Abstract
Cartilage and chondrocytes experience loading that causes alterations in chondrocyte biological activity. In vivo chondrocytes are surrounded by a pericellular matrix with a stiffness of ∼25-200 kPa. Understanding the mechanical loading environment of the chondrocyte is of substantial interest for understanding chondrocyte mechanotransduction. The first objective of this study was to analyze the spatial variability of applied mechanical deformations in physiologically stiff agarose on cellular and sub-cellular length scales. Fluorescent microspheres were embedded in physiologically stiff agarose hydrogels. Microsphere positions were measured via confocal microscopy and used to calculate displacement and strain fields as a function of spatial position. The second objective was to assess the feasibility of encapsulating primary human chondrocytes in physiologically stiff agarose. The third objective was to determine if primary human chondrocytes could deform in high-stiffness agarose gels. Primary human chondrocyte viability was assessed using live-dead imaging following 24 and 72 hours in tissue culture. Chondrocyte shape was measured before and after application of 10% compression. These data indicate that (1) displacement and strain precision are ∼1% and 6.5% respectively, (2) high-stiffness agarose gels can maintain primary human chondrocyte viability of >95%, and (3) compression of chondrocytes in 4.5% agarose can induce shape changes indicative of cellular compression. Overall, these results demonstrate the feasibility of using high-concentration agarose for applying in vitro compression to chondrocytes as a model for understanding how chondrocytes respond to in vivo loading.
Keywords: Chondrocyte, mechanotransduction, osteoarthritis, confocal microscopy, finite deformation
Introduction
Osteoarthritis (OA) is the most common joint disorder, affecting over 100 million individuals (Woolf and Pfleger 2003). OA is most commonly associated with excessive loading of aging joints (e.g. caused by obesity or injury), leading to deterioration of articular cartilage and joint inflammation. Articular cartilage is located at the surfaces of joints, and serves as a low-friction material between bones. Articular cartilage is composed of articular chondrocytes (cartilage cells), the pericelluar matrix (PCM), and the extracellular matrix (ECM) (Muir 1995). In these regions of the body (e.g. the knee), the articular cartilage, and thus articular chondrocytes, are subjected to almost-constant mechanical loading (e.g. walking, running, etc…). Repetitive action is crucial for joint health, yet excessive loading can lead to OA (Harada, Tomita et al. 2005). Individuals with a history of heavy mechanical work (e.g. heavy lifting) are ∼7-fold less likely to have OA at the age of 90 (Goekoop, Kloppenburg et al. 2011), suggesting that long-duration, but sub-injurious, mechanical loading may induce protective biological responses. Therefore, understanding the biological responses of chondrocytes to mechanical loading are extremely important to improving joint health. These data emphasize the need for development of fundamental knowledge regarding how chondrocytes and other joint cells sense and respond to mechanical loads, a process defined as mechanotransduction (Vincent 2013). This paper characterizes the deformational environment of a stiff 3D hydrogel for use in cartilage mechanotransduction studies.
Exogenous dynamic compression can substantially alter chondrocyte metabolism in both an anabolic and catabolic manner, but the balance between matrix synthesis and matrix degradation is not yet fully understood (Buschmann, Kim et al. 1999; Fitzgerald, Jin et al. 2008). Dynamic compression can induce phosphorylation of multiple enzymes, including MAPK and SEK (Fanning, Emkey et al. 2003; Bougault, Paumier et al. 2008), Akt (Niehoff, Offermann et al. 2008), Erk -1 and -2 (Li, Wang et al. 2003; De Croos, Jang et al. 2007; Ryan, Eisner et al. 2009), and Rho kinase (Haudenschild, D'Lima et al. 2008). Additionally, exogenous loading can alter Superficial Zone Protein expression (Neu, Khalafi et al. 2007), induce transcription of ECM genes (Bougault, Paumier et al. 2008), and activate RhoA (Haudenschild, D'Lima et al. 2008). Cyclic dynamic compression can promote Smad2 phosphorylation (Bougault, Aubert-Foucher et al. 2012), gene expression of MMP-13 (Nebelung, Gavenis et al. 2012), which is the marker for catabolic changes in the ECM, and increases in ATP release (Garcia and Knight 2010). These studies demonstrate the sensitivity of chondrocytes to mechanical loading and indicate that a complete understanding chondrocyte mechanotransduction remains to be determined.
