Abstract
Alcohol is well-known for impairing impulse control as well as its disruptive effects on other aspects of behavioral functioning, such as motor control. Time-course analyses during a single dose show rapid development of acute tolerance to impairment of motor coordination, reaction time, and levels of subjective intoxication, but no acute tolerance to impairment of the ability to inhibit responses. Evidence for a possible lag in tolerance development to the impairing effects of alcohol on inhibitory control suggests that, as drinkers’ blood alcohol concentration (BAC) declines, they might exhibit prolonged impulsivity despite having an unimpaired ability to initiate action. The present study extended the time-course analysis to examine the recovery of inhibitory control under a dose of alcohol as drinkers’ BAC descended from a peak of 80 mg/100 ml to a zero level. Twenty-four healthy adults were tested following 0.65 g/kg alcohol and a placebo in a counterbalanced order. They performed a cued go/no-go task that measured response inhibition. They also performed tasks that assessed reaction time, motor coordination, and completed ratings of their subjective levels of intoxication. Alcohol initially impaired inhibitory control, response time, and motor coordination and increased subjective ratings of intoxication. However, acute tolerance to the impairing effects of alcohol was observed for measures of response time, motor coordination, and ratings of intoxication and these measures returned to sober (i.e., placebo) levels by the time BAC fell to near zero. By contrast, impairment of inhibitory control showed no acute tolerance and remained impaired even when drinkers’ BAC returned to near zero. Taken together, these results indicate that the disinhibiting effects of alcohol are present even when the impairing effects of alcohol on other aspects of behavior have diminished under the dose. These findings could provide a greater understanding of impulsive behaviors during the descending limb of intoxication.
Keywords: Alcohol, Inhibition, Tolerance, Go/No-Go Task
1. Introduction
The prevalence of alcohol abuse in the United States has increased over the past decade despite considerable concern over its social costs. Alcohol use is particularly prevalent among young adults, with over half of men and women between 18 and 25 years of age reporting frequent alcohol use (Substance Abuse and Mental Health Services Administration, 2004). Moreover, the typical pattern of alcohol use reported by this demographic is often characterized by periods of heavy alcohol consumption referred to as “binges,” which are usually defined as consuming five or more drinks during a single occasion (Wechsler & Nelson, 2001). There is growing evidence that acute changes in fundamental mechanisms of impulse control contribute importantly to the transition from social drinking to abusive drinking (e.g., Fillmore, 2003; 2007; Lyvers, 2000). As such, researchers have sought to gain a better understanding of how mechanisms of impulsivity operate to promote the abuse of alcohol.
One fundamental component of impulsivity concerns the ability to inhibit inappropriate or maladaptive actions or behaviors. Inhibitory control refers to the ability to inhibit a response that has already been instigated (see Logan & Cowan, 1984). This mechanism of behavior affords an individual control over where and when responses are expressed. Thus, the inhibition of behavioral responses is a necessary function for situations in which an individual needs to exert self-restraint and regulation over behavior. As such, deficits in inhibitory control have been implicated in a wide array of impulsive behaviors including heavy, binge drinking (e.g., Goudriaan et al., 2007; Marczinski et al., 2007). Human laboratory studies have employed stop-signal and cued go/no-go models to evaluate behavioral control as the ability to quickly activate and inhibit preopotent (i.e., instigated) responses (Logan 1994; Miller et al., 1991). These models are based on reaction time tasks requiring individuals to quickly activate a response to a go-signal and inhibit a response to stop or no-go signals. Studies have shown that these mechanisms of behavioral control are sensitive to the disruptive effects of alcohol. Indeed, alcohol increases inhibitory failures and slows response activation in a dose-dependent manner (Fillmore, et al., 2005; Fillmore & Weafer, 2004). However, studies provide evidence that inhibitory mechanisms are more sensitive to alcohol’s impairing effects compared with response activation. For example, studies have consistently found that inhibitory control is impaired at relatively low blood alcohol concentrations (BAC) that fail to slow response times (e.g., Fillmore & Vogel-Sprott, 1999; de Wit et al., 2000).
Studies examining the speed with which behaviors recover from alcohol’s impairing effects have also provided evidence of the sensitivity of inhibitory mechanisms to the drug’s effects (e.g., Fillmore et al., 2005; Ostling & Fillmore, 2010; Fillmore & Weafer, 2012). The term tolerance refers to the observation that the intensity of a behavioral response to a drug diminishes with repeated administrations of the drug (Kalant et al., 1971). Although alcohol tolerance can develop as a function of chronic, heavy consumption, it can also be observed following a single dose of alcohol. Acute tolerance refers to the diminished response to alcohol during the time-course of a single dose. This effect was first documented early last century by Mellanby (1919), who compared the intensity of alcohol impairment at a given BAC on the ascending and descending limbs of the blood alcohol curve. He observed that alcohol-induced ataxia in dogs was less intense at a given BAC during the descending versus the ascending limb of the BAC curve. This acute tolerance might be due to an adaptive process occurring during physiological exposure to the drug over time (e.g., Kalant et al, 1971).
In humans, acute tolerance to the impairing effects of alcohol has been observed for several behaviors such as motor coordination, reaction time, and subjective ratings of intoxication (Beirness & Vogel-Sprott, 1984; Schweizer et al., 2004; Fillmore et al., 2005; Fillmore & Vogel-Sprott, 1996; Marczinski & Fillmore, 2009; Schewiezer et al., 2004). In the past, acute tolerance was thought to develop uniformly across behaviors. However, several laboratory studies have failed to observe the development of acute alcohol tolerance for measures of inhibitory control (e.g., Fillmore et al., 2005; Ostling & Fillmore, 2010; Fillmore & Weafer, 2012). In one such study, Fillmore et al. (2005) compared the development of acute tolerance to the impairing effects of alcohol on response activation to the impairing effects on response inhibition. Participants performed the cued go/no-go task twice: once on the ascending limb and once on the descending limb of the BAC curve following 0.65 mg/kg alcohol. Both tests were performed at comparable BACs of approximately 80 mg/100 ml. The study showed that alcohol impaired behavioral activation by slowing reaction time and impaired response inhibition by increasing failures to inhibit responses to no-go targets. With regard to acute tolerance, the study found rapid recovery of behavioral activation. That is, reaction times measured on the descending limb of the blood alcohol curve had returned to sober levels. However, inhibitory control remained as impaired on the descending limb as it was on the ascending limb of the blood alcohol curve. Such findings show that inhibitory mechanisms are especially slow to recover from the impairing effects of alcohol.
