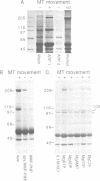
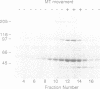
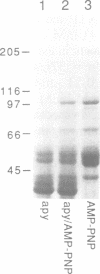
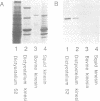
Abstract
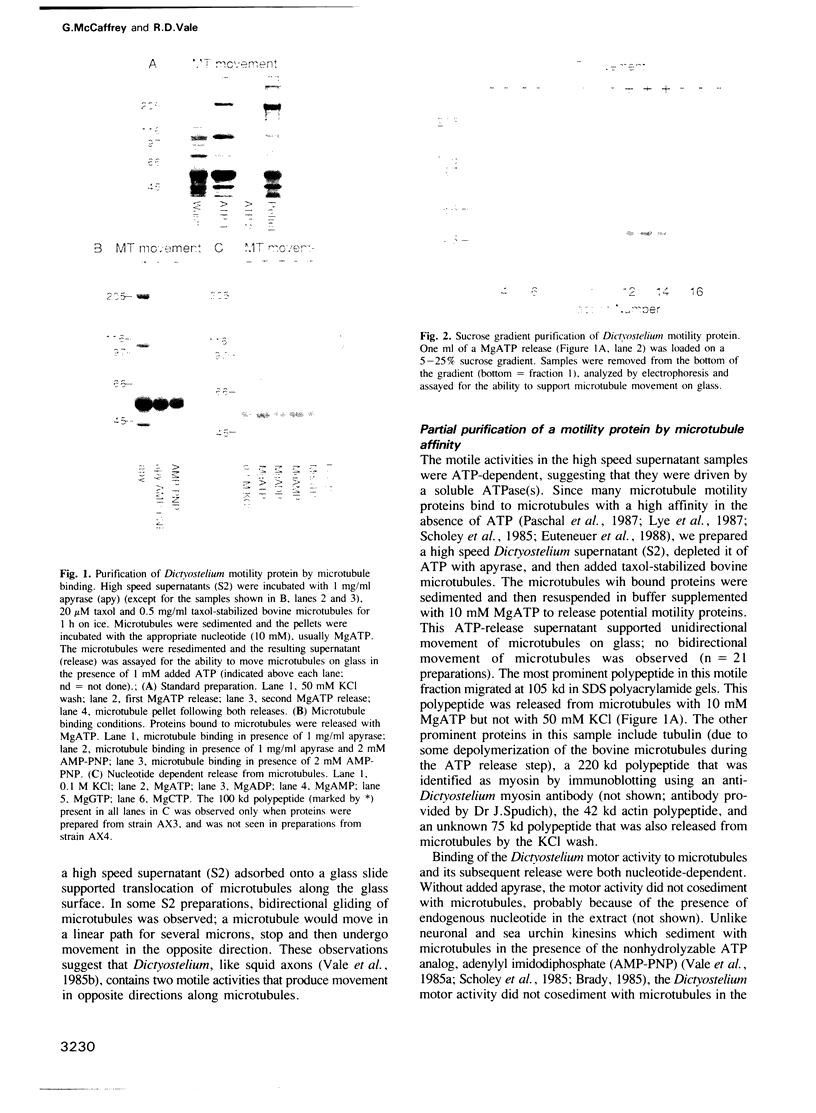
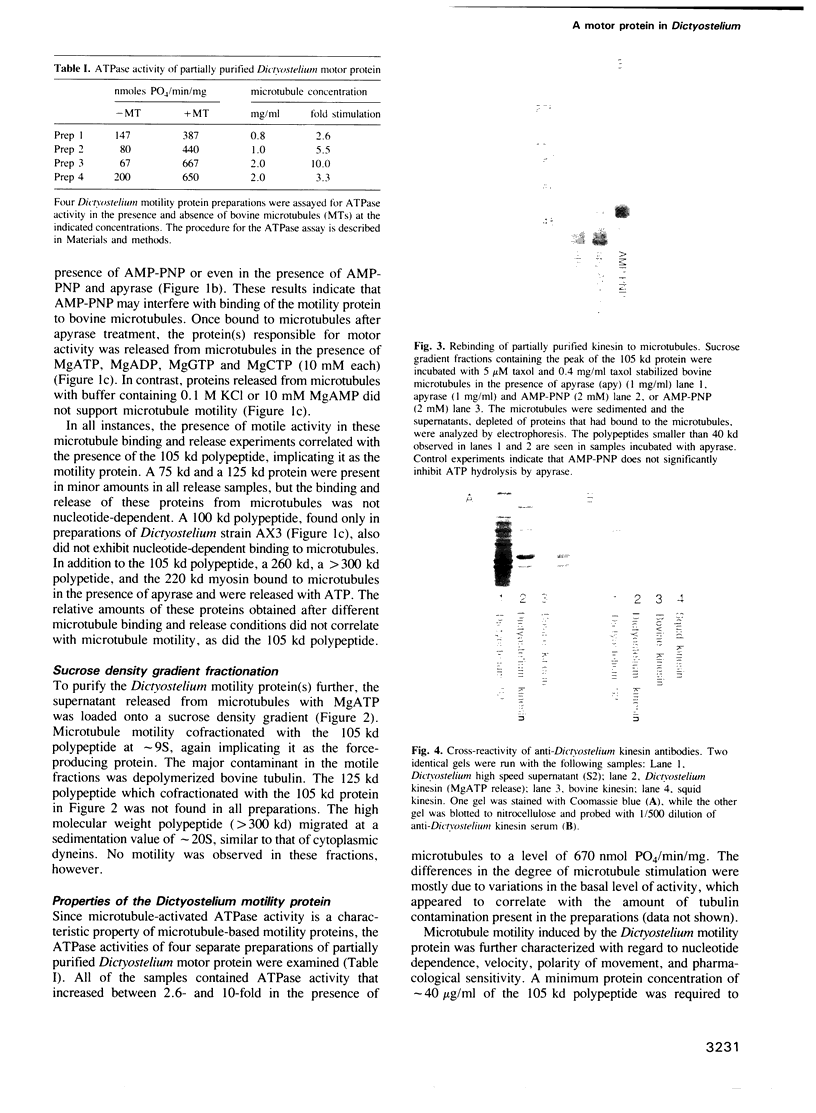
Dictyostelium discoideum, a unicellular eukaryote amenable to both biochemical and genetic dissection, provides an attractive system for studying microtubule-based transport. In this work, we have identified microtubule-based motor activities in Dictyostelium cell extracts and have partially purified a protein that induces microtubule translocation along glass surfaces. This protein, which sediments at approximately 9S in sucrose density gradients and is composed of a 105 kd polypeptide, generates anterograde movement along microtubules that is insensitive to 5 mM NEM (N-ethyl-maleimide) but sensitive to 200 microM vanadate, and has similar nucleotide-dependent microtubule binding properties to those of kinesins purified from mammals, sea urchin and Drosophila. This kinesin-like molecule from Dictyostelium, however, is immunologically distinct from bovine and squid neuronal kinesins and supports microtubule movement on glass at four-fold greater velocities (2.0 versus 0.5 microns/sec). Furthermore, AMP-PNP (adenylyl imidodiphosphate), which promotes attachment of previously characterized kinesins to microtubules, decreases the affinity of the Dictyostelium kinesin homolog for microtubules. Thus, an AMP-PNP-induced rigor binding may not be a characteristic of kinesins from lower eukaryotes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Weiss D. G., Hayden J. H., Brown D. T., Fujiwake H., Simpson M. Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol. 1985 May;100(5):1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988 May 3;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Cohn S. A., Ingold A. L., Scholey J. M. Correlation between the ATPase and microtubule translocating activities of sea urchin egg kinesin. Nature. 1987 Jul 9;328(6126):160–163. doi: 10.1038/328160a0. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., Koonce M. P., Pfister K. K., Schliwa M. An ATPase with properties expected for the organelle motor of the giant amoeba, Reticulomyxa. Nature. 1988 Mar 10;332(6160):176–178. doi: 10.1038/332176a0. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Allen R. D., Sloboda R. D. Translocation of vesicles from squid axoplasm on flagellar microtubules. Nature. 1985 May 16;315(6016):245–248. doi: 10.1038/315245a0. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Huitorel P., Kirschner M. W. The polarity and stability of microtubule capture by the kinetochore. J Cell Biol. 1988 Jan;106(1):151–159. doi: 10.1083/jcb.106.