A variety of hydrogels have been utilized including photo cross-linked polyethylene glycol (Farnsworth, Antunez et al. 2013), self-assembling peptides (Kisiday, Lee et al. 2009), alginate (Haudenschild, Chen et al. 2011), and agarose (Knight, Toyoda et al. 2006; Vaughan, Grainger et al. 2010). Most existing studies utilize 3D microenvironments (e.g. agarose or alginate) for cell encapsulation with a much lower stiffness (< 5 kPa) than the cartilage pericelluar matrix (25-200 kPa) (Alexopoulos, Williams et al. 2005; Darling, Wilusz et al. 2010). Agarose hydrogels are of particular interest because the stiffness can be selected to match the stiffness of cartilage PCM (Normand, Lootens et al. 2000) without potential complications of UV photocrosslinking (e.g. induction of the DNA damage response (Filatov, Bjorklund et al. 1996)). This study characterizes the deformational environment of high-stiffness (∼35 kPa) agarose gels. To our knowledge, chondrocyte mechanotransduction studies have never been performed using agarose with PCM stiffness.
Cartilage experiences a variety of in vivo loading. The motivation for this study is to characterize the micro-level deformation fields in a physiologically stiff, 3D culture environment, to study how chondrocytes sense and respond to mechanical loading. Using a bioreactor capable of applying sub-micron precision, displacement-controlled loading to agarose hydrogels during confocal microscopy, this study describes (1) the cellular-level deformation fields in agarose hydrogels under mechanical compression, (2) the encapsulation of primary human chondrocytes in agarose hydrogels with stiffness matched to human PCM (25-200 kPa) (Darling, Wilusz et al. 2010; Jutila, Zignego et al. 2013; McLeod, Wilusz et al. 2013), and (3) the ability to apply uniform compression to embedded cells.
To minimize experimental variability when applying in vitro loads to 3D chondrocyte cultures, applied deformations must be spatially homogeneous throughout the hydrogels to avoid spatially-distinct mechanical stimuli. The first objective of this study was to analyze the spatial variability of applied mechanical deformations in physiologically stiff agarose on cellular and sub-cellular length scales. Fluorescent microspheres were used as fiducial markers within agarose hydrogels, which were compressed uniaxially during confocal imaging. Microsphere positions were tracked with 2D-Cartesian coordinates over a range of ∼250 μm. These data were used to calculate the displacement and strain fields at multiple locations with spatial resolution of ∼5 μm.
The second objective was to assess the feasibility of encapsulating primary human chondrocytes in physiologically stiff agarose, and the third objective was to determine if primary human chondrocytes could deform in high-stiffness gels. Primary chondrocytes were isolated from discarded joint replacement tissue and encapsulated in agarose, and viability was assayed via live-dead staining. The results of this study indicate that (1) applied deformations have minimal spatial variability and strain bias, (2) primary chondrocytes can maintain high viability through 72 hours, and (3) human chondrocytes can be deformed in physiologically stiff agarose. Future studies may use this system to elucidate cellular mechanisms of chondrocyte mechanotransduction.
Methods
To track deformations within cylindrical agarose, fluorescent microspheres were used as fiducial markers and encapsulated based on previous techniques (Lee and Knight 2004). Uniaxial compression was applied to samples in 0.15M PBS during simultaneous confocal imaging (Figure S1). The positions of fluorescent microspheres were tracked and used to calculate finite deformation measures including Lagrangian strain using previously validated methods (Geers, DeBorst et al. 1996; Neu, Hull et al. 2005; Chan, Toribio et al. 2012). In separate experiments, primary human chondrocytes were embedded in agarose based on previous methods (Bougault, Paumier et al. 2009). Viability was assessed using standard methods (Ohlendorf, Tomford et al. 1996). Cell-seeded constructs were imaged during deformation following 72 hours of gel equilibration for both 2% and 4.5% agarose (Ng, Ateshian et al. 2009). See supplement for detailed methods.