Evidence that inhibitory control fails to recover from alcohol’s impairing effects at the same rate as other behaviors begs the question of when impaired inhibitory mechanisms return to sober levels. Prolonged impairment of inhibitory mechanisms along the descending limb of the BAC curve could play an important role in the development of alcohol abuse. Drinkers might be prone to engaging in continued impulsive action even as BACs decline, such as resuming alcohol consumption, resulting in excessive binge drinking in a situation. No work has systematically extended the time-course analysis of the disinhibiting effects of alcohol along the BAC curve to determine when behavioral impairment might show full recovery. Thus, the present study compared the recovery of alcohol-induced impairment of inhibitory control with the recovery of other behaviors that have demonstrated acute tolerance to alcohol. The study employed an extended time-course approach to examine the recovery of inhibitory control, reaction time, motor coordination, and ratings of subjective intoxication following a dose of 0.65 g/kg alcohol as drinkers’ BAC descended from a peak of approximately 80 mg/100 ml to a near-zero level. As a control, performance was also tested following a placebo dose. Consistent with our previous research (e.g., Fillmore et al., 2005; Ostling & Fillmore, 2010), it was hypothesized that reaction time, motor coordination, and subjective intoxication would display acute tolerance to the effects of alcohol, and that complete recovery would also be evident once BACs returned to near-zero levels. However, we predicted that there would be no evidence of acute tolerance for inhibitory control, and that given this lag in recovery, we might fail to observe complete recovery of this impairment as BACs approach zero.
2. Method
2.1 Participants
Twenty-four individuals (12 men and 12 women) between the ages of 21 and 29 (mean age = 23.2, SD = 2.6) participated in this study. Volunteers were recruited by flyers, posters, and newspaper/online advertisements seeking adults for studies of the effects of alcohol on cognitive functions. Volunteers were screened using health questionnaires and a medical history interview. Volunteers who reported any contraindication to alcohol, impaired cardiovascular functioning, seizure, head trauma, or central nervous system (CNS) tumors, were excluded from participation. Volunteers were also asked about past histories or present diagnoses of psychiatric disorder (i.e., Axis I, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM-IV, American Psychiatric Association, 2000]). Participants who reported a diagnosis of a DSM-IV Axis I disorder, past or present use of psychotropic medication, and/or past or present participation in counseling or therapy were also excluded from participation.
Volunteers had to report drinking at least once per month in an amount of at least two drinks to participate. Volunteers who reported alcohol dependence, as determined by a score of 5 or higher on the Short-Michigan Alcoholism Screening Test (S-MAST; Selzer et al., 1975) were excluded from the study. Any other high-risk indicators of alcohol dependence, including prior treatment for an alcohol use disorder or conviction for driving under the influence also precluded participation. With regard to other drug use, the majority of the sample reported using caffeine (n = 20). Thirteen participants reported smoking cigarettes in the amount of less than a pack of cigarettes a day. Nine reported occasional past month use of marijuana on a less-than-weekly basis. No other drug use in the past month, including stimulants, opiates, or cocaine, was reported. Participants were in good health with no contraindications to drinking. The University of Kentucky Medical Institutional Review Board approved the study, and participants received $85.
2.2 Materials and Measures
2.2.1 Personal drinking habits questionnaire (PDHQ; Vogel-Sprott, 1992)
This questionnaire provided three measures of the quantity and frequency of typical consumption: the number of drinking occasions per week, the typical drinks consumed per drinking occasion, and the typical BAC attained during a drinking episode. Typical BAC was calculated based on self-reported number of drinks usually consumed in a drinking episode, the type of alcohol usually consumed (beer, wine, or liquor), and the typical hourly duration of the drinking episode. This information, along with gender and weight in kilograms, was entered into an anthropometric formula to calculate peak BAC obtained during the typical drinking episode of each participant (McKim, 2007).
2.2.2 Cued go/no-go task
Inhibitory control was measured using a computerized cued go/no-go model used in previous research (e.g., Marczinski & Fillmore, 2003; Fillmore et al. 2005) and was operated by E-Prime experiment generation software (Schneider et al., 2002). A trial began with a fixation point (+) for 800 ms, followed by a blank screen for 500 ms. A rectangular-shaped cue was then displayed for one of four randomly occurring stimulus onset asynchronies (SOAs = 100, 200, 400, and 800 ms) before a go or no-go signal appeared for 1000 ms. If the rectangle turned green (the go signal) subjects were to make a computer key press as quickly as possible and if the rectangle turned blue (the no-go signal) they were to inhibit any response. A test consisted of 250 trials with 700 ms inter-trial intervals and required 15 minutes to complete.
The orientation of the rectangular cue signaled the probability that a go or no-go signal would appear. A vertically-oriented rectangle (height = 7.5 cm, width = 2.5 cm) turned green on 80% of the trials and turned blue on 20% of the trials. A horizontally-oriented rectangle (height = 2.5 cm, width = 7.5 cm) turned green on 20% of the trials and turned blue on 80% of the trials. Therefore, vertical and horizontal-oriented rectangles operated as go and no-go cues, respectively. The measure of interest was the proportion (p) of inhibition failures to no-go signals in the go cue condition. Greater p-inhibition failures indicate poorer inhibitory control (i.e., disinhibition). Presentation of the go cue increases response preparation (i.e., produces a response prepotency), making it more difficult to inhibit a response when the no-signal unexpectedly appears. The disinhibiting effects of alcohol are most evident in this cue condition (Marczinski and Fillmore, 2003).
2.2.3 Two-choice reaction time (RT) task
Reaction time (RT) was measured by a computerized choice RT task which was operated using E-prime Experiment Generation software (Schneider et al., 2002) and performed on a personal computer. Participants are required to respond as quickly and accurately as possible to the presentation of targets on the screen. The letters X and O serve as the targets, and participants must press the (“) key in response to the letter O and the (/) key in response to the letter X. A test contains 90 trials. Each trial consisted of the following sequence of events (a) a fixation point (+) displayed for 800 ms; (b) a blank white screen displayed for one of three stimulus onset asynchronies (SOAs = 100, 400, and 900 ms); (c) the stimulus presented for 1,000 ms or until the response occurred; (d) a feedback screen that presented in a random order. A test required approximately 5 minutes to complete.
2.2.4 Grooved Pegboard
Motor coordination was measured by a grooved pegboard task (Lafayette Instruments, Lafayette, IN). The pegboard task consists of a 5 by 5 inch metal surface that contains 25 “keyhole shaped” holes arranged in five rows of five holes each. Each of these holes has a large rounded side and a smaller, square side (a groove). The orientation of the groove in each hole varies such that no two adjacent holes have the same orientation. Participants are required to pick up the pegs one at a time and place them in the holes, filling in one row at a time until all 25 holes have been filled (i.e., one trial). The time to complete a trial (in seconds) is the measure of interest. A test consisted of four trials. The average completion time of the four tests was the dependent measure.
2.2.5. Subjective Intoxication
The degree of subjective intoxication is measured on a visual analogue scale (VAS). Participants rate their degree of subjective intoxication by placing a vertical line at the point representing the extent to which they ‘feel intoxicated’ on a 100-mm horizontal line ranging from 0 mm “not at all” to 100 mm “very much.”
