Abstract
Background
Accumulating evidence supports a role of DNA methylation in the pathogenesis of leukemia. The aim of our study was to evaluate the potential genes with aberrant DNA methylation in the prediction of leukemia risk by a comprehensive meta-analysis of the published data.
Methods
A series of meta-analyses were done among the eligible studies that were harvested after a careful filtration of the searching results from PubMed literature database. Mantel-Haenszel odds ratios and 95% confidence intervals were computed for each methylation event assuming the appropriate model.
Results
A total of 535 publications were initially retrieved from PubMed literature database. After a three-step filtration, we harvested 41 case-control articles that studied the role of gene methylation in the prediction of leukemia risk. Among the involving 30 genes, 20 genes were shown to be aberrantly methylated in the leukemia patients. A further subgroup meta-analysis by subtype of leukemia showed that CDKN2A, CDKN2B, ID4 genes were significantly hypermethylated in acute myeloid leukemia.
Conclusions
Our meta-analyses identified strong associations between a number of genes with aberrant DNA methylation and leukemia. Further studies should be required to confirm the results in the future.
Introduction
Leukemia is a common malignant disease of hematopoietic system, caused by unbalanced hematopoietic cells proliferation and death [1]. The development and progression of leukemia is complex. Based on the speed of disease progression and the types of affected white blood cell, leukemia can be divided into four most common types of leukemia, which comprise acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphocytic leukemia (ALL) and chronic lymphocytic leukemia (CLL) (http://www.nlm.nih.gov/medlineplus/leukemia.html).
Although tremendous efforts have been made in the identification of susceptible factors of leukemia [2], [3], the pathogenesis of leukemia is not fully clarified [4]. Environmental factors, such as high benzene exposure, radiation, electrical work, are shown to be associated with the development of leukemia [5], [6]. Meanwhile, leukemia is known to be associated with the accumulation of defects in a wide range of cancer genes [7].
Many genetic and epigenetic alternations were found to play an important role in leukemia pathogenesis [8], [9]. Previous study has indicated aberrant DNA methylation associated with leukemogenesis [10]. As a typical epigenetic modifications, aberrant DNA methylation was observed in lymphoid/hematopoietic malignancies, including AML [4], [11], CML [12], [13], ALL [1], [14], and CLL [15], [16].
These aberrant patterns of DNA methylation in leukemia can be useful for cancer risk prediction. Recent advances attest to the great promise of DNA methylation markers as powerful tools in the clinic [17]–[19]. Meta-analysis can generate a more objective evaluation of candidate genes DNA methylation and the risks of leukemia, based on the conclusions of uncertainty and disagreements. Here we perform comprehensive meta-analyses based on the accumulating leukemia association studies on DNA methylation to better identify biomarkers with aberrant DNA methylation in leukemia. The goal of our study was to summarize the genes with aberrant DNA methylation as promising biomarkers for leukemia risk prediction.
