Abstract
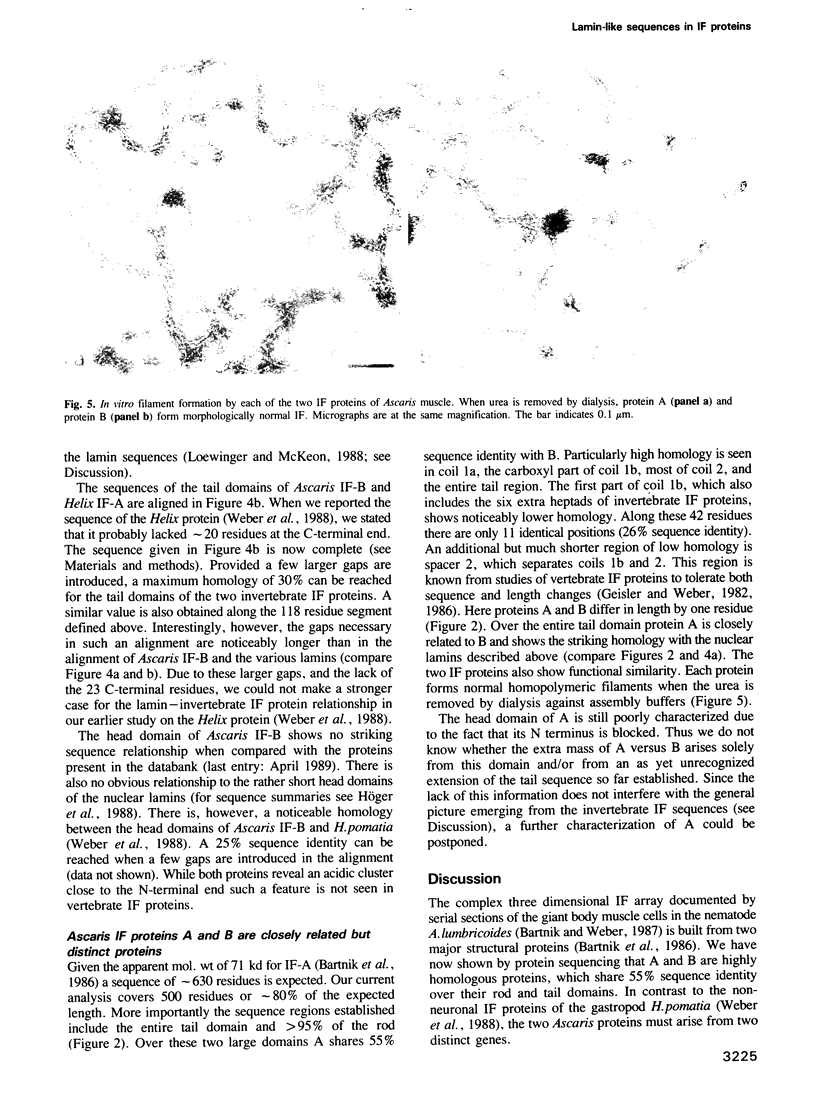
The giant body muscle cells of the nematode Ascaris lumbricoides show a complex three dimensional array of intermediate filaments (IFs). They contain two proteins, A (71 kd) and B (63 kd), which we now show are able to form homopolymeric filaments in vitro. The complete amino acid sequence of B and 80% of A have been determined. A and B are two homologous proteins with a 55% sequence identity over the rod and tail domains. Sequence comparisons with the only other invertebrate IF protein currently known (Helix pomatia) and with vertebrate IF proteins show that along the coiled-coil rod domain, sequence principles rather than actual sequences are conserved in evolution. Noticeable exceptions are the consensus sequences at the ends of the rod, which probably play a direct role in IF assembly. Like the Helix IF protein the nematode proteins have six extra heptads in the coil 1b segment. These are characteristic of nuclear lamins from vertebrates and invertebrates and are not found in vertebrate IF proteins. Unexpectedly the enhanced homology between lamins and invertebrate IF proteins continues in the tail domains, which in vertebrate IF proteins totally diverge. The sequence alignment necessitates the introduction of a 15 residue deletion in the tail domain of all three invertebrate IF proteins. Its location coincides with the position of the karyophilic signal sequence, which dictates nuclear entry of the lamins. The results provide the first molecular support for the speculation that nuclear lamins and cytoplasmic IF proteins arose in eukaryotic evolution from a common lamin-like predecessor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Bartnik E., Osborn M., Weber K. Intermediate filaments in muscle and epithelial cells of nematodes. J Cell Biol. 1986 Jun;102(6):2033–2041. doi: 10.1083/jcb.102.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik E., Osborn M., Weber K. Intermediate filaments in non-neuronal cells of invertebrates: isolation and biochemical characterization of intermediate filaments from the esophageal epithelium of the mollusc Helix pomatia. J Cell Biol. 1985 Aug;101(2):427–440. doi: 10.1083/jcb.101.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik E., Weber K. Intermediate filaments in the giant muscle cells of the nematode Ascaris lumbricoides; abundance and three-dimensional complexity of arrangements. Eur J Cell Biol. 1988 Feb;45(2):291–301. [PubMed] [Google Scholar]
- Beck L. A., Hosick T. J., Sinensky M. Incorporation of a product of mevalonic acid metabolism into proteins of Chinese hamster ovary cell nuclei. J Cell Biol. 1988 Oct;107(4):1307–1316. doi: 10.1083/jcb.107.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelsky D., Olson J. F., Koshland D. E., Jr Cell cycle-dependent methyl esterification of lamin B. J Biol Chem. 1987 Mar 25;262(9):4303–4309. [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W. Homology of a conserved sequence in the tail domain of intermediate filament proteins with the loop region of calcium binding proteins. Cell Biol Int Rep. 1987 Nov;11(11):831–831. doi: 10.1016/0309-1651(87)90163-9. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Fischer S., Plessmann U., Weber K. Neurofilament architecture combines structural principles of intermediate filaments with carboxy-terminal extensions increasing in size between triplet proteins. EMBO J. 1983;2(8):1295–1302. doi: 10.1002/j.1460-2075.1983.tb01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Proteinchemical characterization of three structurally distinct domains along the protofilament unit of desmin 10 nm filaments. Cell. 1982 Aug;30(1):277–286. doi: 10.1016/0092-8674(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Related amino acid sequences in neurofilaments and non-neural intermediate filaments. Nature. 1982 Apr 1;296(5856):448–450. doi: 10.1038/296448a0. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Landesman Y., Drees B., Bare J. W., Saumweber H., Paddy M. R., Sedat J. W., Smith D. E., Benton B. M., Fisher P. A. Drosophila nuclear lamin precursor Dm0 is translated from either of two developmentally regulated mRNA species apparently encoded by a single gene. J Cell Biol. 1988 Mar;106(3):585–596. doi: 10.1083/jcb.106.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983 Jul;33(3):915–924. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Höger T. H., Krohne G., Franke W. W. Amino acid sequence and molecular characterization of murine lamin B as deduced from cDNA clones. Eur J Cell Biol. 1988 Dec;47(2):283–290. [PubMed] [Google Scholar]
