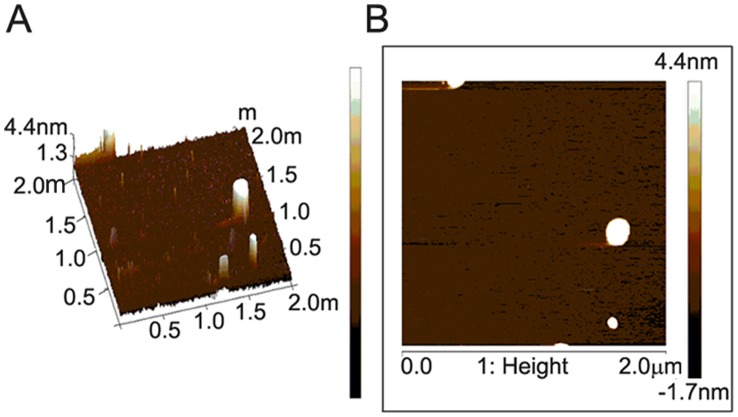
The morphology of H2bdtc-SLNs was assessed using an atomic force microscope (ICON Bruker, USA). The samples were prepared by immersing freshly cleaved mica (Muscovite Mica Substrates Sheets, SPI Supplies, China) in SLNs aqueous dispersion and stored overnight, at room temperature, to complete the drying process. The samples were evaluated by AFM in the intermittent contact mode (tapping mode) by scanning the surface of mica (2 µm×2 µm in area) using a rectangular silicon cantilever with a spring constant of 40 N m−1 vibrating at a frequency of 320 kHz. Imaging was performed at room temperature, and the topology image was used to determine the morphology of H2bdtc-SLNs [33].
Drug entrapment efficiency (EE%) determination
The total H2bdtc content in the H2bdtc-SLNs was determined by UV-vis spectroscopy at 400 nm (UV Spectrophotometer UV 1800, Shimadzu, Japan). First, a defined amount of H2bdtc-SLNs was dissolved in dimethyl sulfoxide. The amount of encapsulated drug was indirectly measured after centrifuging the H2bdtc-loaded SLNs for 40 min at 6000 rpm (1605 G), at 25°C in a centrifuge (Heraeus Megafuge 16 R Thermo Scientific, USA) equipped with a membrane concentrator (Amicon Ultra 15, MWCO 100 K, Millipore Corporation, USA). The filtrate was diluted with dimethyl sulfoxide (1∶1), and the concentration of free H2bdtc in the diluted filtrate was determined using the same conditions employed to measure the total H2bdtc content used during the loading procedure (section 2.2). The amount of H2bdtc loaded into SLNs was calculated by subtracting the amount of free H2bdtc in the filtrate from the total amount of H2bdtc used during loading (26). EE (%) was determined using the following equation [26], [34].
Partition coefficient of H2bdtc (K octanol/water)
Partition coefficients for the H2bdtc were determined in triplicate in an n- octanol/water system following a published procedure [35]. Measurements of H2bdtc n-octanol/water partition coefficients were carried out using the shake-flask method. H2bdtc was dissolved in aqueous solution previously saturated with n-octanol at a concentration of 1 mg/mL and mixed with the same volume of octanol also previously saturated with water. Samples were stirred for 30 min, separate in two phases, and centrifugated for 10 min at 2000 rpm. The amount of H2bdtc in the aqueous phase was quantified by UV-visible spectroscopy.
Mice
Female Swiss mice (6 to 8 weeks old) were bred and maintained at the Department of Biochemistry and Immunology, School of Medicine of Ribeirao Preto, University of São Paulo, Ribeirão Preto, Brazil. The mice were maintained in microisolator cages under standard conditions; they were fed with food and water ad libitum.
Ethics statement
All the in vivo procedures were performed in accordance with the guidelines issued by the Brazilian College of Animal experimentation (COBEA) and received prior approval by the Ethics Committee on Animal Experimentation – CETEA (n° 006/2011) of the School of Medicine of Ribeirão Preto.”
Parasites and experimental infection
All the experiments were conducted using the trypomastigote form of the Y strain of T. cruzi (Lineage type II). For the in vitro experiments, parasites were grown in a fibroblast cell line (LLC-MK2). For the in vivo experiments, mice were intraperitoneally inoculated with 2.0×103 bloodstream trypomastigote forms, which had been derived from previously infected Swiss mice.
In vitro evaluation of the trypanocidal activity and cytotoxicity of free H2bdtc and H2bdtc-loaded SLNs
The trypanocidal activities of free H2bdtc, H2bdtc-SLNs, and BZN against the trypomastigote form of the T. cruzi Y strains were evaluated as described previously [36]. To this end, the trypomastigote culture at a concentration of 6.5×106 parasites mL−1 was re-suspended in RPMI 1640 medium with 5% FBS. Triplicate cultures were treated with one of the investigated drugs and maintained at 37.0±0.1°C in a humidified atmosphere of 5% CO2. To test parasite viability, the number of motile forms was determined using a previously described method [37]. The concentration of compound corresponding to 50% trypanocidal activity after 24 h of incubation was expressed as the IC50try (inhibitory concentration for the trypomastigote form).
Spleen cells isolated from C57BL/6 mice, macerated in RPMI 1640 medium (Gibco), and filtered using a 100-µm pore filter were used to evaluate the cytotoxicity in vitro. The isolated cells were centrifuged at 1500 rpm for 10 min, and erythrocytes were lysed in lysis buffer for 5 min, at room temperature. Cells were washed, counted, and resuspended at 6.5×106 mL−1 in RPMI medium containing 5% fetal bovine serum. The spleen cells were seeded to a 96-well microplate (n = 2) and incubated for 24 h with H2bdtc diluted in dimethyl sulfoxide (DMSO, final H2bdtc concentration not exceeding 0.5%) or H2bdtc-SLNs (concentrations ranging from 125 µM to 0.24 µM in serial dilutions). BZN (Roche) was used as the reference drug; Tween 20 was employed as positive control for cell death. After the incubation period, the cells were washed and incubated with propidium iodide at a final concentration of 10 µg mL−1. Cell cytotoxicity was measured on a flow cytometer (FACSCantoII - BD), and the data were analyzed using the FlowJo program (Tree Star).