Results
The spatial homogeneity of each gel concentration was evaluated by comparing the final loading step displacement and strain values at six unique locations in each gel. For each gel concentration we found strain fields with minimal variability from all six gel locations. Axial finite strains, (Eyy) were 2.01 ± 0.08, 1.65 ± 0.11, 1.33 ± 0.10, 1.11 ± 0.08, and 0.98 ± 0.07 for 3, 3.5, 4, 4.5, and 5% agarose, respectively (Figures 1-2 and Table S1). Transverse finite strains (Exx) and shear strains (Exy) were minimal for all agarose concentrations (Figures 1-2, Table S1). Error analysis indicated that the observed displacement precision resulted in errors in Eyy strain of ∼1% (Figure S3). We found high viability which was independent of spatial position, indicating a homogeneous distribution of viable cells (Figure 3, Table S2). All average viability measures were ≥ 96.2%. Deformations were applied to agarose constructs containing embedded primary chondrocytes. Cellular geometry was identified with Calcein AM fluorescence, and compressed images showed the cross sections of uncompressed cells to be circular (width/height of 1.00 ± 0.00) and the compressed cells to be ellipsoidal (Figure 4). Cells in 4.5% agarose exhibited greater deformation than cells in 4.5% agarose as quantified by the aspect ratio of chondrocyte width to height (p < 0.001. 2%: 1.07 ± 0.06. 4.5%: 1.28 ± 0.04.). For chondrocytes in 4.5% agarose, the coefficient of variation of the aspect ratio was 3.1% indicating the uniformity of applied compression to the embedded cells.
Figure 1.
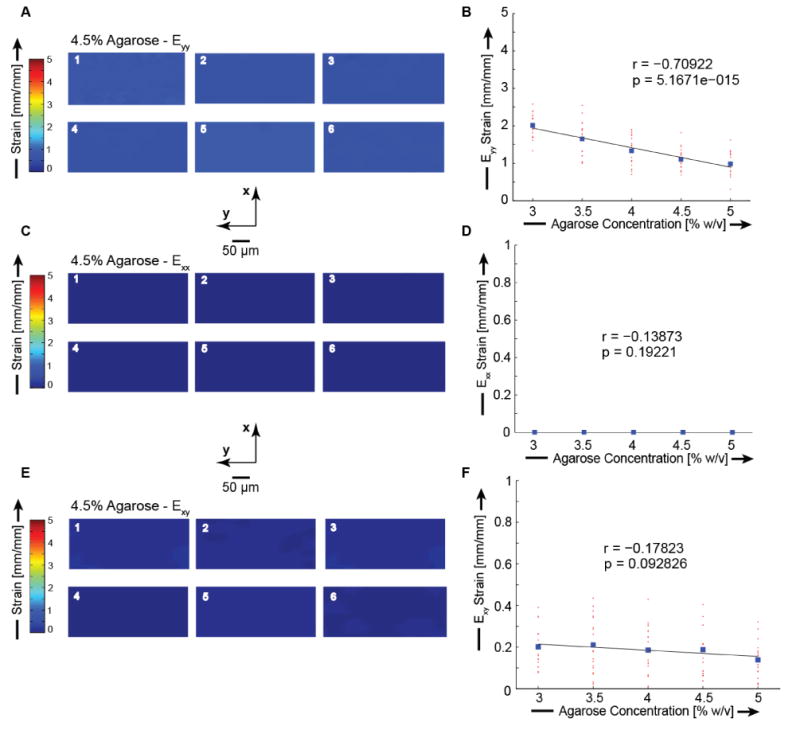
Finite deformation Lagrangian strain fields within 4.5% agarose hydrogel. Strains were calculated using a finite deformation code in Matlab (Geers et al., 1996). The axial (Eyy), transverse (Exx), and shear (Exy) strain fields for 4.5% agarose are plotted. Axial strains were calculated to be 1.11 ± 0.08 [mm/mm]. Transverse strains were calculated to be 0.01 ± 0.00 [mm/mm]. Shear strains were calculated to be 0.18 ± 0.02 [mm/mm]. (A) Representative axial strain image Eyy. (B) Axial strain Eyy as a function of agarose concentration. (C) Representative transverse strain Exx. (D) Transverse strain Exx as a function of concentration. (E) Representative shear strain Exy.(F) Shear strain Exy as a function of concentration.