2.3. Procedure
Participants were tested individually in the Behavioral Pharmacology Laboratory of the Department of Psychology between 10 a.m. and 6 p.m. Sessions were scheduled at least 24 hours apart and were completed within a two week time period. Participants were instructed to fast for four hours prior to each session, as well as to refrain from consuming alcohol or any psychoactive drugs for at least 24 hours before all sessions. Prior to each session, participants provided urine samples and were tested for drug metabolites including amphetamine, barbiturates, benzodiazepines, cocaine, opiates, and tetrahydrocannabinol (On Trak TesTstiks, Roche Diagnostics Coorporation, Indianapolis, IN, USA) and in women, human chorionic gonadotrpin (hCG hormone), to verify that they were not pregnant (Mainline Confirms HGL, Mainline Technology, Ann Arbor, MI, USA). Any participants who tested positive for recent drug use or for pregnancy were excluded from participating. Breath samples were also provided at the beginning of each session to verify a zero BAC.
2.3.1. Familiarization session
After providing informed consent, participants provided proof of age to verify that they were at least 21 years old. They completed questionnaires concerning health status, drinking habits, and demographic characteristics. Finally, they performed shortened versions of each test to become acquainted with the task requirements.
2.3.2. Test Sessions
Performance was tested under two doses of alcohol: 0.0 g/kg (placebo) and 0.65 g/kg. Each dose was administered during a separate test session, and dose order was counterbalanced across participants. Participants were blind to the doses they received at each session. Sessions were separated by a minimum of one day and a maximum of one week. The alcohol dose was calculated on the basis of body weight and administered as absolute alcohol mixed with three parts carbonated soda divided equally into two glasses. Participants had two minutes to finish each glass, and the glasses were served four minutes apart. The placebo consisted of a volume of absolute alcohol that matched the total volume of the 0.65 g/kg alcohol drink. A small amount (3 ml) of alcohol was floated on the surface of the beverage. It was sprayed with an alcohol mist that resembles condensation and provides a strong alcoholic scent as the beverage is consumed. Previous research has shown that individuals report that this beverage contains alcohol (Fillmore & Vogel-Sprott, 1998).
Subjects were tested on the cued go/no-go, choice RT, and pegboard tasks and completed the subjective rating scale at three times: 30 minutes (time 1), 90 minutes (time 2), and 320 minutes (time 3) after drinking began. Based on prior studies of the active alcohol dose (e.g., Marczinski & Fillmore, 2003; Fillmore & Vogel-Sprott, 1998), subjects were expected to obtain a BAC of 65 mg/100 ml at 30 (time 1) minutes that would continue to rise to an approximate peak of 80 mg/100 ml at 60 minutes, and descend back to 65 mg/100 ml by 90 minutes (time 2). Based on the average rate of BAC decline per minute, BACs were predicted to return to near zero levels by 320 minutes after drinking (time 3). BAC was measured at 30-minute intervals throughout the session, including immediately prior to and immediately following each test. The intervals were as follows: 30, 60, 90, 120, 150, 180, 210, 240, 270, 290, and 320 minutes after drinking began. Breath samples were also obtained at these times during the placebo session ostensibly to measure subjects’ BACs. Participants remained at leisure in a waiting room and were provided with a light snack as their BACs fell between time 2 and time 3. After the final test (time 3), they were provided with transportation home. After the final session they were paid and debriefed.
2.4. Criterion measures
2.4.1. Inhibitory Control
Response inhibition was measured as participants’ failures to inhibit responses to no-go targets (failure of response inhibition). Failure of response inhibition was measured as the proportion (p) of no-go targets in the go cue condition in which a participant failed to inhibit a response (i.e., p-inhibition failures)
2.4.2. Reaction Time
The two-choice RT task measured participants’ RT as the mean RT to targets in milliseconds, with fewer milliseconds indicating faster RTs.
2.4.3. Motor Coordination
The grooved pegboard task measured motor coordination as the time in seconds required to insert all of the pegs into the board averaged across the four trials. Faster mean completion times indicated greater motor coordination.
2.4.4. Subjective Intoxication
Greater degree of intoxication was indicated by higher ratings on the subjective intoxication VAS.
2.5. Data analyses
All dependent measures were analyzed by 2 dose (0.0 g/kg and 0.65 g/kg) X 3 time (times 1, 2, and 3) repeated-measures analyses of variance (ANOVA). Acute tolerance to alcohol was tested by a priori simple effects comparisons of time 1 and time 2 following 0.65 g/kg using pairwise t tests. Planned comparisons of performance at time 3 between placebo and alcohol conditions were also used to determine if complete recovery was evident as BACs approached zero following the active dose.
3. Results
3.1. Drinking habits
Subjects reported drinking 1.8 (SD = 0.8) days per week and consuming 5.3 (SD = 2.4) drinks per occasion. Subjects reported typically drinking to a BAC of 82.6 (SD = 44.4) mg/100 ml. Two-sample t tests revealed that men drank more frequently than women (p < 0.05). Men reported drinking 2.2 (SD = 0.6) days per week compared to 1.5 (SD = 0.8) for women. Men also reported consuming more drinks per drinking occasion compared with women (p < 0.01), with men typically consuming 6.8 (SD = 2.3) drinks and women consuming 3.8 (SD = 1.6) drinks. However, once body weight differences were taken into account, there were no significant differences between men and women with respect to the typical BACs attained per drinking episode p = 0.07.