In vivo evaluation of the cytotoxicity and trypanocidal activity of free H2bdtc and H2bdtc-SLNs
Female Swiss mice aged between 6 and 8 weeks, weighing between 20 and 25 g, were infected with 2.0×103 blood trypomastigotes per animal. A total of four experimental groups consisting of seven Swiss mice each were included in the study. Treatment started at day 5 post-inoculation (p.i.). BZN, free H2bdtc and H2bdtc-SLNs were orally administered at 4 µmol kg−1 (BZN 1.0 mg kg−1 day-1/free H2bdtc and H2bdtc-SLNs 1.4 mg kg−1 day−1) per day for 10 consecutive days. The following treatments were applied: Group 1 = PBS control group; infected and not treated, Group 2 = infected and treated with BZN, Group 3 = infected and treated with free H2bdtc, and Group 4 = infected and treated with H2bdtc-SLNs. To evaluate parasitaemia and mortality, seven animals from each group were used. Seven animals were killed at day 22 p.i. (early mortality), to quantify inflammation of the heart and liver and to measure creatine kinase-MB (CK-MB) and glutamic-pyruvic transaminase (GPT) production.
Parasitemia and mortality
Parasitemia was analyzed on alternate days from day 7 p.i.; to this end, 5 µL of fresh blood was collected from the animal tail. The count of 100 fields was performed via direct observation under a light microscope [38]. Mortality was inspected on a daily basis until day 60.
Histological analysis
Groups of seven mice were euthanized at day 20 p.i., and portions of the heart and liver were fixed in paraffin for histological analysis. To assess inflammatory infiltration via light microscopy DP71 (Olympus Optical Co, Japan), tissues were sectioned at a 5-µm thickness and stained with hematoxylin-eosin (H&E). Each tissue section was imaged 25 times, and the percentage of the area occupied by cellular infiltrates was determined using the Image J program.
Quantitative real-time PCR (qPCR)
Quantitative PCR was used to determine the amount of parasitic DNA in heart tissues. Briefly, DNA was purified from 25 mg of heart tissue using a QIAamp DNA Mini Kit (Qiagen), according to the manufacturer's instructions. Each PCR reaction comprised 40 ng of genomic DNA; 0.3 µM of the T. cruzi-specific primers TCZ-F 5′-GCTCTTGCCCACAMGGGTGC-3′ (M = A or C) TCZ-R 5′-CCAAGCAGCGGATAGTTCAGG-3′ [39], which amplify a 182-bp product; 7.65 µL of GoTaq qPCR Master Mix; and H2O (final total volume of 15 µL).
The reactions were performed using the Real-Time PCR System. The cycling program involved a denaturation cycle of 95.0°C for 10 min, followed by 40 cycles of the three steps of the amplification phase: 95.0°C for 15 s, 55.0°C for 30 s, and 72.0°C for 15 s. The melting phase was performed at 95.0°C for 15 s and at 60.0°C for 1 min, followed by a 0.3°C ramp and then 95.0°C for 15 s. During the melting phase, the acquisition setting was set at step. The data were analyzed with StepOne Software version 2.2.2.
Serum activity of creatine kinase isoform MB (CK-MB) and glutamic-pyruvic transaminase (GPT)
The cardiac and hepatic lesions of mice infected with T. cruzi, treated or not, were assessed by measuring the creatine kinase-MB (CK-MB) and glutamic-pyruvic transaminase (GPT) levels, respectively, in the serum at day 22 p.i. The CK-MB levels were measured using a CK-MB kit (Liquiform, Brazil), as previously described [40]. Absorbance was measured on a microplate spectrophotometer (EMAX Molecular Devices Corporation, California, EUA). The color produced from this reaction was measured at a wavelength of 340 nm; the results are expressed in U/I. GPT was analyzed using an ALT/GPT kit (Liquiform, Brazil), according to the manufacturer's instructions. The colorimetric assay determines the amount of pyruvate produced according to the Reitman and Frankel method, from the formation of 2,4-dinitrophenylhydrazine [41]. The color produced by this reaction was measured at a wavelength of 505 nm.
Statistical analyses
Data are expressed as the mean SEM. Student's t-test was used to analyze the statistical significance of the variation between the infected and control assays. Differences were considered statistically significant when P<0.05. The differences in droplet size, dispersity, zeta potential, and entrapment efficiency values achieved during the stability test were evaluated via a one-way ANOVA analysis of variance followed by Tukey post-test analysis. The differences were considered statistically significant when P<0.05. All the analyses were performed using PRISM 5.0 software (Graph Pad, San Diego, CA, US).
Results
Preparation and characterization of H2bdtc-SLNs
The H2bdtc showed a lipophilic character (Log P (o/w) = 2.69±0.03) and were efficiently encapsulated in this manner in SLNs. On the basis of Photon Correlation Spectroscopy (PCS), H2bdtc-SLNs had diameter of 127.4±10.2 nm and dispersity lower than 0.3; the zeta potential revealed a negative surface charge (−56.1±4.4 mV) (Table 1). The entrapment efficiency was 98.16±1.12, showing that the drug dispersed well within the lipid matrix. Atomic force microscopy images revealed that H2bdtc-SLNs particles were spherical, with an average diameter of approximately 180 nm (Figure 2), agreeing with the PCS results.
Table 1. Particle size, zeta potential, dispersity index and entrapment efficiency of SLNs (mean ± SD, n = 3).
| Formulation | ||||
| Compounds | Mean Particle (nm) | D-Stroke | Zeta Potential (mV) | Entrapment Efficiency (%) |
| SLN | 132.3±10.2 | 0.260±0.0098 | −60.20±2.30 | 98.16±1.12 |
| H2bdtc-SLN | 127.4±0.130 | 0.229±0.130 | −56.10±4.40 | |
*Dispersity (D-stroke). SLNs: SLNs without drug loaded and H2bdtc loaded in SLNs: H2bdtc loaded in SLNs.
Figure 2. Atomic Force Microscopy micrographs of H2bdtc loaded in SLNs (A) 2 µm×2 µm three-dimensional image.
(B) 2 µm×2 µm planar image.