Materials and Methods
Search Strategy
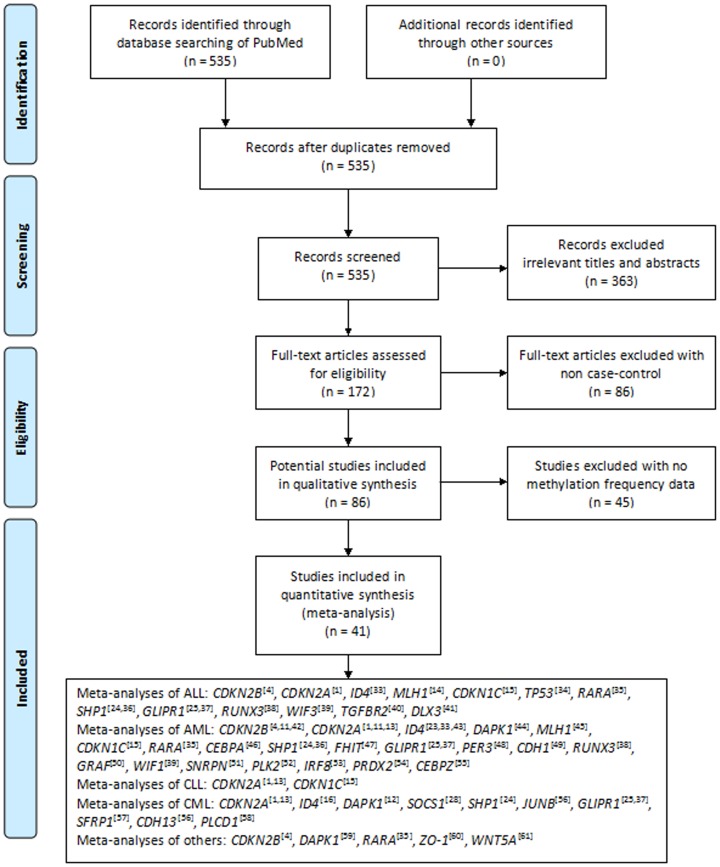
A systematic literature search was performed in PubMed by using “leukemia” and “DNA methylation” as the search terms for articles updated until December 25, 2013. The search was limited to articles published in English and Chinese. Articles in the search output were surveyed according to their titles and abstracts. Studies were selected if they met the following criteria: 1) they were case-control associations of gene methylation with the risk of leukemia in humans; 2) they had sufficient methylation information to calculate the odd ratios (ORs) and 95% confidential intervals (CIs) for the meta-analysis. The selection procedures of studies were illustrated in the flow chart of Figure 1.
Figure 1. Flow diagram of the stepwise selection from relevant studies.
Data Extraction
For the eligible articles, we extracted the following information: first author’s name, published year, PubMed ID, disease category (AML, ALL, CML, CLL or others), the numbers of cases and controls. All the data were extracted by four authors (DJ, YX, HZ and CX). A consensus was reached through a rigorous discussion when there existed conflicting evaluations.
Meta-analysis
Review manager 5 was used for meta-analysis. Mantel-Haenszel ORs and their 95% CIs were computed for each gene to evaluate the contribution of gene methylation to the risk of leukemia. Heterogeneity of the studies in the meta-analysis was evaluated by I2 metric [20], [21]. A random-effect model was used when there existed heterogeneity in the meta-analysis (I2>50%), otherwise a fixed-effect model was applied for the meta-analysis [22].
Results
Study Characteristics
A total of 535 studies were retrieved from the PubMed literature database after searching the keywords of “leukemia” and “DNA methylation”. After a series of selection procedure shown in Figure 1, we excluded 363 irrelevant studies, 86 non-case-control studies, and 45 studies without methylation data. Finally, 41 case-control studies were qualified for our meta-analyses. These comprised 15 ALL studies, 31 AML studies, 4 CLL studies, 13 CML studies, and 5 other studies (including 1 on acute promyelocytic leukemia, and 4 on undefined leukemia). The 41 case-control studies were involved with 30 genes among 1640 healthy individuals and 2587 leukemia patients. Among the tested genes, there were 19 genes with only one report, 3 genes with 2 reports, and 8 genes with 3 or more reports (Table 1).
Table 1. Genes differently methylated in case-control studies from leukemia subjects.