3.2. Blood alcohol concentrations
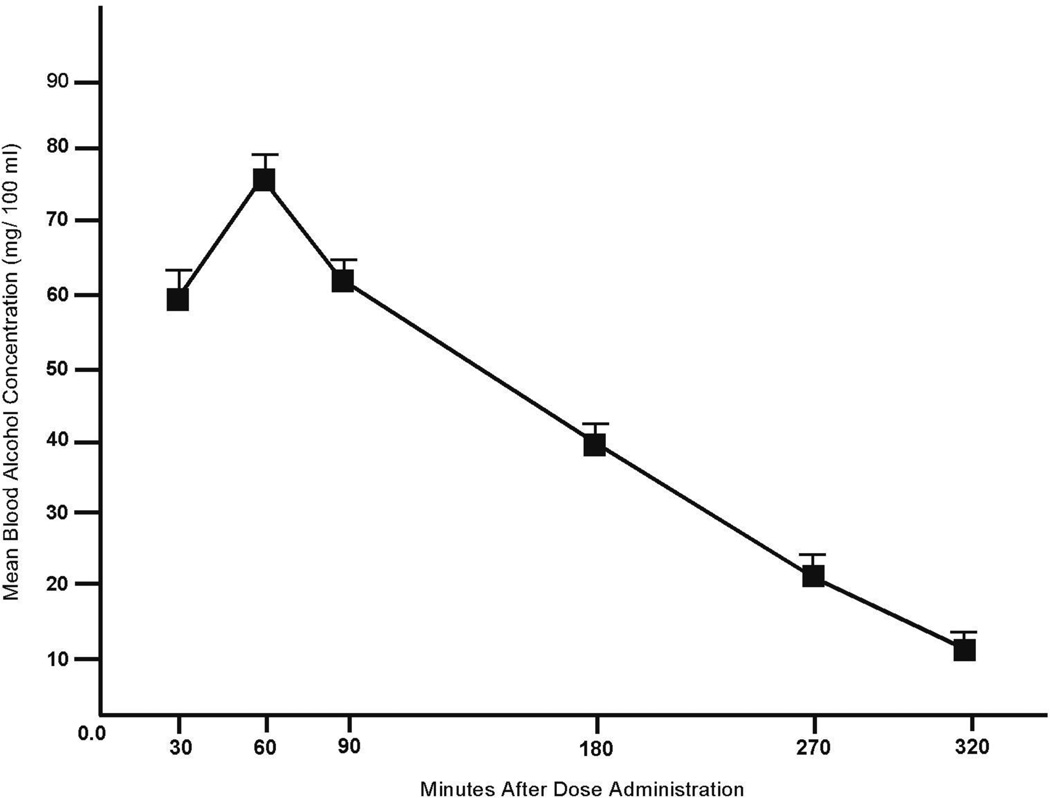
No detectable BACs were observed following placebo. Figure 1 plots the mean BACs following alcohol when BACs were obtained. The figure shows that BACs ascended to a peak of 75.4 mg/100 ml (SD = 12.7) at 60 minutes after drinking began. Potential gender differences were examined by a 2 (gender) X 3 (time) ANOVA. No main effect or interaction involving gender was observed (ps > 0.30). There was a main effect of test, owing to the higher BACs at times 1 and 2 compared with time 3. Indeed, for the entire sample, the mean BAC at time 1 (30 minutes), BAC was 59.9 mg/100 ml (SD = 15.9). At time 2 (90 minutes) the mean BAC was 61.6 mg/ 100 ml (SD = 10.8). A paired-sample t test revealed no difference in BAC at time 1 versus time 2, p = 0.5. At test 3, the mean BAC (320 minutes), the mean BAC was 11.7 mg/ 100 ml (SD = 10.1).
Figure 1.
Mean blood alcohol concentrations (BAC) under alcohol at intervals when breath samples were obtained. Capped vertical lines show standard error of the mean.
3.3 Task performance
3.3.1 Inhibitory Control
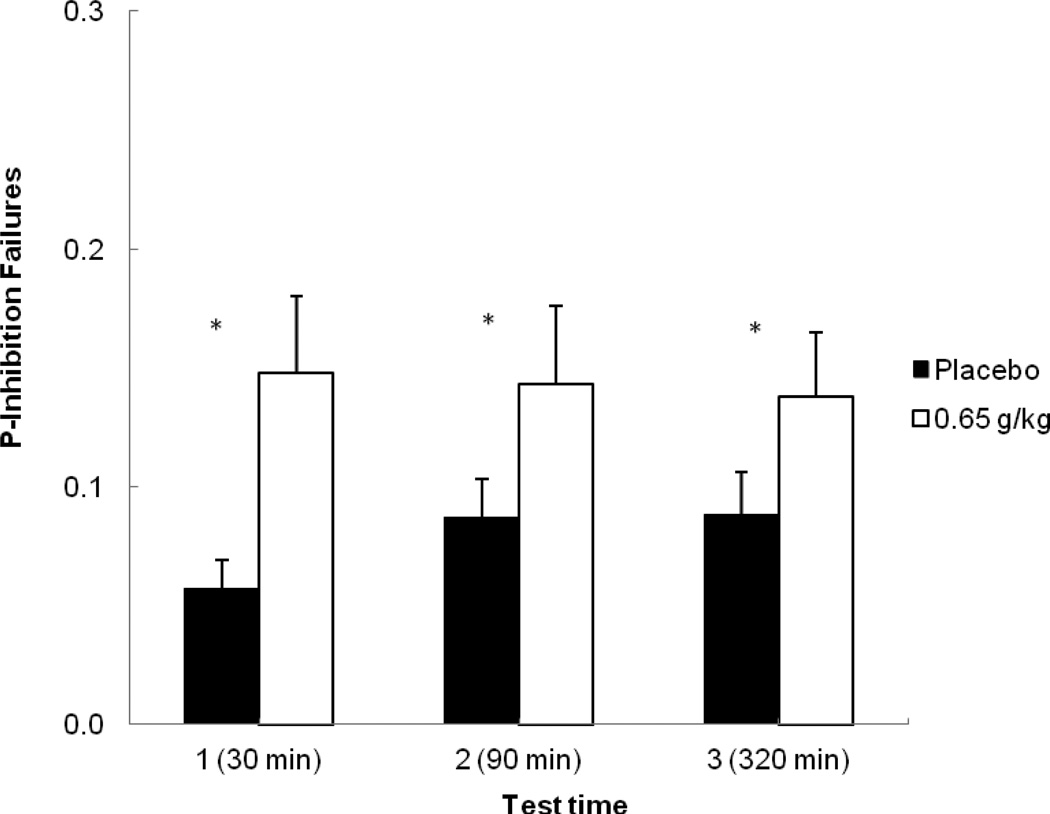
Figure 2 shows the mean p-inhibition failures on the cued go/no-go task following placebo and alcohol for the three tests. The figure shows greater inhibitory failures in response to alcohol compared with placebo for each test. A 2 (dose) X 3 (time) repeated-measures ANOVA of p-inhibition failures revealed a significant main effect of dose, F (1, 23) = 12.02, p < 0.01, η2 = 0.34. There was no main effect of time, F (2, 46) = 0.58, p = 0.56, η2 partial = 0.02, nor an interaction, F (2, 46) = 0.88, p = 0.42, η2 partial = 0.04. Planned comparison tests confirmed that at time 1, alcohol significantly increased inhibitory failures compared with placebo, t (23) = 2.83, p < 0.01, and that this impairment remained at time 2 (the descending limb), t (23) = 2.08, p < 0.05. A planned test also compared performance at time 1 at time 2 following alcohol. This test revealed no difference in inhibitory failures between time 1 and time 2 following alcohol, p = 0.83, indicating no acute recovery of inhibitory control from the ascending to descending limb. Finally, a comparison of inhibitory failures at time 3 between placebo and alcohol conditions revealed that there were still significantly more errors following alcohol compared with placebo at this time, t (23) = 2.39, p < 0.05.
Figure 2.
Mean proportion of failures to inhibit responses on the cued go/no-go task at each test time in response to placebo and alcohol. Capped vertical lines show the standard error of the mean. Asterisks (*) indicate a significant increase in inhibitory failures from placebo to alcohol, p < 0.05.