In vitro evaluation of the trypanocidal activity and cytotoxicity of free H2bdtc and H2bdtc- SLNs
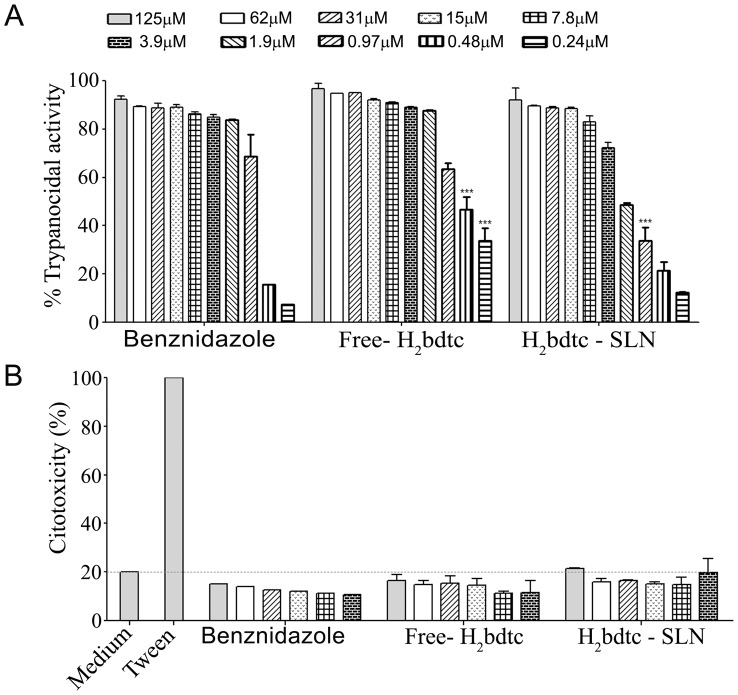
We assessed the in vitro trypanocidal activity of free H2bdtc, H2bdtc-SLNs and BZN after 24 h of incubation with T. cruzi trypomastigotes forms. Free H2bdtc presents IC50try (inhibitory concentrations against bloodstream trypomastigote) as 0.50±0.12, H2bdtc-SLNs as 1.83±0.18 and BZN 0.50±0.39 µM (Figure 3A). We also measured the cytotoxicity of free H2bdtc and H2bdtc-SLNs in spleen cells of Swiss mice; none of the tested drugs was significantly cytotoxic (Figure 3B). Hence, both free H2bdtc and H2bdtc-SLNs displayed similar in vitro trypanocidal activity to BZN; this activity was not associated with general cytotoxicity but rather with specific activity against the parasite.
Figure 3. In vitro evaluation of the trypanocidal activity and cytotoxicity of H2bdtc – (concentrations range from 125 µM to 0.24 µM in serial dilutions).
(A) Percentage of trypanocidal activity of free H2bdtc and H2bdtc loaded in SLNs against the Y strain of T. cruzi analyzed by quantifying viable parasites via microscopy after 24 h post-treatment. (B) Percentage of cytotoxicity of free H2bdtc and H2bdtc loaded in SLNs in spleen cells derived from mice after 24 h via propidium iodide treatment and FACS analysis. The mean + SEM is shown and is representative of three independent experiments (n = 2). Statistically significant differences compared with the control (BZN). Anova, Bonferroni post - test: ***p<0.001
In vivo activity of free H2bdtc and H2bdtc-SLNs
We performed in vivo experiments to investigate the controlled release behavior of H2bdtc from H2bdtc-SLNs; we also compared the activities of free H2bdtc and H2bdtc-SLNs against T. cruzi. We decided to use an H2bdtc-SLNs concentration of 4 µmol kg−1 day−1. During the in vivo treatments on the basis of preliminary in vivo results obtained for the Y strain of T. cruzi, which revealed that H2bdtc had low level of parasitemia (Supporting Information: Figure S1). In all the infected groups, parasitemia peaked at day 9 p.i., with gradual parasite elimination from the bloodstream after day 11 p.i. It is worth noting that we employed BZN concentrations 100 times lower than that used for Chagas patients. H2bdtc-SLNs eliminated 70% of the circulating parasites at the peak of infection, whereas free H2bdtc and the positive control BZN eliminated 48 and 15% of the parasites, respectively, as compared with the control group treated with PBS (Figure 4A). In agreement with the data revealing reduced parasitemia, mice treated with H2bdtc-SLNs presented 100% survival rate (Figure 4B), similar to the result achieved with BZN administered at a clinical dose of 400 µmol kg−1 day−1 (100 times more concentrated than the concentration used herein). Compared with the control group (PBS), groups treated with free H2bdtc and BZN exhibited a survival rate of 57%.
Figure 4. Parasitaemia and survival rates of mice infected with T. cruzi and treated with free H2bdtc, H2bdtc encapsulated in SLNs and BZN.
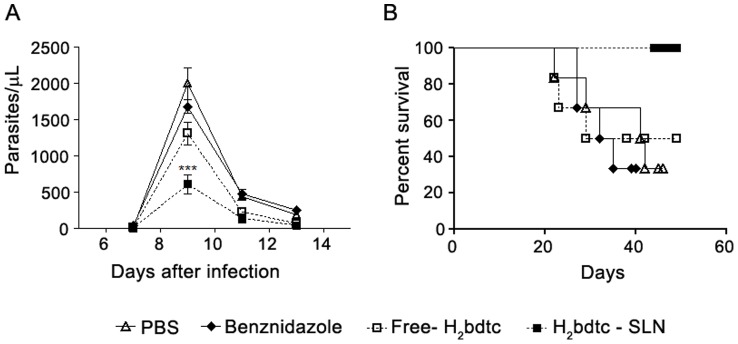
(A) Parasitaemia was monitored on days 7, 9, 11 and 13 after infection. (B) Survival was monitored daily for 60 consecutive days. The mean + SEM is shown and is representative of three independent experiments (n = 7). Statistically significant differences compared with the control (BZN). T student test: ***p<0.001
Cardiac and liver lesions
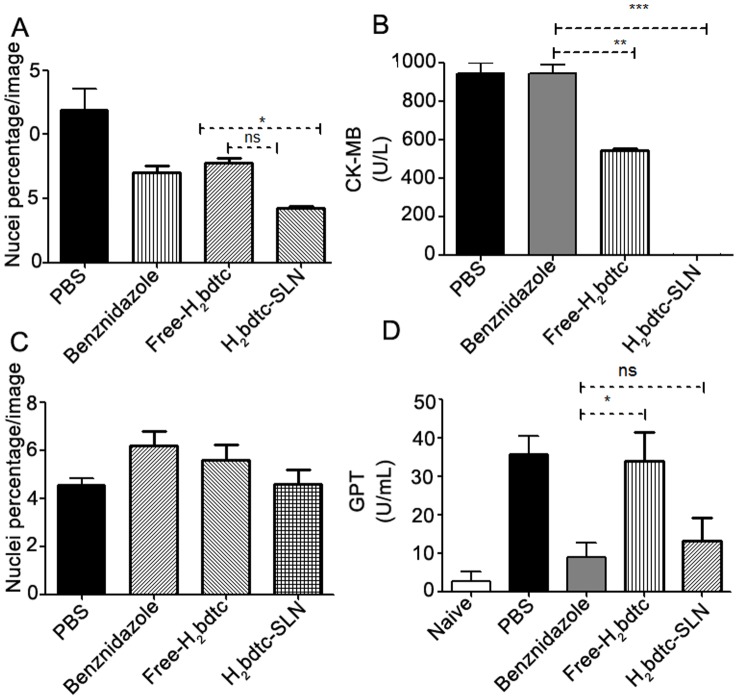
Encouraged by the in vivo results, we evaluated how free H2bdtc, H2bdtc-SLNs, and BZN affected the cardiac and hepatic tissues of the surviving animals. Infected mice treated with H2bdtc-SLNs presented reduced cardiac inflammation (Figure 5A) and heart lesions were absent (Figure 5B), as established by the absence of CK-MB, the enzyme released into plasma during cardiac lesion. Treatment with free H2bdtc diminished cardiac damage by 50% as compared with therapies with BZN or PBS. Concerning the ability of the tested compounds to reduce the liver damage caused by the parasite, H2bdtc-SLNs decreased inflammatory infiltration in the liver and hepatic toxicity more effectively, as assessed by measuring the glutamic-pyruvic transaminase (GTP) levels in the serum (Figure 5C, 5D). Considering all these results, it is possible to infer that treatment with H2bdtc per se reduced exacerbation of the inflammatory response on T. cruzi target organs and, consequently, tissue damage. Loading of H2bdtc into nanoparticles afforded even better results, producing no lesion in the heart tissue.
