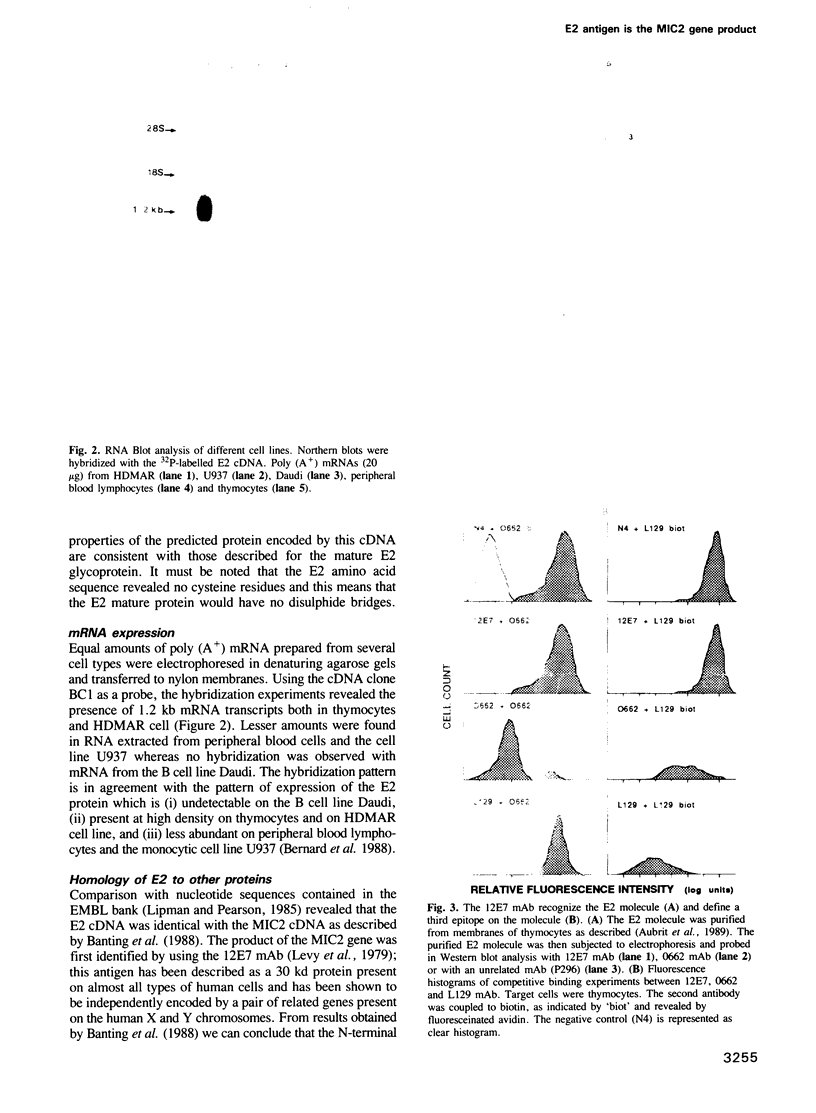
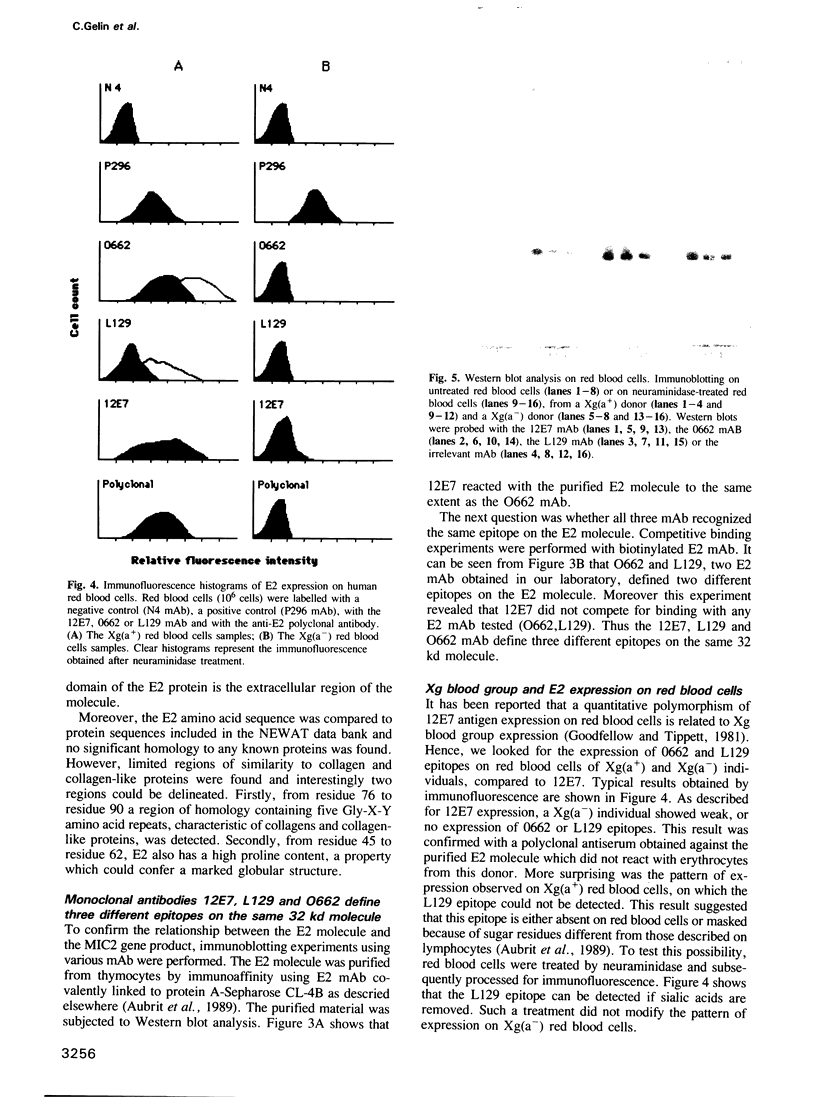
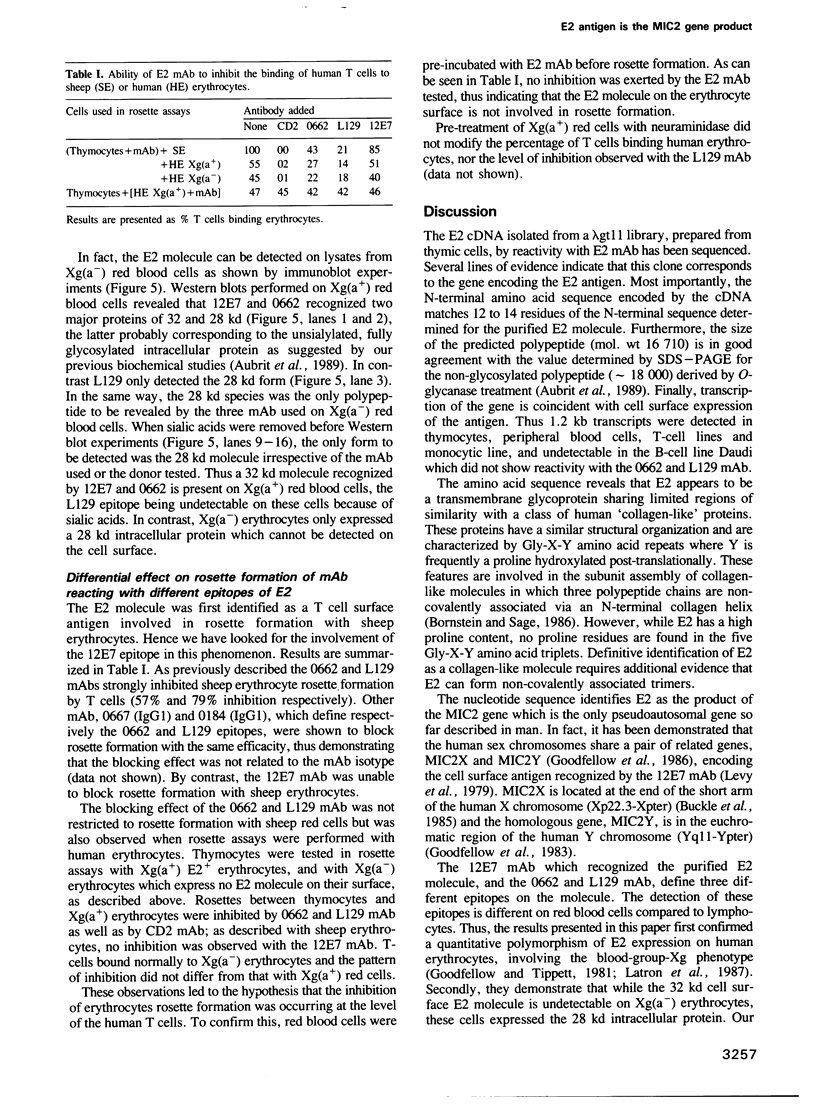
Abstract
E2 is a 32 kd human T-cell surface glycoprotein involved in spontaneous rosette formation with erythrocytes. A 1.11 kb cDNA was isolated from a lambda gt11 expression library by screening with monoclonal antibodies directed against E2. The primary structure of E2, deduced from the nucleotide sequence of its gene, comprises 185 amino acids and is devoid of N-linked glycosylation sites. The E2 protein is rich in proline residues and displays an organization typical of an integral membrane protein. Northern blotting showed a good correlation between mRNA abundance, E2 surface density and the level of T cell differentiation. In fact, nucleotide sequencing revealed that E2 is the MIC2 gene product, previously identified with the 12E7 Mab. Xg(a-) female individuals have no E2 molecule on the surface of their red cells, in contrast with Xg(a+) individuals, but have the molecule in their cytoplasm, in the form of the 28 kd precursor. These findings show that the E2 antigen, a cell surface molecule involved in T cell adhesion processes, is the product of the MIC2 gene, the only pseudoautosomal gene to be described in man.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banting G. S., Pym B., Darling S. M., Goodfellow P. N. The MIC2 gene product: epitope mapping and structural prediction analysis define an integral membrane protein. Mol Immunol. 1989 Feb;26(2):181–188. doi: 10.1016/0161-5890(89)90100-4. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat H., Mitrani-Rosenbaum S., Gamliel H., Naparstek E., Leizerowitz R., Korkesh A., Sagi M., Voss R., Kohn G., Polliack A. Establishment in continuous culture of a T-lymphoid cell line (HD-Mar) from a patient with Hodgkin's lymphoma. Int J Cancer. 1980 May 15;25(5):583–590. doi: 10.1002/ijc.2910250506. [DOI] [PubMed] [Google Scholar]
- Bernard A., Aubrit F., Raynal B., Pham D., Boumsell L. A T cell surface molecule different from CD2 is involved in spontaneous rosette formation with erythrocytes. J Immunol. 1988 Mar 15;140(6):1802–1807. [PubMed] [Google Scholar]
- Bernard A., Gelin C., Raynal B., Pham D., Gosse C., Boumsell L. Phenomenon of human T cells rosetting with sheep erythrocytes analyzed with monoclonal antibodies. "Modulation" of a partially hidden epitope determining the conditions of interaction between T cells and erythrocytes. J Exp Med. 1982 May 1;155(5):1317–1333. doi: 10.1084/jem.155.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A., Tran H. C., Boumsell L. Three different erythrocyte surface molecules are required for spontaneous T cell rosette formation. J Immunol. 1987 Jul 1;139(1):18–23. [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Brottier P., Boumsell L., Gelin C., Bernard A. T cell activation via CD2 [T, gp50] molecules: accessory cells are required to trigger T cell activation via CD2-D66 plus CD2-9.6/T11(1) epitopes. J Immunol. 1985 Sep;135(3):1624–1631. [PubMed] [Google Scholar]
- Brown M. H., Krissansen G. W., Totty N. F., Sewell W. A., Crumpton M. J. Purification and N-terminal amino acid sequence of the human T lymphocyte CD2 (T11) surface antigen. Eur J Immunol. 1987 Jan;17(1):15–20. doi: 10.1002/eji.1830170104. [DOI] [PubMed] [Google Scholar]