3.3.2. Reaction Time
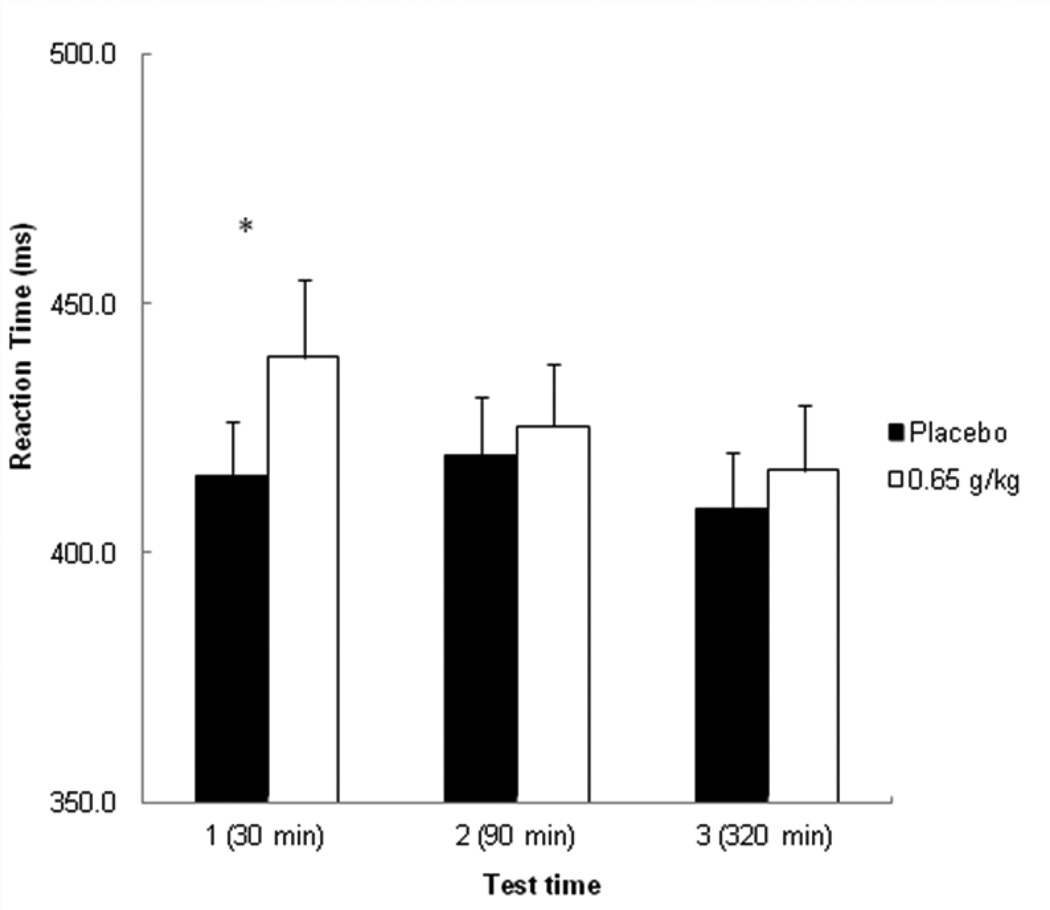
Figure 3 plots the mean RTs on the two-choice reaction time for each test following placebo and alcohol. A 2 (dose) X 3 (time) repeated-measures ANOVA revealed a significant main effect of dose, F (1, 23) = 8.19, p < 0.01, η2 partial= 0.26, a main effect of time, F (2, 46) = 9.52, p < 0.001, η2 partial= 0.29, and an interaction, F (2. 46) = 4.11, p < 0.05, η2 partial = 0.15. Planned comparison tests confirmed that at time 1, alcohol significantly slowed RTs compared with placebo, t (23) = 3.34, p < 0.001. However, following alcohol, RTs were significantly faster at time 2 compared with time 1, t (23) = 2.24, p < 0.05. Moreover, at time 2, RTs following alcohol did not differ from RTs following placebo, p = 0.24, indicating acute recovery of RT on the descending limb. Finally, a comparison of RT at time 3 between placebo and alcohol also confirmed that there was no significant difference in RTs at this time, p = 0.17.
Figure 3.
Mean reaction time (RT) in milliseconds during the choice reaction time task at each test time in response to placebo and alcohol. Capped vertical lines show the standard error of the mean. Asterisks (*) indicate a significant increase in RT from placebo to alcohol, p < 0.001.
3.3.3 Motor Coordination
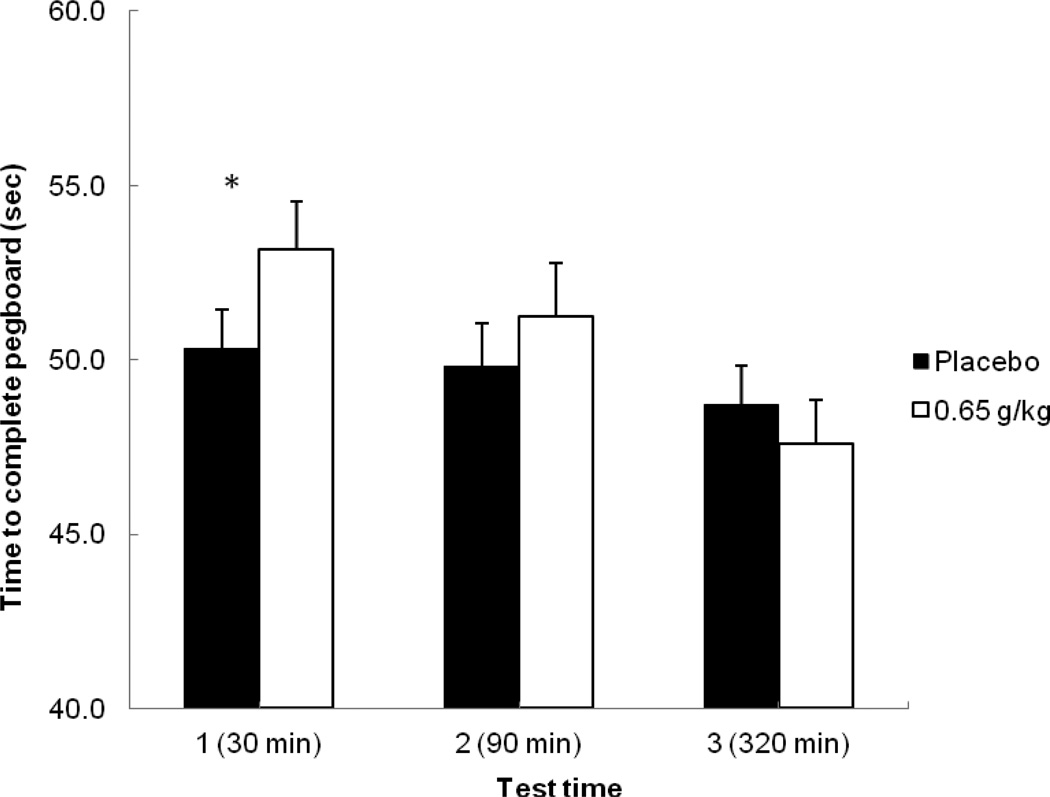
Figure 4 plots the mean time to complete the pegboard task in seconds for each test following placebo and alcohol. A 2 (dose) X 3 (time) repeated-measures ANOVA revealed a main effect of time, F (2, 46) = 36.25, p < 0.001, η2 = 0.61, and an interaction, F (2, 46) = 23.04, p < 0.001, η2 = 0.50. There was no main effect of dose, F (1, 23) = 3.64, p = 0.07, η2 = 0.14. Planned comparison tests showed that at time 1, alcohol significantly slowed completion time compared with placebo, t (23) = 4.55, p < 0.001. There was a significant decrease in completion time from time 1 to time 2 following alcohol, t (23) = 3.50, p < 0.001, indicating acute tolerance. At time 2, there was no statistical difference observed in completion time between alcohol and placebo, p = 0.06, indicating acute recovery of motor coordination on the descending limb. Finally, a comparison at time 3 between placebo and alcohol also showed no significant difference in completion time, p = 0.09.