Figure 5. Cardiac and liver lesions of T. cruzi- infected animals after treatment with H2bdtc encapsulated in solid lipid nanoparticles.
(A) Quantification of cellular nuclei per 50 µm2 of heart tissues derived from non-treated and treated animals. (B) Quantification of CK-MB in the serum of infected and treated mice. (C) Quantification of cellular nuclei per 50 µm2 of liver tissues derived from non-treated and treated animals. (D) Quantification of glutamic-pyruvic transaminase (GPT) levels. The mean + statistically significant differences compared with the control are denoted by: *p<0.05, **p<0.01 e ***p<0.001, T student test.
Because it is well established that parasites play an important role in cardiac damage during T. cruzi infection [42], [43], we quantified T. cruzi DNA derived from the heart tissues of mice treated with the tested compounds via real-time PCR. Treatment with H2bdtc-SLNs reduced the parasite burden significantly more effectively as compared with the other tested drugs (Figure 6), indicating that killing the parasites is most likely the mechanism through which H2bdtc-SLNs acts to diminish tissue lesions and enhance mice survival.
Figure 6. Quantification of the parasites load in cardiac tissues via real-time PCR.
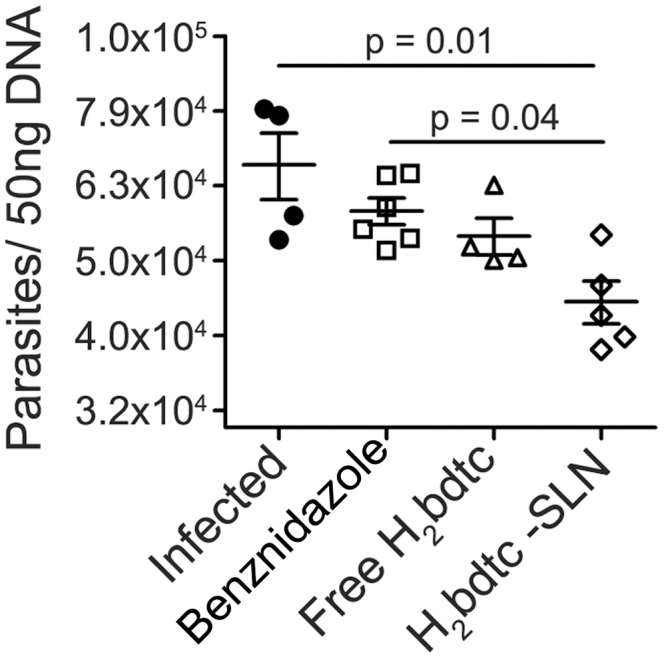
The presence of T. cruzi in infected heart tissue mice were analyzed by PCR 22days after infection. Statistically significant differences were showed in the figure.
Discussion
Chagas disease has often been pointed out as being a major neglected disease; the drugs that are currently available to treat this disease are little effective [5], [12]. Efforts have been made to provide the affected populations with new compounds to treat the disease. In the past few years, researchers have tested many substances against T. cruzi. In particular, H2bdtc, which belongs to the class of S-dithiocarbazates, is efficient against the parasite [21]. A 24-h UV-vis study into the stability of H2bdtc in aqueous solution did not evidence any changes in the spectrum of this compound. Nevertheless, this drug is poorly soluble in water (1.50×10−6 M), which has limited its use to treat Chagas disease. Because H2bdtc is lipophilic (Log P (o/w) = 2,69±0,03) and SLNs constitute effective oral drug delivery systems, we loaded H2bdtc into this type of lipid.
We prepared the SLNs by the microemulsion method [31], [44], to avoid the use of organic solvents. The resulting SLNs had diameter of approximately 120 nm, dispersity lower than 0.3, and spherical shape, which made these lipids suitable for oral administration [45], [46], [47], [48]. The zeta potential measurement allowed us to predict the stability of the colloidal dispersion. Charged particles have high zeta potential–negative or positive–and usually do not aggregate [49]. The zeta potential results revealed that the SNPs prepared here had a negative surface charge (−56.1±4.4 mV), indicating that the system was physically stable. Loaded and unloaded SNPs had similar zeta potentials, attesting that the tested drug was completely and uniformly dispersed inside the lipid matrix [50].
Encapsulation did not change the in vitro trypanocidal activity of H2bdtc, which was higher than the activity of BZN at the same concentration used here. The IC50 values obtained for H2bdtc against the trypomastigote form of the Y strain of T. cruzi were comparable with or superior to those of previously reported active compounds [51], [52]. Other papers have also described the use of colloidal drug carriers such as liposomes and nanoparticles to treat Chagas diseases [53], [54]. Treatment of T. cruzi infection with a BZN-loaded liposome increased BZN levels in the liver and blood. Intravenous administration of free BZN and BZN-encapsulated liposome at 0.2 mg of BZN per kilogram of body weight revealed three-fold higher BZN accumulation in the liver in the second case. Nevertheless, encapsulation failed to improve the in vivo BZN efficacy [55].