- Buckle V., Mondello C., Darling S., Craig I. W., Goodfellow P. N. Homologous expressed genes in the human sex chromosome pairing region. Nature. 1985 Oct 24;317(6039):739–741. doi: 10.1038/317739a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fröland S. S. Binding of sheep erythrocytes to human lymphocytes. A probable marker of T lymphocytes. Scand J Immunol. 1972;1(3):269–280. doi: 10.1111/j.1365-3083.1972.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Gelin C., Boumsell L., Bernard A. Control of cell proliferation within the human thymus. A very limited thymocyte subpopulation generates a suppressive activity. Eur J Immunol. 1986 Oct;16(10):1209–1216. doi: 10.1002/eji.1830161005. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. J., Darling S. M., Thomas N. S., Goodfellow P. N. A pseudoautosomal gene in man. Science. 1986 Nov 7;234(4777):740–743. doi: 10.1126/science.2877492. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Tippett P. A human quantitative polymorphism related to Xg blood groups. Nature. 1981 Jan 29;289(5796):404–405. doi: 10.1038/289404a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Sheer D., Ropers H. H., Caine A., Ferguson-Smith M. A., Povey S., Voss R. Genetic evidence that a Y-linked gene in man is homologous to a gene on the X chromosome. Nature. 1983 Mar 24;302(5906):346–349. doi: 10.1038/302346a0. [DOI] [PubMed] [Google Scholar]