Figure 2.
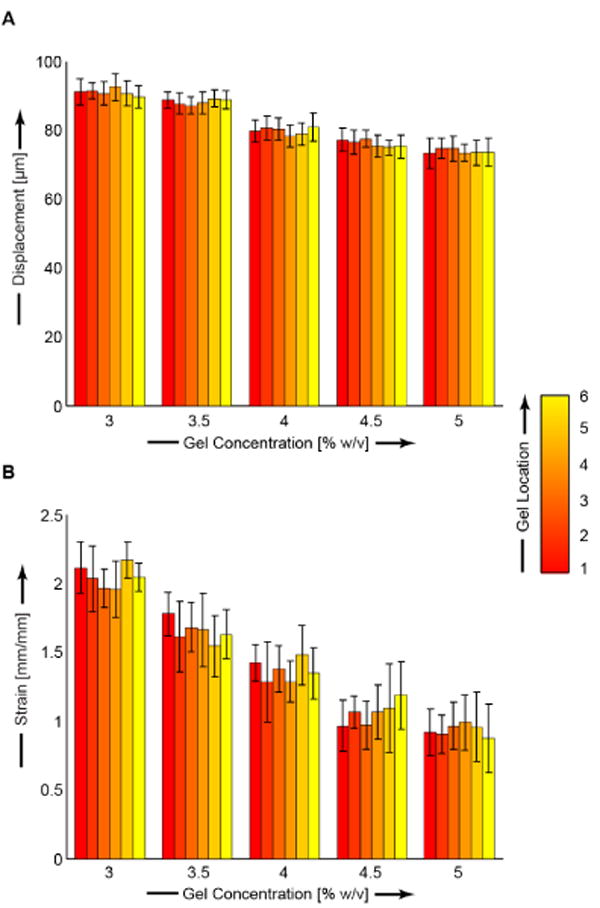
Displacement (A) and strain (B) as functions of gel position and agarose concentration. We found a significant relationship for Uy (r = -0.8466 and p < 0.001) and Eyy (r = -0.71 and p < 0.001) between agarose gel concentration and bead deformations, and indicating that the stiffer, higher-concentration gels resulted in smaller bead displacements and strains, as expected. Locations as defined in Figure S1.
Figure 3.

Viability of primary human chondrocytes in high concentration agarose gels after 24 and 72 hours. Primary human chondrocytes were harvested from joint replacement tissue (Bridger Orthopedic, Bozeman, MT), digested in Type IV collagenase (2 mg/mL for 12-14 hrs. at 37°C), cultured, encapsulated in physiologically-stiff agarose (3-5% w/v), and assessed for viability after 24 and 72 hours using confocal microscopy. Live cells were identified with Calcein AM fluorescence (ex. 497 em. 516) and dead cells with propidium iodide fluorescence (ex. 537 em. 617). All six gel locations were imaged showing minimal variability in cell viability throughout the gel. Average viability was >97% and >95% for 24 and 72 hour time periods, respectively.
Figure 4.