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S. Video image processing greatly enhances contrast, quality, and speed in polarization-based microscopy. J Cell Biol. 1981 May;89(2):346–356. doi: 10.1083/jcb.89.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Albanesi J. P., Fujisaki H., Korn E. D. Extensive purification from Acanthamoeba castellanii of a microtubule-dependent translocator with microtubule-activated Mg2+-ATPase activity. J Biol Chem. 1987 Nov 25;262(33):16180–16185. [PubMed] [Google Scholar]
- Katz K. S., Ratner D. I. Homologous recombination and the repair of double-strand breaks during cotransformation of Dictyostelium discoideum. Mol Cell Biol. 1988 Jul;8(7):2779–2786. doi: 10.1128/mcb.8.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg E. A., Shanina N. A., Magretova N. N., Chernyak V. Y., Gelfand V. I. The quaternary structure of bovine brain kinesin. EMBO J. 1988 Feb;7(2):353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985 Aug 15;316(6029):645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Lye R. J., Porter M. E., Scholey J. M., McIntosh J. R. Identification of a microtubule-based cytoplasmic motor in the nematode C. elegans. Cell. 1987 Oct 23;51(2):309–318. doi: 10.1016/0092-8674(87)90157-7. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Titus M. A., De Lozanne A., Spudich J. A. Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 1989 Mar;8(3):923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. R., Warner F. D. Interactions of dynein arms with b subfibers of Tetrahymena cilia: quantitation of the effects of magnesium and adenosine triphosphate. J Cell Biol. 1980 Oct;87(1):84–97. doi: 10.1083/jcb.87.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murofushi H., Ikai A., Okuhara K., Kotani S., Aizawa H., Kumakura K., Sakai H. Purification and characterization of kinesin from bovine adrenal medulla. J Biol Chem. 1988 Sep 5;263(25):12744–12750. [PubMed] [Google Scholar]
- Neighbors B. W., Williams R. C., Jr, McIntosh J. R. Localization of kinesin in cultured cells. J Cell Biol. 1988 Apr;106(4):1193–1204. doi: 10.1083/jcb.106.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Shpetner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987 Sep;105(3):1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Vallee R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987 Nov 12;330(6144):181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. E., Scholey J. M., Stemple D. L., Vigers G. P., Vale R. D., Sheetz M. P., McIntosh J. R. Characterization of the microtubule movement produced by sea urchin egg kinesin. J Biol Chem. 1987 Feb 25;262(6):2794–2802. [PubMed] [Google Scholar]