- Huet S., Wakasugi H., Sterkers G., Gilmour J., Tursz T., Boumsell L., Bernard A. T cell activation via CD2 [T, gp50]: the role of accessory cells in activating resting T cells via CD2. J Immunol. 1986 Sep 1;137(5):1420–1428. [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Jackson P. R., Pappas M. G., Hansen B. D. Fluorogenic substrate detection of viable intracellular and extracellular pathogenic protozoa. Science. 1985 Jan 25;227(4685):435–438. doi: 10.1126/science.2578226. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Latron F., Blanchard D., Cartron J. P. Immunochemical characterization of the human blood cell membrane glycoprotein recognized by the monoclonal antibody 12E7. Biochem J. 1987 Nov 1;247(3):757–764. doi: 10.1042/bj2470757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Dilley J., Fox R. I., Warnke R. A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6552–6556. doi: 10.1073/pnas.76.12.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Greene W. C., Hoover R. G., Nowell P. C. Monoclonal antibody OKT11A inhibits and recombinant interleukin 2 (IL 2) augments expression of IL 2 receptors at a pretranslational level. J Immunol. 1985 Oct;135(4):2478–2482. [PubMed] [Google Scholar]
- Reisner Y., Kapoor N., Kirkpatrick D., Pollack M. S., Cunningham-Rundles S., Dupont B., Hodes M. Z., Good R. A., O'Reilly R. J. Transplantation for severe combined immunodeficiency with HLA-A,B,D,DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983 Feb;61(2):341–348. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Hercend T., Fox D. A., Bensussan A., Bartley G., Daley J. F., Schlossman S. F., Reinherz E. L., Ritz J. The role of interleukin 2 and T11 E rosette antigen in activation and proliferation of human NK clones. J Immunol. 1985 Jul;135(1):672–678. [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]