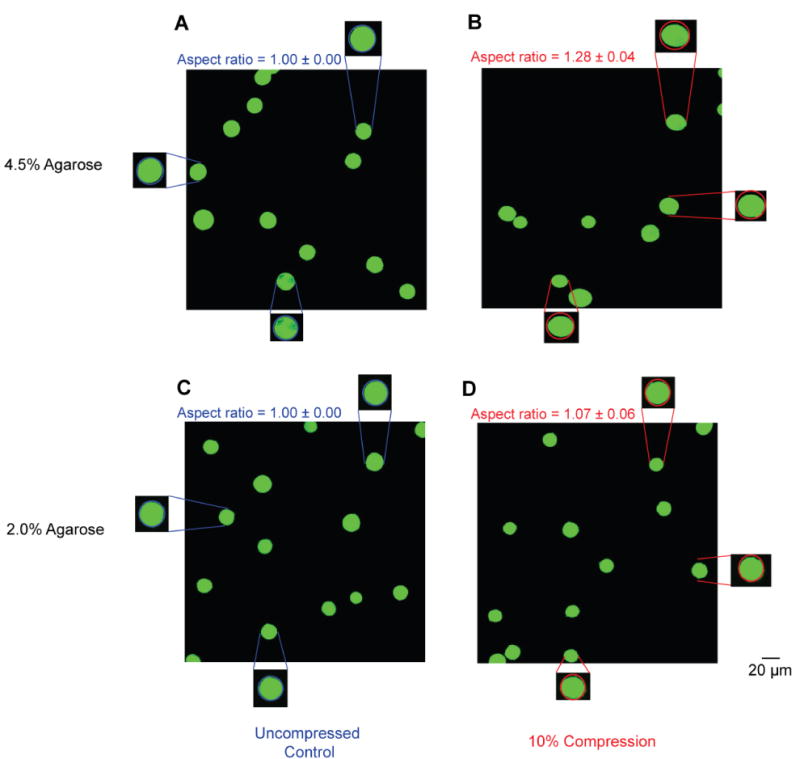
Deformation of primary human chondrocytes in 2.0% and 4.5% agarose. To examine deformations to chondrocytes in typical stiffness (2.0%) and high stiffness (4.5%) agarose, primary human chondrocytes were cast into agarose hydrogels, equilibrated in culture for 72 h, and imaged before and after a 10% nominal compression. Deformations were quantified via the aspect ratio (width / height). Aspect ratios were significantly greater following 10% compression in 4.5% agarose compared with 2.0% (p < 0.001). Left panels (A and C) show representative uncompressed images. Right panels (B and D) show images following 10% nominal compression. Top row (A and B) shows data for 4.5% agarose. Bottom row (C and D) shows data for 2.0% agarose. A total of n ∼ 150 cells were measured for aspect ratio in each condition.
Discussion
The objectives of this study were to (1) assess the spatial variability of mechanical deformations in physiologically stiff agarose on a sub-cellular length scale, and (2) determine if it is possible to encapsulate live primary human chondrocytes in physiologically-stiff agarose, maintain viability, and induce deformations on the embedded cells. We determined the spatial homogeneity in each gel by assessing displacements and strains at six spatial locations in each gel over an array of pre-determined agarose concentrations (3-5% w/v) which result in stiffness values in the range of the human PCM (Darling, Wilusz et al. 2010; Jutila, Zignego et al. 2013; McLeod, Wilusz et al. 2013).
When computing strain fields from experimental data, noise is capable of skewing results and requires preliminary filtering of the displacement data (Geers, DeBorst et al. 1996). In this study, displacements were calculated directly from individual particle locations within the hydrogels during the twenty steps of applied compression. Displacements at each step were calculated with respect to the undeformed particle locations of the first loading step. A 5×5 box-sized Gaussian filter was applied to the displacement data in order to increase the precision of the calculated strain fields (Chan, Toribio et al. 2012). Once the data was filtered, it was input into a finite deformation code in Matlab for strain field calculation (Geers, DeBorst et al. 1996).
The finite deformation code utilizes a 2D-continuum mechanics approach where Green-Lagrange strain fields are calculated from discretely sampled displacement fields. The code calculates the finite deformation tensor, F, and the Green-Lagrange strain tensor, E, from experimental particle position data. The random spacing of the beads may induce error into the in the strain calculation because larger distances between neighboring particles, will result in larger discretization error. While this may affect the magnitude of the strain values, the objective of this study was to evaluate the homogeneity of the displacement and strain fields, and this error was mitigated by the displacement field smoothing prior to strain calculation.