Liposome instability prevents their use as drug delivery systems [56]. Fortunately, we verified that free H2bdtc and H2bdtc-SLNs were not toxic to the spleen cells of Swiss mice, which encouraged us to directly test the effect of H2bdtc formulations in vivo using a murine model of acute Chagas disease.
Treatment started with a relatively low oral dose of free H2bdtc and H2bdtc-SLNs (4 µmol kg−1 day−1) as compared with currently employed doses of the commercially available BZN and compounds tested in the literature [57], [58]. H2bdtc-SLNs, Free H2bdtc and BZN reduced the presence of parasites in the blood of infected mice in 70, 48 and 15% respectively. H2bdtc-SLNs maintained 100% survival rate of infected mice, whereas 43% of the mice treated with free H2bdtc or BZN at the same concentration succumbed to the disease. It is noteworthy that free SLN and PBS elicited similar levels of parasitemia (Supporting Information: Figure S2). Therefore, the use of SLNs as a drug delivery system increased the oral bioavailability of the target drug, as previously described [59], [60], [61], [62], [63]. H2bdtc loading into SLNs overcame the problems inherent to the poor water solubility of the compound and may be could make it more accessible to the parasite (however, detailed pharmacokinetic data will be presented in a separate forthcoming paper). Additionally, some authors have proposed that drugs loaded into SLNs measuring 20–500 nm are absorbed by lymphatic transport, which reduces the first-pass metabolism [62], [63].
Analysis of histological sections of liver and heart tissues (Supporting Information: Figure: Figure S3 and Figure S4) revealed that the inflammatory infiltrate decreased in all the treated groups as compared with the control. The reduction was more pronounced in mice treated with H2bdtc-SLNs, possibly because parasitemia was lower in this case. This corroborated with findings from previous studies [64], [65] and confirmed that the parasite elicited intense inflammation especially in the cardiac tissues. The fact that the heart tissues of mice treated with H2bdtc-SLNs were perfectly preserved agreed with the notion that the presence of inflammatory infiltrates is associated with cardiac tissue damage [66], [67] and also with parasitic load [42], [43]. Indeed, mice treated with H2bdtc-SLNs exhibited significantly lower parasite burden as compared with the other groups. Hence, the reduced parasitism elicited by H2bdtc-SLNs helps to preserve the heart tissues of mice infected with T. cruzi, allowing us to conclude that H2bdtc is a potent trypanocidal agent.
Investigation into how H2bdtc interacts with possible targets represents a theme for future studies. For the time being, we must bear in mind that triazoles and thiosemicarbazones are well known for inhibiting cruzain, a protein belonging to the family of cysteine proteases and which is the most abundant protein in T. cruzi. Cruzain is essential for parasite development and survival within host cells [68]. H2bdtc bears pyrazole and dithiocarbazate parts, which are similar to triazoles and thiosemicarbazones, respectively, and could account for its trypanocidal action.
A mechanism of action similar to that of BZN probably does not occur. The BZN mode of action involves intracellular reduction of the nitro group, to produce highly reactive free radicals and/or electrophilic metabolites that could affect other systems, especially host systems, contributing to the cytotoxic effects observed in BZN-treated patients [69].
It is worth noting that cysteine proteases are very important for parasites; however, the lack of redundancy with respect to their mammalian hosts makes these proteases interesting targets for the development of new therapeutic agents [70]. Altogether, our findings show that H2bdtc-SLNs are a possible drug candidate to treat Chagas disease: it is more efficient against T. cruzi than the drugs used in current therapies.
Supporting Information
In vivo evaluations of the trypanocidal activity free-H2bdtc and H2bdtc-SLNs – (concentrations 4 µM, 40 µM and 80 µM) (_____) Parasitaemia rate of mice infected with T. cruzi and treated with free-H2bdtc. (----) Parasitaemia rate of mice infected with T. cruzi and treated with H2bdtc encapsulated in solid lipid nanoparticles. Parasitaemia was monitored on days 7, 9, 11 and 13 after infection. The mean + SEM is shown and is representative of three independent experiments (n = 7). Statistically significant differences compared with the free-H2bdtc. T student test: **p<0.01 and ***p<0.001.
(TIF)
Parasitaemia of mice infected with T. cruzi and treated with free SLNs and PBS. Parasitaemia was monitored on days 7, 9, 11 and 13 after infection.
(TIF)
Cardiac lesions of T. cruzi-infected animals after treatment with H2bdtc encapsulated in SLNs. The sections represent of heart tissues inflammatory process composed of various cell types (21 days after infection).
(TIF)
Liver lesions of T. cruzi-infected animals after treatment with H2bdtc encapsulated in SLNs. The sections represent of liver tissues inflammatory process composed of various cell types (21 days after infection).
(TIF)
Acknowledgments
We give special thanks to Dr. Denise Brufato Ferraz and Professor Roberto S. da Silva for their technical support.
Funding Statement
This work was supported by the Research Foundation of the State of São Paulo” (FAPESP 2010/20610-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lountos GT, Tropea JE, Waugh DS (2013) Structure of the Trypanosoma cruzi protein tyrosine phosphatase TcPTP1, a potential therapeutic target for Chagas' disease. Molecular and Biochemical Parasitology 187 1–8: doi.org/10.1016/j.molbiopara.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coura JR, Vinas PA (2010) Chagas disease: a new worldwide challenge. Nature 465: S6–S7. [DOI] [PubMed] [Google Scholar]
- 3. Clayton J (2010) Chagas disease 101. Nature 465: S4–S5. [DOI] [PubMed] [Google Scholar]
- 4. Petherick A Ventura-Garcia L, Roura M, Pell C, Posada E (2013) Socio-Cultural Aspects of Chagas Disease: A Systematic Review of Qualitative Research. PLoS Negl Trop Dis 7: e2410 10.1371/journal.pntd.0002410.t002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reidpath D, Allotey P, Pokhrel S (2011) Social sciences research in neglected tropical diseases 2: A bibliographic analysis. Health Res Policy and Systems 9: 1 10.1186/1478-4505-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (2010) First WHO report on neglected tropical diseases. Available: http://www.who.int/neglected_diseases/2010report/en/. Accessed in 23 August 2013.