- Kretsinger R. H. Calcium coordination and the calmodulin fold: divergent versus convergent evolution. Cold Spring Harb Symp Quant Biol. 1987;52:499–510. doi: 10.1101/sqb.1987.052.01.057. [DOI] [PubMed] [Google Scholar]
- Krohne G., Wolin S. L., McKeon F. D., Franke W. W., Kirschner M. W. Nuclear lamin LI of Xenopus laevis: cDNA cloning, amino acid sequence and binding specificity of a member of the lamin B subfamily. EMBO J. 1987 Dec 1;6(12):3801–3808. doi: 10.1002/j.1460-2075.1987.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon F., Lemonnier M., Benarous R., Huc C., Fiszman M., Gros F., Portier M. M. Multiple mRNAs encode peripherin, a neuronal intermediate filament protein. EMBO J. 1989 Jun;8(6):1719–1726. doi: 10.1002/j.1460-2075.1989.tb03564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Gorham J. D., Cole P., Greene L. A., Ziff E. B. A nerve growth factor-regulated messenger RNA encodes a new intermediate filament protein. J Cell Biol. 1988 Jan;106(1):181–193. doi: 10.1083/jcb.106.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Anomalous placement of introns in a member of the intermediate filament multigene family: an evolutionary conundrum. Mol Cell Biol. 1986 May;6(5):1529–1534. doi: 10.1128/mcb.6.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger L., McKeon F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. EMBO J. 1988 Aug;7(8):2301–2309. doi: 10.1002/j.1460-2075.1988.tb03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin T. M., Hatzfeld M., Franke W. W. Analysis of cytokeratin domains by cloning and expression of intact and deleted polypeptides in Escherichia coli. EMBO J. 1987 Sep;6(9):2607–2615. doi: 10.1002/j.1460-2075.1987.tb02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Conway J. F., Steinert P. M. Structural studies on lamin. Similarities and differences between lamin and intermediate-filament proteins. Biochem J. 1986 Aug 15;238(1):305–308. doi: 10.1042/bj2380305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Rihs H. P., Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989 May;8(5):1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stick R. cDNA cloning of the developmentally regulated lamin LIII of Xenopus laevis. EMBO J. 1988 Oct;7(10):3189–3197. doi: 10.1002/j.1460-2075.1988.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treston A. M., Mulshine J. L. Peptide structure. Beyond transcriptional events. Nature. 1989 Feb 2;337(6206):406–406. doi: 10.1038/337406a0. [DOI] [PubMed] [Google Scholar]
- Vorburger K., Lehner C. F., Kitten G. T., Eppenberger H. M., Nigg E. A. A second higher vertebrate B-type lamin. cDNA sequence determination and in vitro processing of chicken lamin B2. J Mol Biol. 1989 Aug 5;208(3):405–415. doi: 10.1016/0022-2836(89)90505-6. [DOI] [PubMed] [Google Scholar]
- Weber K. Link between lamins and intermediate filaments. Nature. 1986 Apr 3;320(6061):402–402. doi: 10.1038/320401a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Dodemont H., Kossmagk-Stephan K. Amino acid sequences and homopolymer-forming ability of the intermediate filament proteins from an invertebrate epithelium. EMBO J. 1988 Oct;7(10):2995–3001. doi: 10.1002/j.1460-2075.1988.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda S. L., Glomset J. A. Evidence for modification of lamin B by a product of mevalonic acid. J Biol Chem. 1988 May 5;263(13):5997–6000. [PubMed] [Google Scholar]