Conclusions
These data demonstrate the ability to apply compressions to physiologically stiff agarose hydrogels with absolute displacement precision of ∼1% and absolute strain precision of ∼6.5% (e.g. application of nominal 10% strain would result in strains between 9.35 and 10.65%). This minimal variability in displacement and strain fields between different spatial locations in each gel implies spatial homogeneity and demonstrate the utility of using this system to apply well-defined strains to primary human chondrocytes and other cells to study cellular mechanotransduction. This study also demonstrated the feasibility of encapsulating primary human chondrocytes in physiologically stiff agarose gels while maintaining high viability. Finally, this study found that 4.5% agarose was capable of physically deforming primary human chondrocytes. These results provide a robust methodological foundation for future studies investigating how chondrocytes respond to mechanical cues: using this system, we can now apply a uniform mechanical stimulus which is necessary to minimize biological variability when dissecting mechanisms of mechanotransduction.
Supplementary Material
Acknowledgments
The authors thank Professor Marc Geers from Eindhoven University of Technology, The Netherlands, for providing his custom strain computation code and Ms. Betsey Pitts, Montana State University, Center for Biofilm Engineering, for assistance with the confocal imaging. This work was funded by NIH P20 GM103394 and startup funds from Montana State University, Vice President for Research.
Role of Funding Source: The funding source was not involved in study design or performance.
Footnotes
Conflict of Interest: The authors have no conflict of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos LG, Williams GM, et al. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38(3):509–517. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Bougault C, Aubert-Foucher E, et al. Dynamic Compression of Chondrocyte-Agarose Constructs Reveals New Candidate Mechanosensitive Genes. Plos One. 2012;7(5):11. doi: 10.1371/journal.pone.0036964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougault C, Paumier A, et al. Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. BMC Biotechnol. 2008;8:71. doi: 10.1186/1472-6750-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougault C, Paumier A, et al. Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. Bmc Biotechnology. 2008;8:10. doi: 10.1186/1472-6750-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougault C, Paumier A, et al. Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures. Nat Protoc. 2009;4(6):928–938. doi: 10.1038/nprot.2009.63. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Kim YJ, et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366(1):1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- Chan DD, Toribio D, et al. Displacement smoothing for the precise MRI-based measurement of strain in soft biological tissues. Comput Methods Biomech Biomed Engin. 2012 doi: 10.1080/10255842.2011.641178. [DOI] [PubMed] [Google Scholar]
- Darling EM, Wilusz RE, et al. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J. 2010;98(12):2848–2856. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Croos JN, Jang B, et al. Membrane type-1 matrix metalloproteinase is induced following cyclic compression of in vitro grown bovine chondrocytes. Osteoarthritis Cartilage. 2007;15(11):1301–1310. doi: 10.1016/j.joca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Fanning PJ, Emkey G, et al. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J Biol Chem. 2003;278(51):50940–50948. doi: 10.1074/jbc.M305107200. [DOI] [PubMed] [Google Scholar]
- Farnsworth NL, Antunez LR, et al. Dynamic compressive loading differentially regulates chondrocyte anabolic and catabolic activity with age. Biotechnol Bioeng. 2013 doi: 10.1002/bit.24860. [DOI] [PubMed] [Google Scholar]
- Filatov D, Bjorklund S, et al. Induction of the mouse ribonucleotide reductase R1 and R2 genes in response to DNA damage by UV light. Journal of Biological Chemistry. 1996;271(39):23698–23704. doi: 10.1074/jbc.271.39.23698. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, et al. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283(11):6735–6743. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- Garcia M, Knight MM. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res. 2010;28(4):510–515. doi: 10.1002/jor.21025. [DOI] [PubMed] [Google Scholar]