- 7. Guedes PM, Silva GK, Gutierrez FR, Silva JS (2011) Current status of Chagas disease chemotherapy. Expert Rev Anti-infective Therapy 9: 609–620 10.1586/eri.11.31 [DOI] [PubMed] [Google Scholar]
- 8. Urbina JA (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115: 55–68 10.1016/j.actatropica.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 9. Murcia L, Carrilero B, Segovia M (2012) Limitations of currently available Chagas disease chemotherapy. Rev Española de Quimioterapia 25: 1–3. [PubMed] [Google Scholar]
- 10.Viotti R, Alarcón de Noya B, Araujo-Jorge T, Grijalva MJ, Guhl F, López MC, Ramsey JM, Ribeiro I, Schijman AG, Sosa-Estani S, Torrico F, Gascon J (2013) Towards a Paradigm Shift in the Treatment of Chronic Chagas Disease. Antimicrobial Agents and Chemotherapy 58: : 263–269. Doi 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selzer PM (2013)Trypanosomatid Diseases: Molecular Routes to Drug Discovery, Drug Discovery in Infectious Diseases: European Cooperation in Science and Tecnology 450 p. [Google Scholar]
- 12. Castro A, Meca MM, Bartel LC (2006) Toxic Side Effects of Drugs Used to Treat Chagas'Disease (American Trypanosomiasis). Human Exp toxicol 25: 471–479 10.1191/0960327106het653oa [DOI] [PubMed] [Google Scholar]
- 13. Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H (2011) Adverse Events After the Use of Benznidazole in Infants and Children With Chagas Disease. Pediatrics 127: e212–e218 10.1542/peds.2010-1172 [DOI] [PubMed] [Google Scholar]
- 14. Abad-Franch F, Diotaiuti L, Gurgel-Gonçalves R, Gürtler R E (2013) Certifying the interruption of Chagas disease transmission by native vectors: cui bono? Mem. Inst. Oswaldo Cruz 108: 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guedes PMM, Gutierrez FRS, Nascimento MSL, Do-Valle-Matta MA, Silva JS (2012) Antiparasitical chemotherapy in Chagas' disease cardiomyopathy: current evidence. Trop Med and Int Health 17: 1057–1065 10.1111/j.1365-3156.2012.03025.x [DOI] [PubMed] [Google Scholar]
- 16. Beshir AB, Guchhai SK, Gascón JA, Fenteany G (2008) Synthesis and structure–activity relationships of metal–ligand complexes that potently inhibit cell migration. Bioorg Med Chem Lett 18: 498–504 10.1016/j.bmcl.2007.11.099 [DOI] [PubMed] [Google Scholar]
- 17. Tarafder MTH, Jin KT, Crouse KA, Ali AM, Yamin BM, et al. (2002) Coordination chemistry and bioactivity of Ni2+, Cu2+, Cd2+ and Zn2+ complexes containing bidentate Schiff bases derived from S-benzyldithiocarbazate and the X-ray crystal structure of bis[S-benzyl-β-N-(5-methyl-2-furylmethylene) dithiocarbazato] cadmium (II). Polyhedron 21: 2547–2554. [Google Scholar]
- 18. Pavan FR, Maia PIS, Leite SRA, Deflon VM, Batista AA, et al. (2010) Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti – Mycobacterium tuberculosis activity and cytotoxicity. Eur J Med Chem 45: 1898–1905 10.1016/j.ejmech.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 19. Maurya MR, Kumar A, Bhat AR, Azam A, Bader C, et al. (2006) Dioxo- and Oxovanadium(V) Complexes of Thiohydrazone ONS Donor Ligands: Synthesis, Characterization, Reactivity, and Antiamoebic Activity. J Inorg Chem 45: 1260–1269 10.1021/ic050811 [DOI] [PubMed] [Google Scholar]
- 20. Tampouris K, Coco S, Yannopoulos A, Koinis S (2007) Palladium(II) complexes with S-benzyl dithiocarbazate and S-benzyl-N-isopropylidenedithiocarbazate: Synthesis, spectroscopic properties and X-ray crystal structures. Polyhedron 26: 4269–4275 10.1016/j.poly.2007.05.039 [DOI] [Google Scholar]
- 21. Maia PIS, Fernandes AGA, Silva JJN, Andricopulo AD, Lemos SS, et al. (2010) Dithiocarbazate complexes with the [M(PPh3)]2+ (M = Pd or Pt) moiety: Synthesis, caracterization and anti-Tripanosoma cruzi activity. J Inorg Biochem 104: 1276–1282 10.1016/j.jinorgbio.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 22. Silva AC, Kumar A, Wild W, Ferreira D, Santos D, et al. (2012) Long-term stability, biocompatibility and oral delivery potential of Risperidone-loaded solid nanoparticles. Int J Pharm 15: 798–805 10.1016/j.ijpharm.2012.07.058 [DOI] [PubMed] [Google Scholar]
- 23. Chakraborty S, Shukla D, Mishra B, Singh S (2009) Lipid: an emerging plataform for oral delivery of drugs with poor bioavailability. Eur J Pharm and Biopharm 75: 1–15 10.1016/j.ejpb.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 24. Muller RH, Mehnert W, Lucks JS, Schawarz C, Zur Muhlen A, et al. (1995) Solid lipid nanoparticles (SLNs) – an alternative colloidal carrier system for controlled drug delivery. Eur J Pharm and Biopharm 41: 62–69. [Google Scholar]
- 25. Muller RH, Radtke M, Wissing SA (2002) Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Delivery Revs 54: 131–155 10.1016/S0169-409X(02)00118-7 [DOI] [PubMed] [Google Scholar]
- 26. Taveira SF, Araújo LMPC, de Santana DCAS, Nomizo A, Freitas LAP, Lopez RFV (2012) Development of Cationic Solid Lipid Nanoparticles with Factorial Design-Based Studies for Topical Administration of Doxorubicin. J Biomed Nanotechnol 8: 219–228 10.1166/jbn.2012.1383 [DOI] [PubMed] [Google Scholar]
- 27. Morales-Neto R, Hulshof L, Ferreira CV, Gadelha FR (2009) Distinct Phosphatase Activity Profiles in Two Strains of Trypanosoma cruzi. Journal of Parasitology 95: 1525–1531 10.1645/GE-1899.1 [DOI] [PubMed] [Google Scholar]
- 28. Buscaglia CA, Di Noia JM (2003) Trypanosoma cruzi clonal diversity and the epidemiology of Chagas' disease. Microbes and Infection 5: 419–427 10.1016/S1286-4579(03)00050-9 [DOI] [PubMed] [Google Scholar]
- 29. Ekambaram P, Sathali Aah, Priyanka K (2012) Solid Lipid Nanoparticles: A Review. Sciences Reviews Chemical Communcation 2: 80–102. [Google Scholar]