| Gene | Studies | Overall OR (95% CI)a | P value |
| CDKN2A | 12 | 3.53 [1.43, 8.73] | <0.01 |
| GLIPR1 | 6 | 5.96 [2.29, 15.46] | <0.01 |
| CDKN2B | 5 | 9.67 [2.48, 37.75] | <0.01 |
| SHP1 | 5 | 29.27 [6.80, 125.99] | <0.01 |
| ID4 | 5 | 45.24 [11.02, 185.78] | <0.01 |
| DAPK1 | 3 | 28.85 [5.54, 150.14] | <0.01 |
| CDKN1C | 3 | 6.16 [0.66, 57.72] | 0.11 |
| RARA | 3 | 3.46 [0.65, 18.39] | 0.15 |
| RUNX3 | 2 | 11.91 [1.45, 97.86] | 0.02 |
| WIF1 | 2 | 14.15 [1.78, 112.81] | 0.01 |
| MLH1 | 2 | 5.93 [0.27, 130.34] | 0.26 |
| SOCS1 | 1 | 0.10 [0.01, 0.78] | 0.03 |
| JUNB | 1 | 55.00 [1.86, 1622.60] | 0.02 |
| ZO-1 | 1 | 45.00 [2.01, 1006.75] | 0.02 |
| WNT5A | 1 | 121.51 [7.08, 2085.83] | <0.01 |
| PER3 | 1 | 104.27 [6.33, 1718.74] | <0.01 |
| CDH1 | 1 | 33.00 [1.78, 610.61] | 0.02 |
| GRAF | 1 | 81.54 [4.82, 1379.56] | <0.01 |
| SNRPN | 1 | 25.00 [1.39, 449.48] | 0.03 |
| PLK2 | 1 | 67.34 [3.77, 1204.54] | <0.01 |
| PRDX2 | 1 | 22.32 [1.32, 377.72] | 0.03 |
| PLCD1 | 1 | 39.38 [2.21, 702.41] | 0.01 |
| CEBPZ | 1 | 35.84 [2.12, 604.80] | 0.01 |
| TP53 | 1 | 3.40 [0.16, 73.57] | 0.44 |
| CEBPA | 1 | 2.53 [0.14, 47.18] | 0.53 |
| FHIT | 1 | 4.80 [0.27, 85.35] | 0.29 |
| SFRP1 | 1 | 1.54 [0.17, 14.09] | 0.70 |
| CDH13 | 1 | 11.00 [0.46, 263.53] | 0.14 |
| DLX3 | 1 | 1.66 [0.48, 5.65] | 0.42 |
| IRF8 | 1 | 6.41 [0.34, 120.24] | 0.21 |
Odds ratio (OR) describes the likelihood of gene methylation observed in leukemia cases compared to controls.
Meta-analysis of the Association Studies between Gene and the Risk of Leukemia
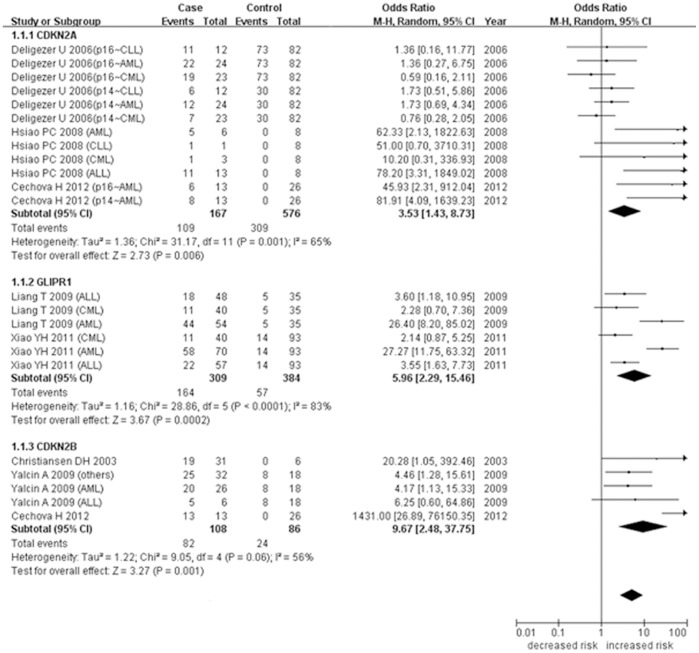
As shown in Figure 2, the meta-analysis of CDKN2A methylation was involved with 12 case-control studies among 576 controls and 167 cases. Our results showed a significant heterogeneity among the 12 studies (I2 = 65%). In addition, the meta-analysis indicated that hypermethylation of CDKN2A gene was associated with the increased risk of leukemia (P = 0.006, OR = 3.53, 95% CI = 1.43–8.73). Meta-analysis between GLIPR1 methylation and leukemia was involved with 6 case-control studies among 384 controls and 309 cases (Figure 2). The meta-analysis showed a significant heterogeneity (I2 = 83%) among these studies, and revealed that GLIPR1 hypermethylation was associated with the increased risk of leukemia (P = 0.0002, OR = 5.96, 95% CI = 2.29–15.46). Meta-analysis of CDKN2B methylation with leukemia was carried out in 5 studies among 86 controls and 108 cases (Figure 2). Our results showed a significant heterogeneity among the 5 case-control studies (I2 = 56%), and revealed that hypermethylation of CDKN2B gene was associated with the increased risk of leukemia (P = 0.001, OR = 9.67, 95% CI = 2.48–37.75).