Figure 4.
Mean time to complete the pegboard task (in seconds) at each test time in response to placebo and alcohol. Capped vertical lines show the standard error of the mean. Asterisks (*) indicate a significant increase in completion time from placebo to alcohol, p < 0.001.
3.3.4. Subjective Intoxication
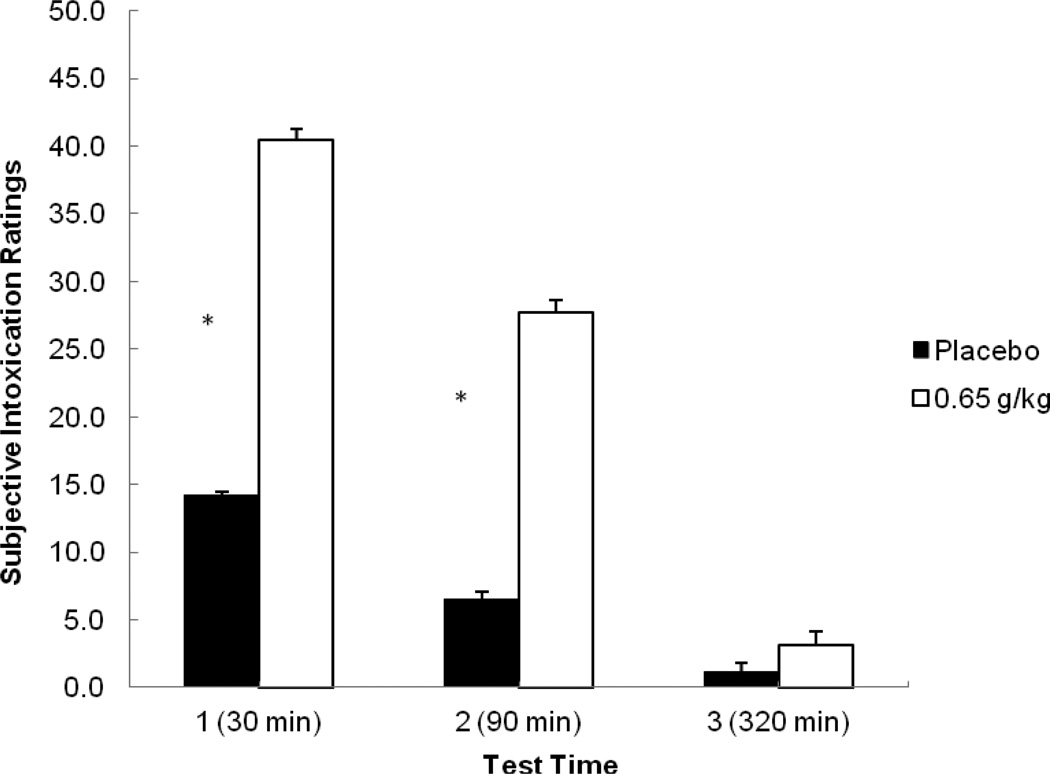
Figure 5 plots the mean subjective intoxication ratings for each time following placebo and alcohol. A 2 (dose) X 3 (time) repeated-measures ANOVA of ratings revealed a main effect of dose, F (1, 23) = 33.03, p < 0.001, η2 = 0.59, of time, F (2, 46) = 32.63, p < 0.001, η2 = 0.59, and an interaction, F (2, 46) = 23.87, p < 0.001, η2 = 0.51. Planned comparison tests revealed that alcohol increased intoxication ratings compared with placebo at time 1, t (23) = 5.56, p < 0.001, and time 2, t (23) = 5.49, p < 0.001. There was also a significant decrease in ratings from time 1 to time 2 following alcohol, t (23) = 5.20, p < 0.001. Finally, a comparison at time 3 between placebo and alcohol showed no significant difference in intoxication ratings, p = 0.08.
Figure 5.
Mean subjective intoxication ratings (1 – 100) at each test time in response to placebo and alcohol. Capped vertical lines show the standard error of the mean. Asterisks (*) indicate a significant increase in intoxication ratings from placebo to alcohol, p < 0.001.
3.3.5 Reliability/Stability of Task Measures
The degree to which each task reliably assessed participants’ performance over time during a session was also tested. For each task, we analyzed the intraclass correlation coefficient (Cronbach’s Alpha) based on performance at each of the three testing times following placebo. Cronbach’s alphas for inhibitory control, motor coordination, and reaction time measures were 0.78, 0.98, and 0.98, respectively. These results confirm high test-retest reliability of each measure, and that the individual differences among participants’ performance level on a task showed a high degree of consistency over tests within the session.
4. Discussion
The present study sought to determine the degree to which alcohol-induced impairment of inhibitory control recovers as BACs decline to a near zero level. Inhibitory control was measured by performance on a cued go/no-go task in a group of young adult, social drinkers. Subjects also performed tasks measuring reaction time and motor coordination, and provided subjective ratings of intoxication. Subjects performed all tasks in response to placebo and 0.65 g/kg alcohol, and performance was tested at three time points after drinking: at comparable BACs of approximately 65 mg/100 ml on the ascending and descending limbs of the blood alcohol curve, and then over five hours after drinking began, when BACs were nearly zero.
The study showed that alcohol significantly impaired performance on all tasks and increased subjective ratings of intoxication on the ascending limb of the BAC curve compared with placebo. Acute tolerance was observed for RT, motor coordination, and subjective intoxication. That is, comparisons of performance at comparable BACs on the descending versus ascending limb of the BAC curve showed significant decreases in impairment of RT and motor coordination and a reduction in ratings of intoxication. This recovery continued to the third test of performance, as BACs approached zero. By contrast, we observed no evidence of acute tolerance for inhibitory control. Indeed, alcohol continued to increase inhibitory failures on the descending limb to the same general degree as was observed on the ascending limb, which is consistent with previous findings from our laboratory (Fillmore et al., 2005; Ostling & Fillmore, 2010; Fillmore & Weafer, 2012). Although this study design did not permit a straightforward approach to formally test whether the time by dose interaction significantly differed by measure, the effect size for the time by dose interaction for inhibitory control was considerably smaller compared with the other measures. Moreover, not only had the disinhibiting effects of alcohol failed to recover on the descending limb, but the study showed that at time 3, nearly five hours after drinking, inhibitory control remained significantly impaired at a magnitude similar to the degree of impairment observed much earlier under the dose during times 1 and 2.