- 30. Salomon CJ (2012) First century of chagas' disease: An overview on novel approaches to nifurtimox and benznidazole delivery systems. J Pharm Sci 101: 888–894 10.1002/jps.23010 [DOI] [PubMed] [Google Scholar]
- 31. Marquele-Oliveira F, Santana DCA, Taveira SAF, Vermeulen DM, Oliveira ARM, et al. (2010) Development of nitrosyl ruthenium complex-loaded lipid carriers for topical administration: improvement in skin stability and in nitric oxide release by visible light irradiation. J Pharm and Biom Anal 53: 843–851 10.1016/j.jpba.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 32. Kovačević AB, Müller RH, Savić SD, Vuleta GM, Keck CM (2014) Solid lipid nanoparticles (SLN) stabilized with polyhydroxy surfactants: Preparation, characterization and physical stability investigation. Colloids and Surfaces A: Physicochem. Eng. Aspects 444: 15–25 10.1016/j.colsurfa.2013.12.023 [DOI] [Google Scholar]
- 33. Potta SG, Minemi S, Nukala RK, Peinado C, Lamprou DA, et al. (2011) Preparation and characterization of ibuprofen solid lipid nanoparticles with enhanced solubility. J Microencapsulation 28: 47–81 10.3109/02652048.2010.529948 [DOI] [PubMed] [Google Scholar]
- 34. Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N (2009) Preparation and Evaluation of Miconazole Nitrate-Loaded Solid Lipid Nanoparticles for Topical Delivery. AAPS PharmSciTech 10: 289–296 Doi: 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopes RF, Collett JH, Bentley MV (2000) Influence of cyclodextrin complexation on the in vitro permeation and skin metabolism of dexamethasone. Int J Pharm 200: 127–132 10.1016/S0378-5173(00)00365-3 [DOI] [PubMed] [Google Scholar]
- 36. Silva JJ, Osakabe AL, Pavanelli WR, Silva JS, Franco DW (2007) In vitro and in vivo antiproliferative and trypanocidal activities of ruthenium NO donors. Br J Pharmacol 152 (1): 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop 4: 389–396. [PubMed] [Google Scholar]
- 38. Brener Z (1962a) Observations on immunity to superinfections inmice experimentally inoculated with Trypanosoma cruzi and subjected to treatment. Rev Inst Med Trop 4: 119–123. [PubMed] [Google Scholar]
- 39. Cummings KL, Tarleton RL (2003) Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol Biochem Parasitol 129: 53–59 10.1016/S0166-6851(03)00093-8 [DOI] [PubMed] [Google Scholar]
- 40. Maekawa M, Sugiura A, Iwahara K, Sakai Y, Kishi K (2012) Effect of the Inhibition of mitochondrial Creatine Kinase Activity on the Clinical Diagnosis of Suspected Acute Myocardial Infarction. The Open Clin Chem J 5: 1–6. [Google Scholar]
- 41. Reitman S, Frankel S (1957) Glutamic – pyruvate transaminase assay by colorimetric method. Am J Clin pathol 28: 56–63. [DOI] [PubMed] [Google Scholar]
- 42. Borges DC, Araujo NM, Cardoso CR, Chica JEL (2012) Different parasite inocula determine the modulation of the immune response and outcome of experimental Trypanosoma cruzi infection. Immunology 138: 145–156 10.1111/imm.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corral RS, Guerrero NA, Cuervo H, Gironès N, Fresno M (2013) Trypanosoma cruzi Infection and Endothelin-1 Cooperatively Activate Pathogenic Inflammatory Pathways in Cardiomyocytes. PLoS Negl Trop Dis 7: e2034 10.1371/journal.pntd.0002034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Z, Bu H, Gao Z, Huang Y, Gao F, et al. (2010) The characteristics and mechanism of simvastatin loaded lipid nanoparticles to increase oral bioavailability in rats. Int J Pharm 394: 147–153 10.1016/j.ijpharm.2010.04.039 [DOI] [PubMed] [Google Scholar]
- 45. Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H (2009) Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Controlled Release 133: 238–244 10.1016/j.jconrel.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 46. Mehwert W, Mader K (2012) Solid lipid nanoparticles: production, characterization and applications. Adv drug delivery revs 64: 83–101 10.1016/S0169-409X(01)00105-3 [DOI] [PubMed] [Google Scholar]
- 47. Gasco MR (1993) Method for producing solid lipid nanoespheres have a narrow size distribution. US Patent 5: 250–236. [Google Scholar]
- 48. Chen CC, Tsai TH, Huang ZR, Fang JY (2010) Effects of lipophilic emulsifier on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur J Pharm and Biopharm 74: 474–482 10.1016/j.ejpb.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 49. Monshi A, Salehi R, Fathi MH, Karbasi S, Pieles U, Daniels AU (2012) Preparation, chemistry and physical properties of bone-derived hydroxyapatite particles having a negative zeta potential. Mater Chem Phys 132: 446–452 10.1016/j.matchemphys.2011.11.051 [DOI] [Google Scholar]
- 50. Varshosaz J, Eskandari S, Tabbakhian M (2012) Freeze - drying of nanostructure lipid carriers by different carbohydrate polymers used as cryoprotectants. Carbohydr Polym 88: 1157–1163 10.1016/j.carbpol.2012.01.051 [DOI] [Google Scholar]
- 51. Moreira DRM, Costa SPM, Hernandes MZ, Rabello MM, Filho GBO (2012) Structural Investigation of Anti-Trypanosoma Cruzi 2-Iminothiazolidin-4-ones Allows the Identification of agents with Efficacy in Infected Mice. Med Chem Res 55: 10918–10936 10.1021/jm301518v [DOI] [PubMed] [Google Scholar]
- 52. Caputto ME, Ciccarelli A, Frank F, Moglionia AG, Moltrasio GY (2012) Synthesis and biological evaluation of some novel 1-indanone thiazolylhydrazone derivatives as anti-Trypanosoma cruzi agents. Eur J Med Chem 55: 155–163 10.1016/j.ejmech.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 53. González-Martin G, Merino I, Rodríguez-Cabezas M, Torres M, Núñez R (1998) Characterization and tripanocidal activity of nifurtimox-containing and empty nanoparticles of polyethylcianoacrylates. J Pharm and Pharmacool 50: 29–35. [DOI] [PubMed] [Google Scholar]