Figure 2. Correlation between CDKN2A/GLIPR1/CDKN2B methylation and leukemia in the meta-analysis.
Meta-analysis of SHP1 methylation was involved with 5 case-control studies among 46 controls and 104 cases. Our results indicated that hypermethylation of SHP1 gene was associated with the increased risk of leukemia (P<0.00001, OR = 29.27, 95% CI = 6.80–125.99). Meta-analysis between ID4 methylation and leukemia was involved with 5 case-control studies among 78 controls and 165 cases. The results revealed that ID4 hypermethylation was associated with the increased risk of leukemia (P<0.00001, OR = 45.24, 95% CI = 11.02–185.78). Meta-analysis of DAPK1 methylation with leukemia was carried out in 3 studies among 29 controls and 169 cases. Our results showed that hypermethylation of DAPK1 gene was associated with the increased risk of leukemia (P<0.0001, OR = 28.85, 95% CI = 5.54–150.14). In addition, our results were unable to observe significant association of the methylation of CDKN1C and RARA genes with leukemia.
Meta-analysis of the Association Studies between Gene and the Risk of AML
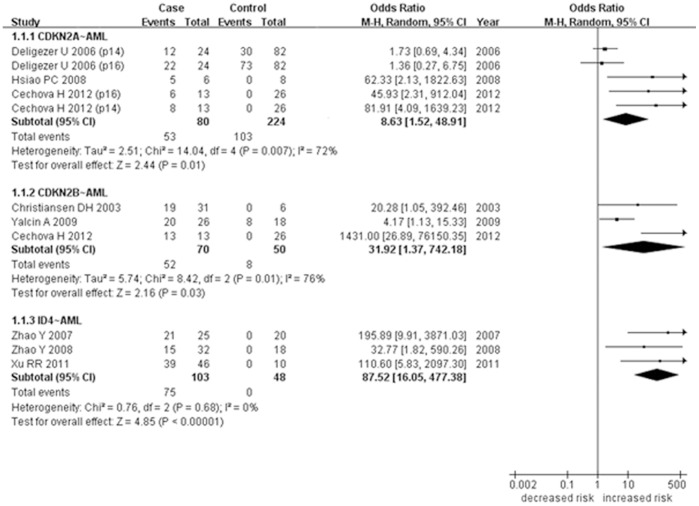
We further performed a breakdown meta-analysis by leukemia subtypes in the above-mentioned studies (Table 2). As shown in Figure 3, meta-analysis of CDKN2A methylation in AML was involved with 5 case-control studies among 80 cases and 224 controls. A significant heterogeneity was observed among the 5 studies (I2 = 72%). Our results showed that CDKN2A hypermethylation was associated with the increased risk of AML (P = 0.01, OR = 8.63, 95% CI = 1.52–48.91). The meta-analysis between CDKN2B methylation and AML was involved with 3 studies among 70 cases and 50 controls (Figure 3). There existed significant heterogeneity among the 3 studies in the meta-analysis of CDKN2B methylation (I2 = 76%). Our results showed CDKN2B hypermethylation was associated with the increased risk of AML (P = 0.03, OR = 31.92, 95% CI = 1.37–742.18). As shown in Figure 3, the meta-analysis of ID4 methylation was involved with 3 studies among 103 cases and 48 controls. The results showed that ID4 hypermethylation was associated with the increased risk of AML (P<0.00001, OR = 87.52, 95% CI = 16.05–477.38).