This study is the first to examine whether alcohol-induced deficits of inhibitory control fully recover as BACs decline to zero. It is not clear why drinkers remained substantially disinhibited in the study despite having near-zero BACs. There is a growing body of research that suggests that inhibitory control is especially sensitive to the disruptive effects of alcohol compared with other behavioral functions. This sensitivity is especially evident when examining the development of tolerance to alcohol impairment. With regard to chronic tolerance, it is generally assumed that heavier drinkers should display reduced reactions to a dose of alcohol (i.e., tolerance), whereas lighter drinkers should be more affected by the same dose. While this is the case for measures, such as RT, motor coordination, and subjective intoxication, we fail to observe tolerance to the disinhibiting effects of alcohol as a function of recent, heavy consumption (i.e., Marczinski et al., 2007; Miller et al., 2012). Moreover, as shown by the present study and by others, within a single drinking episode, inhibitory control fails to adapt to and recover from the impairing effects of an acute dose of alcohol (Fillmore et al., 2005; Ostling & Fillmore, 2010; Fillmore & Weafer, 2012). What is new from the present research is that we have provided additional evidence showing that alcohol-induced impairment of inhibitory mechanisms continues even after BACs become essentially negligible after drinking.
It is unlikely that the persistent impairment of inhibition observed after five hours post-alcohol was the result of boredom or fatigue, as participants’ response activation and psychomotor performance recovered fully by this time. Moreover, the testing regime was not arduous for the subjects as they were required to complete the test battery only three times during the entire test session with ample time between tests. Instead, the prolonged impairment of inhibitory control might represent a protracted pharmacological/physiological effect of alcohol. In fact, there is some evidence to suggest that the effects of alcohol on cognition and behavior can be observed the day following alcohol consumption, long after alcohol has been eliminated from the body (for a review, see Prat et al., 2008). However, evidence for the hangover effect has generally been inconsistent. For example, several studies have failed to show any protracted impairment from alcohol on simple, psychomotor skills, such as RT and coordination (e.g., Chait & Perry, 1994; Finnigan et al., 1998; Kruisselbrink et al., 2006). A lack of prolonged impairment of motor performance is consistent with our current findings that showed the initial alcohol-induced impairments of these behaviors began to recover early during the time-course on the descending limb of the curve even while BACs were still elevated (i.e., > 50 mg/100 ml). By contrast, prolonged, day-after impairments have been observed for more complex behaviors, such as those requiring divided and/or sustained attention (Finnigan et al., 2005; Roehrs & Roth, 2001). However, to date, no work has shown whether alcohol-induced impairments of inhibitory mechanisms of behavioral control are subject to such a “day-after” hangover effects. The protracted impairment of inhibitory control observed in the present study could suggest that cognitive operations involving the inhibition of actions are likely to show protracted impairments from alcohol, possibility even day-after impairments, such as carryover or hangover effects. Such a possibility awaits to be examined.
To better understand why alcohol has such protracted effects on inhibitory control compared with other aspects of behavior, it is important to consider the neural underpinnings of behavioral control. Event-related human brain potentials (ERPs) have been used to identify the neural mechanisms underlying cognitive processes, including inhibitory control. To do this, ERPs are recorded as participants perform a cued go/no-go task. Findings have shown that the successful inhibition of responses on the task generates heightened P3 (or P300) waves located at midline central sites (i.e., Bokura et al., 2001; Falkenstein et al., 1999; Kok et al., 2004), whereas reduced P3 waves are associated with disinhibition (e.g., Bauer and Hesselbrock, 1999; Iacono et al., 2003; Patrick et al., 2006). What is more, P3 waves are consistently reduced following moderate doses of alcohol (Barthalow et al., 2003; Rorhbaugh, et al., 1987). Indeed, Easdon et al. (2005) have shown that a moderate dose of alcohol increased inhibitory failures and reduced P3 amplitudes specifically in instances when participants exhibited inhibition failures. Given that the P3 component of the ERP has been of particular interest in the study of alcohol abuse and inhibition, a potential extension of this work would be to continue to record ERPs throughout the time course of the BAC curve. This would provide an important psychophysiological counterpart to the present study’s behavioral findings, and offer insight into the duration of alcohol’s effects on neural functioning even as BACs begin to dissipate.
There are some potential limitations of the current study which might inform future research. First, the study’s sample was comprised of primarily college-aged, young adults. The sample reported consuming an average of 5.3 drinks per drinking occasion, and 12 participants (50% of the sample), reported drinking to a 0.08% BAC on a regular basis. These habits are typical of this demographic (e.g., Johnston et al., 2010), and do not reflect abnormally heavy, or dependent drinkers. Moreover, regression analyses revealed no relationship between any of the drinking habits we measured (frequency, quantity, and typical BAC) and the degree to which inhibitory control was impaired by alcohol (ps > 0.36). That said, future work might be aimed toward extending the present findings to populations with different patterns of consumption.
Another possible limitation of this study is that we only focused on one aspect of behavioral control (i.e., the ability to inhibit a prepotent response). However, our findings raise the question of whether alcohol results in a prolonged impairment across of broad range of inhibitory functions. Indeed, alcohol has been shown to impair other aspects of inhibitory control, such as mechanisms of attentional control. In these studies, alcohol disrupts attentional control, resulting in a decreased ability to direct attention from distractions (Fillmore et al. 2000; Abroms and Fillmore 2004). Moreover, such impairments in mechanisms of attentional control have also been implicated as a factor that might contribute to alcohol abuse (Tarter et al 2004; Blume et al 2005). As such, it is important to examine whether alcohol-induced impairments of attentional control also fail to recover from alcohol’s disinihibiting effects along the timecourse of the BAC curve. Moreover, such a possibility will also provide assurance that the failure of recovery that we have observed in the present study is related not only to inhibitory failures on a go/no-go task, but to the general construct of inhibitory mechanisms of behavioral control.
Finally, it is important to consider the implications of such a prolonged impairment of inhibitory mechanisms following alcohol consumption. In particular, the results might lead to a better understanding of the impulsive behavior and poor decision making commonly observed under alcohol. Studies suggest that alcohol-induced impairment of inhibitory control contributes to alcohol abuse by promoting excessive or binge drinking (e.g., Marczinski et al., 2007; Weafer & Fillmore, 2008), and alcohol-induced disinhibition is also related to other impulsive, aggressive, and socially inappropriate behaviors (Fillmore 2003, 2007; Jentsch & Taylor, 1999). Thus, the recovery of behavioral activation following a dose of alcohol coupled with continued impairment of inhibitory mechanisms might result in the prolonged display of impulsive behavior even as BACs decline considerably. For example, following an initial drink, individuals might decide to extend a drinking session, leading to a binge episode given that they feel sober and detect no impairment of motor coordination, yet continue to be significantly disinhibited. Additionally, other risky decisions might follow, such as decisions to drive or engage in risky sexual or other aggressive behaviors. As such, future studies aimed at better identifying the mechanisms by which this prolonged impairment of impulse control persists will prove beneficial.