- 54. Sánchez G, Cuellar D, Zulantay I, Gajardo M, González-Martin G (2002) Cytotoxicity and trypanocidal activity of nifurtimox encapsulated in ethylcyanoacrylate nanoparticles. Biol Res 35: 39–45. [DOI] [PubMed] [Google Scholar]
- 55. Morilla MJ, Montanari JA, Prieto MJ, Lopez MO, Petray PB (2004) Intravenous liposomal BZN as trypanocidal agent: increasing drug delivery to liver is not enough. Int J Pharm 8: 8278–280 10.1016/j.ijpharm.2004.03.025 [DOI] [PubMed] [Google Scholar]
- 56. Date AA, Joshi MD, Patravale VB (2007) Parasitic diseases: Liposomes and polymeric nanoparticles versus lipid nanoparticles. Advanced drug delivery reviews 59: 505–521 10.1016/j.addr.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 57. Coura JR (2009) Present situation and new strategies for Chagas disease chemotherapy - a proposal. Mem Inst Oswaldo Cruz 104: 549–554 10.1590/S0074-02762009000400002 [DOI] [PubMed] [Google Scholar]
- 58. Zhu X, Liu Q, Yang S, Parman T, Green CE (2012) Evaluation of Arylimidamides DB1955 and DB1960 as Candidates against Visceral Leishmaniasis and Chagas' Disease: In Vivo Efficacy, Acute Toxicity, Pharmacokinetics, and Toxicology Studies. Antimicrob agents and chemother 56: 3690–3699 10.1128/AAC.06404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burra M, Jukanti R, Janga KY, Sunkavali S, Velpula A (2013) Enhanced intestinal absorption and bioavailability of raloxifene hydrochloride via lyophilized solid lipid nanoparticles. Adv powder technol 24: 393–402 10.1016/j.apt.2012.09.002 [DOI] [Google Scholar]
- 60. Singh A, Ahmad I, Akhter S, Jain GK, Iqbal Z (2013) Nanocarriers based formulation of Thymoquinone improves oral delivery: stability assessment, in vitro and in vivo studies. Colloids and Surfaces B: Biointerfaces 102: 822–832 10.1016/j.colsurfb.2012.08.038 [DOI] [PubMed] [Google Scholar]
- 61. Chalikwar SS, Belgamwar VS, Talele VR, Surana SJ, Patil MU (2012) Formulation and evaluation of Nimmodipine-loaded solid lipd nanoparticles delivered via lymphatic transport system. Colloids and Surfaces B: Biointerfaces 97: 109–116 10.1016/j.colsurfb.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 62. Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R (2012) Design and evaluation of polymer coated carvedilol loaded solid lipid nanoaprticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids and Surfaces B: Biointerfaces 95: 1–9 10.1016/j.colsurfb.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 63. Tiwari R, Pathak K (2011) Nanostructured lipid carriers versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharmacol 415: 232–243 10.1016/j.ijpharm.2011.05.044 [DOI] [PubMed] [Google Scholar]
- 64. Machado MPR, Rocha AM, Oliveira LF, Cuba MB, Loss LO (2012) Autonomic nervous system modulation affects the inflammatory immune response in mice with acute Chagas disease. Exp Physiol 97: 1186–1202 10.1113/expphysiol.2012.066431 [DOI] [PubMed] [Google Scholar]
- 65. Torrecilhas ACT, Tonelli RR, Pavanelli WR, Silva JS, Schumacher RI (2009) Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect 11: 29–39 10.1016/j.micinf.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 66. Bonney KM, Gifford KM, Taylor JM, Chen CI, Engman DM (2013) Cardiac damage induced by immunization with heat-killed Trypanosoma cruzi is not antibody mediated. Parasite Immunol 35: 1–10 10.1111/pim.12008 [DOI] [PubMed] [Google Scholar]
- 67. Berríos AM, Estrada CC, Lapier M, Duaso J, Kemmerling U (2013) BZN prevents endothelial damage in experimental model of Chagas disease. Acta Trop 127: 6–13 10.1016/j.actatropica.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 68. Dias LC, Dessoy MA, Silva JJN, Thiemann OH, Oliva G (2009) Chemotherapy of Chagas' disease: state of the art and perspectives for the development of new drugs. Quim Nova 32: 2444–2457 10.1590/S0100-40422009000900038 [DOI] [Google Scholar]
- 69. Castro JA, Mecca MM, Bartel LC (2006) Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Human Exp Toxicol 25: 471–479. [DOI] [PubMed] [Google Scholar]
- 70. Chena YT, Liraa R, Hansellc E, McKerrowc JH, Rousha WR (2008) Synthesis of macrocyclic trypanosomal cysteine protease inhibitors. Bioorg Med Chem Lett 18: 5860–5863 10.1016/j.bmcl.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo evaluations of the trypanocidal activity free-H2bdtc and H2bdtc-SLNs – (concentrations 4 µM, 40 µM and 80 µM) (_____) Parasitaemia rate of mice infected with T. cruzi and treated with free-H2bdtc. (----) Parasitaemia rate of mice infected with T. cruzi and treated with H2bdtc encapsulated in solid lipid nanoparticles. Parasitaemia was monitored on days 7, 9, 11 and 13 after infection. The mean + SEM is shown and is representative of three independent experiments (n = 7). Statistically significant differences compared with the free-H2bdtc. T student test: **p<0.01 and ***p<0.001.
(TIF)
Parasitaemia of mice infected with T. cruzi and treated with free SLNs and PBS. Parasitaemia was monitored on days 7, 9, 11 and 13 after infection.
(TIF)
Cardiac lesions of T. cruzi-infected animals after treatment with H2bdtc encapsulated in SLNs. The sections represent of heart tissues inflammatory process composed of various cell types (21 days after infection).
(TIF)
Liver lesions of T. cruzi-infected animals after treatment with H2bdtc encapsulated in SLNs. The sections represent of liver tissues inflammatory process composed of various cell types (21 days after infection).
(TIF)