Table 2. Genes differently methylated in case-control studies from different kinds of leukemia subjects.
| Gene∼Disease | Studies | Overall OR (95% CI)a | P value |
| CDKN2A∼AML | 5 | 8.63 [1.52, 48.91] | 0.01 |
| CDKN2B∼AML | 3 | 31.92 [1.37, 742.18] | 0.03 |
| CDKN2A∼CML | 3 | 0.82 [0.38, 1.74] | 0.6 |
| CDKN2A∼CLL | 3 | 2.04 [0.74, 5.59] | 0.17 |
| ID4∼AML | 3 | 87.52 [16.05, 477.38] | <0.01 |
Odds ratio (OR) describes the likelihood of gene methylation observed in leukemia cases compared to controls.
Figure 3. Correlation between CDKN2A/CDKN2B/ID4 methylation and AML in the meta-analysis.
Discussion
Numerous studies have found that DNA methylation of different genes was associated with the risk of leukemia [11]–[13], [23]–[25], which implicated a potential role of DNA methylation in the prediction and prognostication for leukemia.
De novo methylation of the 5′CpG island has been reported as an alternative mechanism of inactivation for tumor suppressor genes CDKN2A and CDKN2B [26]. De novo methylation of CDKN2B and CDKN2A CpG islands is frequent in malignant transformation [11]. According to the results of our meta-analysis, the aberrant DNA methylation at CDKN2A gene and CDKN2B gene were risk factors for leukemia, especially for AML. In the subgroup analysis, we found that DNA methylation of CDKN2A gene was significantly associated with AML, but not with CML or CLL. A microarray analysis in 2011 identified that glioma pathogenesis-related protein 1 (GLIPR1) was a methylation-silenced gene in the AML patients, and might serve as a marker to monitor the therapeutic effect of AML [25]. Our analysis also demonstrated that DNA methylation of GLIPR1 gene was a risk factor for leukemia. GLIPR1 is a pleiotropic protein involved in cell proliferation, tumor growth and apoptosis, and it may affect G protein signaling and cell cycle regulation [27]. SOCS1 is an important protein in the JAK/STAT pathway, and plays a key role in the downstream regulation of BCR-ABL protein kinase [28]. Our results showed that DNA methylation of SOCS1 gene was a protective factor for leukemia. SHP1 is tumor suppressor gene involved in the regulation of cell cycle control and apoptosis [29]. SHP1 is negative regulator of the Jak/STAT signaling pathway that is implicated in leukemogenesis. Promoter methylation of SHP1 gene is able to silence its gene expression, and was frequently detected in various kinds of leukemias and lymphoma [24]. Our results showed that aberrant DNA methylation of SHP1 gene was a risk factor for leukemia. Inhibitor of DNA binding protein 4 (ID4) is a member of the dominant-negative basic helix-loop-helix transcription factor family that lacks DNA binding activity and has tumor suppressor function. Promoter of ID4 is consistently methylated to various degrees in CLL cells, and increased promoter methylation in a univariable analysis was shown to be correlated with shortened patient survival [30]. In our results of analysis, the aberrant DNA methylation at ID4 gene was a risk factor for leukemia, especially for AML. Death-associated protein kinase 1 (DAPK1), a tumor suppressor, is a rate-limiting effecter in an endoplasmic reticulum stress-dependent apoptotic pathway [31]. Aberrant DNA methylation and concomitant transcriptional silencing of DAPK1 have been demonstrated to be key pathogenic events in CLL [32]. Our study identified the aberrant DNA methylation at DAPK1 gene was a risk factor for leukemia.