- Roos U. P., De Brabander M., De Mey J. Indirect immunofluorescence of microtubules in Dictyostelium discoideum. A study with polyclonal and monoclonal antibodies to tubulins. Exp Cell Res. 1984 Mar;151(1):183–193. doi: 10.1016/0014-4827(84)90367-7. [DOI] [PubMed] [Google Scholar]
- Saxton W. M., Porter M. E., Cohn S. A., Scholey J. M., Raff E. C., McIntosh J. R. Drosophila kinesin: characterization of microtubule motility and ATPase. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1109–1113. doi: 10.1073/pnas.85.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey J. M., Heuser J., Yang J. T., Goldstein L. S. Identification of globular mechanochemical heads of kinesin. Nature. 1989 Mar 23;338(6213):355–357. doi: 10.1038/338355a0. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Porter M. E., Grissom P. M., McIntosh J. R. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985 Dec 5;318(6045):483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Block S. M., Spudich J. A. Myosin movement in vitro: a quantitative assay using oriented actin cables from Nitella. Methods Enzymol. 1986;134:531–544. doi: 10.1016/0076-6879(86)34118-1. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Paschal B. M., Vallee R. B. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP 1C). J Cell Biol. 1988 Sep;107(3):1001–1009. doi: 10.1083/jcb.107.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Mitchison T., Steuer E., Reese T. S., Sheetz M. P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell. 1985 Dec;43(3 Pt 2):623–632. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Toyoshima Y. Y. Microtubule translocation properties of intact and proteolytically digested dyneins from Tetrahymena cilia. J Cell Biol. 1989 Jun;108(6):2327–2334. doi: 10.1083/jcb.108.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Toyoshima Y. Y. Rotation and translocation of microtubules in vitro induced by dyneins from Tetrahymena cilia. Cell. 1988 Feb 12;52(3):459–469. doi: 10.1016/s0092-8674(88)80038-2. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Wall J. S., Paschal B. M., Shpetner H. S. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988 Apr 7;332(6164):561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- Wagner M. C., Pfister K. K., Bloom G. S., Brady S. T. Copurification of kinesin polypeptides with microtubule-stimulated Mg-ATPase activity and kinetic analysis of enzymatic properties. Cell Motil Cytoskeleton. 1989;12(4):195–215. doi: 10.1002/cm.970120403. [DOI] [PubMed] [Google Scholar]
- Witke W., Nellen W., Noegel A. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987 Dec 20;6(13):4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]