- Geers MGD, DeBorst R, et al. Computing strain fields from discrete displacement fields in 2D-solids. International Journal of Solids and Structures. 1996;33(29):4293–4307. [Google Scholar]
- Goekoop RJ, Kloppenburg M, et al. Determinants of absence of osteoarthritis in old age. Scandinavian Journal of Rheumatology. 2011;40(1) doi: 10.3109/03009742.2010.500618. [DOI] [PubMed] [Google Scholar]
- Harada Y, Tomita N, et al. Effect of low loading and joint immobilization for spontaneous repair of osteochondral defect in the knees of weightless (tail suspension) rats. Journal of Orthopaedic Science. 2005;10(5):508–514. doi: 10.1007/s00776-005-0931-7. [DOI] [PubMed] [Google Scholar]
- Haudenschild DR, Chen J, et al. Vimentin contributes to changes in chondrocyte stiffness in osteoarthritis. J Orthop Res. 2011;29(1):20–25. doi: 10.1002/jor.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, D'Lima DD, et al. Dynamic compression of chondrocytes induces a Rho kinase-dependent reorganization of the actin cytoskeleton. Biorheology. 2008;45(3-4) [PubMed] [Google Scholar]
- Jutila AA, Zignego DZ, et al. Encapsulation of Chondrocytes in Physiologically Stiff Agarose. 2013 Submitted. [Google Scholar]
- Kisiday JD, Lee JH, et al. Catabolic responses of chondrocyte-seeded peptide hydrogel to dynamic compression. Ann Biomed Eng. 2009;37(7):1368–1375. doi: 10.1007/s10439-009-9699-9. [DOI] [PubMed] [Google Scholar]
- Knight MM, Toyoda T, et al. Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. Journal of Biomechanics. 2006;39(8) doi: 10.1016/j.jbiomech.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lee DA, Knight MM. Mechanical loading of chondrocytes embedded in 3D constructs: in vitro methods for assessment of morphological and metabolic response to compressive strain. Methods Mol Med. 2004;100:307–324. doi: 10.1385/1-59259-810-2:307. [DOI] [PubMed] [Google Scholar]
- Li KW, Wang AS, et al. Microenvironment regulation of extracellular signal-regulated kinase activity in chondrocytes: effects of culture configuration, interleukin-1, and compressive stress. Arthritis Rheum. 2003;48(3):689–699. doi: 10.1002/art.10849. [DOI] [PubMed] [Google Scholar]
- McLeod MA, Wilusz RE, et al. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J Biomech. 2013;46(3):586–592. doi: 10.1016/j.jbiomech.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995;17(12):1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- Nebelung S, Gavenis K, et al. Simultaneous anabolic and catabolic responses of human chondrocytes seeded in collagen hydrogels to long-term continuous dynamic compression. Ann Anat. 2012 doi: 10.1016/j.aanat.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Neu CP, Hull ML, et al. Heterogeneous three-dimensional strain fields during unconfined cyclic compression in bovine articular cartilage explants. Journal of Orthopaedic Research. 2005;23(6):1390–1398. doi: 10.1016/j.orthres.2005.03.022.1100230622. [DOI] [PubMed] [Google Scholar]
- Neu CP, Khalafi A, et al. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56(11):3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- Ng KW, Ateshian GA, et al. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Eng Part A. 2009;15(9):2315–2324. doi: 10.1089/ten.tea.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehoff A, Offermann M, et al. Dynamic and static mechanical compression affects Akt phosphorylation in porcine patellofemoral joint cartilage. J Orthop Res. 2008;26(5):616–623. doi: 10.1002/jor.20542. [DOI] [PubMed] [Google Scholar]
- Normand V, Lootens DL, et al. New insight into agarose gel mechanical properties. Biomacromolecules. 2000;1(4) doi: 10.1021/bm005583j. [DOI] [PubMed] [Google Scholar]
- Ohlendorf C, Tomford WW, et al. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res. 1996;14(3):413–416. doi: 10.1002/jor.1100140311. [DOI] [PubMed] [Google Scholar]
- Ryan JA, Eisner EA, et al. Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. J Tissue Eng Regen Med. 2009;3(2):107–116. doi: 10.1002/term.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan NM, Grainger J, et al. The potential of pulsed low intensity ultrasound to stimulate chondrocytes matrix synthesis in agarose and monolayer cultures. Med Biol Eng Comput. 2010;48(12):1215–1222. doi: 10.1007/s11517-010-0681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