The current meta-analysis has some limitations. Firstly, selection bias is inevitable due to the search strategy restricted to articles published in English or Chinese. Secondly, some large between-study heterogeneity existed in our meta-analyses. This phenomenon may be caused by the facts that different subtypes of leukemia were not separated in the first place due to the limited studies, and that different regions of the same gene were tested for methylation in the involved studies. Thirdly, this analysis was performed at the study level, which limited ability to explore the potential for confounding by various demographic and clinical factors (e.g. ethnicity, hormone, different treatments). Fourthly, most of the studies we selected were performed with Methylation-Specific PCR (MSP) and the status of DNA methylation was qualitative (M+ or M−), and it also limited the scope of our analysis. Finally, the efficiency and accuracy of statistical analysis may be influenced by the moderate amount of subjects. We expect more samples being tested in the future to draw a more reliable conclusion.
In conclusion, the results of this study indicated that certain genes DNA methylation was independently associated with the risk of leukemia, especially some kinds of leukemia. Also more studies should be required to confirm the results in the future. DNA methylation has a very strong potential to be a useful biomarker for predicting, prognostication and prediction of response to chemotherapy of leukemia.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
The research was supported by the grants from National Natural Science Foundation of China (31100919 and 81371469), Natural Science Foundation of Zhejiang Province (LR13H020003), K. C. Wong Magna Fund in Ningbo University, and Ningbo Social Development Research Projects (2010C50019 and 2012C50032). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hsiao PC, Liu MC, Chen LM, Tsai CY, Wang YT, et al. (2008) Promoter methylation of p16 and EDNRB gene in leukemia patients in Taiwan. Chin J Physiol 51: 27–31. [PubMed] [Google Scholar]
- 2. Olme CH, Finnon R, Brown N, Kabacik S, Bouffler SD, et al. (2013) Live cell detection of chromosome 2 deletion and Sfpi1/PU1 loss in radiation-induced mouse acute myeloid leukaemia. Leuk Res 37: 1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lupo PJ, Nousome D, Kamdar KY, Okcu MF, Scheurer ME (2012) A case-parent triad assessment of folate metabolic genes and the risk of childhood acute lymphoblastic leukemia. Cancer Causes Control 23: 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yalcin A, Serin MS, Emekdas G, Tiftik N, Aslan G, et al. (2009) Promoter methylation of P15(INK4B) gene is possibly associated with parvovirus B19 infection in adult acute leukemias. Int J Lab Hematol 31: 407–419. [DOI] [PubMed] [Google Scholar]
- 5. Rushton L, Schnatter AR, Tang G, Glass DC (2014) Acute myeloid and chronic lymphoid leukaemias and exposure to low-level benzene among petroleum workers. Br J Cancer 110: 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flodin U, Fredriksson M, Persson B, Hardell L, Axelson O (1986) Background radiation, electrical work, and some other exposures associated with acute myeloid leukemia in a case-referent study. Arch Environ Health 41: 77–84. [DOI] [PubMed] [Google Scholar]
- 7. Balmain A, Gray J, Ponder B (2003) The genetics and genomics of cancer. Nat Genet 33 Suppl: 238–244 [DOI] [PubMed] [Google Scholar]