Highlights.
-
-
Inhibitory control was tested at 3 time points following a dose of alcohol
-
-
No acute tolerance to alcohol was found for inhibitory control
-
-
Inhibition was impaired even five hours after drinking, when BACs were near zero
Acknowledgements
The authors wish to thank Jaime Brown for her help in collecting data.
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA018274 and F31 AA021028.
Role of Funding Source
Funding for this study was provided by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grants R01 AA018274 and F31 AA021028. NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Fillmore and Melissa Miller designed the study, and Melissa Miller wrote the protocol. Melissa Miller oversaw the data collection and coding. Dr. Fillmore and Melissa Miller managed the literature searches and summaries of previous related work, and Melissa Miller undertook the statistical analysis. Melissa Miller and Dr. Fillmore wrote the manuscript. Both authors contributed to and have approved the final manuscript.
Conflict of Interest
Both authors declare that they have no conflicts of interest.
References
- Abroms BD, Fillmore MT. Alcohol-induced impairment of inhibitory mechanisms involved in visual search. Experimental and Clinical Psychopharmacology. 2004;12:243–250. doi: 10.1037/1064-1297.12.4.243. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: 2000. [Google Scholar]
- Bartholow BD, Pearson M, Sher KJ, Wieman LC, Fabianai M, Gratton G. Effects of alcohol consumption and alcohol susceptibility on cognition: A psychophysiological examination. Biological Psychology. 2003;64:167–190. doi: 10.1016/s0301-0511(03)00108-x. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biological Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bierness D, Vogel-Sprott M. The development of alcohol tolerance: Acute recovery as a predictor. Psychopharmacology (Berl) 1984;84:398–401. doi: 10.1007/BF00555220. [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA. Memory, executive function, and readiness to change drinking behavior. Addictive Behaviors. 2005;30:301–314. doi: 10.1016/j.addbeh.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/No-Go task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115:340–349. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- De Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behavioral Neuroscience. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychological. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Cognitive Brain Research. 2005;25:873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: Current approaches and findings. Behavioral and Cognitive Neuroscience Reviews. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Acute alcohol-induced impairment of cognitive functions: Past and present findings. International Journal on Disability and Human Development. 2007;6:115–125. [Google Scholar]
- Fillmore MT, Dixon MJ, Schweizer TA. Alcohol affects processing of ignored stimuli in a negative priming paradigm. Journal of Studies on Alcohol. 2000;61:571–578. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol. 2005;33:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Social drinking history, behavioral tolerance and the expectation of alcohol. Psychopharmacology (Berl) 1996;127:359–364. doi: 10.1007/s002130050098. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: cognitive and pharmacokinetic factors. Alcoholism: Clinical and Experimental Research. 1998;22:1476–1482. [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Experimental and Clinical Psychopharmacology. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–1246. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Acute tolerance to alcohol in at-risk binge drinkers. Psychology of Addictive Behaviors. 2012;20:693–702. doi: 10.1037/a0026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnigan F, Hammersley R, Cooper T. An examination of next-day hangover effects after a 100 mg/100 ml dose of alcohol in heavy social drinkers. Addiction. 1998;93:1829–1838. doi: 10.1046/j.1360-0443.1998.931218298.x. [DOI] [PubMed] [Google Scholar]
- Finnigan F, Schulze D, Smallwood J, Helander A. The effects of self-administered alcohol-induced “hangover” in a naturalistic setting on psychomotor and cognitive performance and subjective scale. Addiction. 2005;100:1680–1689. doi: 10.1111/j.1360-0443.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: A longitudinal study. Alcoholism: Clinical and Experimental Research. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2009. Volume II: College Students and Adults Ages 19 −50. Bethesda, MD: National Institute on Drug Abuse; 2010. NIH Publication No. 10-7585. [Google Scholar]
- Kalant H, Leblanc AE, Gibbons RJ. Tolerance to, and dependence on ethanol. In: Israel Y, Mardonze J, editors. Biological basis of alcoholism. New York: Wiley/Interscience; 1971. pp. 235–269. [Google Scholar]
- Kruisselbrink LD, Martin KL, Megeney M, Fowles JR, Murphy RJ. Physical and psychomotor functioning of females the morning after consuming low to moderate quantities of beer. Journal of Studies of Alcohol. 2006;67:416–420. doi: 10.15288/jsa.2006.67.416. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: A neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychology of Addictive Behaviors. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Acute tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychology of Addictive Behaviors. 2009;23:238–247. doi: 10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- McKim WA. 6th ed. New Jersey: Prentice Hall; 2007. Drugs and behavior: an introduction to behavioral pharmacology. [Google Scholar]
- Mellanby E. Alcohol: Its absorption into and disappearance from the blood under different conditions (Special Report Series Monograph No. 31) London, England: Medical Research Committee; 1919. [Google Scholar]
- Miller J, Schaffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychological. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-r. [DOI] [PubMed] [Google Scholar]
- Miller MA, Hays LR, Fillmore MT. Lack of tolerance to the disinhibiting effects of alcohol in heavy drinkers. Psychopharacology. 2012;4:511–518. doi: 10.1007/s00213-012-2786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostling EW, Fillmore MT. Tolerance to the impairing effects of alcohol on the inhibition and activation of behavior. Psychopharmacology. 2010;212:465–473. doi: 10.1007/s00213-010-1972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, McGue MK. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat G, Adan A, Perez-Pamies M, Sanchez-Turet Miqel. Neurocognitive effects of alcohol hangover. Addictive Behaviors. 2008;33:15–23. doi: 10.1016/j.addbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Research and Health. The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, Adinoff B, Lane EA, Eckardt MJ, Linnoila M. Dose-related effects of ethanol on visual sustained attention and event-related potentials. Alcohol. 1987;4:293–300. doi: 10.1016/0741-8329(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh: Psychology Software Tools; 2002. [Google Scholar]
- Schweizer TA, Jolicoeur P, Vogel-Sprott M, Dixon MJ. Fast, but error-prone, responses during acute alcohol intoxication: Effects of stimulus-response mapping complexity. Alcoholism: Clinical and Experimental Research. 2004;28:643–649. doi: 10.1097/01.alc.0000121652.84754.30. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. Alcohol tolerance and social drinking: Learning the consequences. New York, NY: Guilford Press; 1992. [Google Scholar]
- Wechsler H, Nelson TF. Binge drinking and the American college student: What’s five drinks? Psychology of Addictive Behaviors. 2001;15:287–291. doi: 10.1037//0893-164x.15.4.287. [DOI] [PubMed] [Google Scholar]