- 8. Chen C, Liu Y, Lu C, Cross JR, Morris JPt, et al. (2013) Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev 27: 1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanno S, Maeda N, Tomizawa A, Yomogida S, Katoh T, et al. (2012) Characterization of cells resistant to the potent histone deacetylase inhibitor spiruchostatin B (SP-B) and effect of overexpressed p21waf1/cip1 on the SP-B resistance or susceptibility of human leukemia cells. Int J Oncol 41: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Issa JP, Baylin SB, Herman JG (1997) DNA methylation changes in hematologic malignancies: biologic and clinical implications. Leukemia 11 Suppl 1S7–11. [PubMed] [Google Scholar]
- 11. Cechova H, Lassuthova P, Novakova L, Belickova M, Stemberkova R, et al. (2012) Monitoring of methylation changes in 9p21 region in patients with myelodysplastic syndromes and acute myeloid leukemia. Neoplasma 59: 168–174. [DOI] [PubMed] [Google Scholar]
- 12. Qian J, Wang YL, Lin J, Yao DM, Xu WR, et al. (2009) Aberrant methylation of the death-associated protein kinase 1 (DAPK1) CpG island in chronic myeloid leukemia. Eur J Haematol 82: 119–123. [DOI] [PubMed] [Google Scholar]
- 13. Deligezer U, Erten N, Akisik EE, Dalay N (2006) Methylation of the INK4A/ARF locus in blood mononuclear cells. Ann Hematol 85: 102–107. [DOI] [PubMed] [Google Scholar]
- 14. Matsushita M, Takeuchi S, Yang Y, Yoshino N, Tsukasaki K, et al. (2005) Methylation of the MLH1 gene in hematological malignancies. Oncol Rep 14: 191–194. [PubMed] [Google Scholar]
- 15. Li Y, Nagai H, Ohno T, Yuge M, Hatano S, et al. (2002) Aberrant DNA methylation of p57(KIP2) gene in the promoter region in lymphoid malignancies of B-cell phenotype. Blood 100: 2572–2577. [DOI] [PubMed] [Google Scholar]
- 16. Wang XR, Kang HY, Cen J, Li YH, Wang LL, et al. (2010) [Methylation status of id4 gene promoter in patients with chronic myeloid leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 18: 1402–1404. [PubMed] [Google Scholar]
- 17. Laird PW (2003) The power and the promise of DNA methylation markers. Nat Rev Cancer 3: 253–266. [DOI] [PubMed] [Google Scholar]
- 18. Jiang D, Zheng D, Wang L, Huang Y, Liu H, et al. (2013) Elevated PLA2G7 gene promoter methylation as a gender-specific marker of aging increases the risk of coronary heart disease in females. PLoS One 8: e59752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang LN, Liu PP, Wang L, Yuan F, Xu L, et al. (2013) Lower ADD1 gene promoter DNA methylation increases the risk of essential hypertension. PLoS One 8: e63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DerSimonian R (1996) Meta-analysis in the design and monitoring of clinical trials. Stat Med 15: 1237–1248 discussion 1249–1252. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, et al. (2009) More than numbers: the power of graphs in meta-analysis. Am J Epidemiol 169: 249–255. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Y, Wang QS, Li HH, Bo J, Dou LP, et al. (2008) [Significance of id4 promoter methylation in monitoring AML patients with completely remission]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 16: 476–478. [PubMed] [Google Scholar]
- 24. Oka T, Ouchida M, Koyama M, Ogama Y, Takada S, et al. (2002) Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res 62: 6390–6394. [PubMed] [Google Scholar]
- 25. Xiao YH, Li XH, Tan T, Liang T, Yi H, et al. (2011) Identification of GLIPR1 tumor suppressor as methylation-silenced gene in acute myeloid leukemia by microarray analysis. J Cancer Res Clin Oncol 137: 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martel V, Guerci A, Humbert JC, Gregoire MJ, Chery M, et al. (1997) De novo methylation of tumour suppressor genes CDKN2A and CDKN2B is a rare finding in B-cell chronic lymphocytic leukaemia. Br J Haematol 99: 320–324. [DOI] [PubMed] [Google Scholar]
- 27. Capalbo G, Mueller-Kuller T, Koschmieder S, Klein HU, Ottmann OG, et al. (2013) Endoplasmic reticulum protein GliPR1 regulates G protein signaling and the cell cycle and is overexpressed in AML. Oncol Rep 30: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 28. Hatirnaz O, Ure U, Ar C, Akyerli C, Soysal T, et al. (2007) The SOCS-1 gene methylation in chronic myeloid leukemia patients. Am J Hematol 82: 729–730. [DOI] [PubMed] [Google Scholar]
- 29. Gauffin F, Diffner E, Gustafsson B, Nordgren A, Wingren AG, et al. (2009) Expression of PTEN and SHP1, investigated from tissue microarrays in pediatric acute lymphoblastic, leukemia. Pediatr Hematol Oncol 26: 48–56. [DOI] [PubMed] [Google Scholar]
- 30. Chen SS, Claus R, Lucas DM, Yu L, Qian J, et al. (2011) Silencing of the inhibitor of DNA binding protein 4 (ID4) contributes to the pathogenesis of mouse and human CLL. Blood 117: 862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shanmugam R, Gade P, Wilson-Weekes A, Sayar H, Suvannasankha A, et al. (2012) A noncanonical Flt3ITD/NF-kappaB signaling pathway represses DAPK1 in acute myeloid leukemia. Clin Cancer Res 18: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Claus R, Hackanson B, Poetsch AR, Zucknick M, Sonnet M, et al. (2012) Quantitative analyses of DAPK1 methylation in AML and MDS. Int J Cancer 131: E138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Y, Wang QS, Dou LP, Bo J, Li HH, et al. (2007) [Methylation of Id4 gene promoter in acute leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 15: 1156–1160. [PubMed] [Google Scholar]
- 34. Agirre X, Vizmanos JL, Calasanz MJ, Garcia-Delgado M, Larrayoz MJ, et al. (2003) Methylation of CpG dinucleotides and/or CCWGG motifs at the promoter of TP53 correlates with decreased gene expression in a subset of acute lymphoblastic leukemia patients. Oncogene 22: 1070–1072. [DOI] [PubMed] [Google Scholar]
- 35. Chim CS, Wong SY, Pang A, Chu P, Lau JS, et al. (2005) Aberrant promoter methylation of the retinoic acid receptor alpha gene in acute promyelocytic leukemia. Leukemia 19: 2241–2246. [DOI] [PubMed] [Google Scholar]
- 36. Chim CS, Wong AS, Kwong YL (2004) Epigenetic dysregulation of the Jak/STAT pathway by frequent aberrant methylation of SHP1 but not SOCS1 in acute leukaemias. Ann Hematol 83: 527–532. [DOI] [PubMed] [Google Scholar]
- 37. Liang T, Tan T, Xiao Y, Yi H, Li C, et al. (2009) [Methylation and expression of glioma pathogenesis-related protein 1 gene in acute myeloid leukemia]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 34: 388–394. [PubMed] [Google Scholar]
- 38. Lin DJ, Fan RF, Liu XF (2008) [Significance of DNA methylation status of runx3 gene promoter region in acute leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 16: 263–266. [PubMed] [Google Scholar]
- 39. Wang Y, Zhu CS, Bi KH, Xu WW, Dong L, et al. (2011) [Study of WIF-1 promoter methylation with expressions of beta-catenin in acute leukemia]. Zhonghua Yi Xue Za Zhi 91: 2858–2860. [PubMed] [Google Scholar]
- 40. Scott S, Kimura T, Ichinohasama R, Bergen S, Magliocco A, et al. (2003) Microsatellite mutations of transforming growth factor-beta receptor type II and caspase-5 occur in human precursor T-cell lymphoblastic lymphomas/leukemias in vivo but are not associated with hMSH2 or hMLH1 promoter methylation. Leuk Res 27: 23–34. [DOI] [PubMed] [Google Scholar]
- 41. Campo Dell’Orto M, Banelli B, Giarin E, Accordi B, Trentin L, et al. (2007) Down-regulation of DLX3 expression in MLL-AF4 childhood lymphoblastic leukemias is mediated by promoter region hypermethylation. Oncol Rep 18: 417–423. [PubMed] [Google Scholar]
- 42. Christiansen DH, Andersen MK, Pedersen-Bjergaard J (2003) Methylation of p15INK4B is common, is associated with deletion of genes on chromosome arm 7q and predicts a poor prognosis in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia 17: 1813–1819. [DOI] [PubMed] [Google Scholar]
- 43. Xu RR, Liu F, Cui X, Zhang XW, Wang Y (2011) [ID4 promoter methylation in acute myeloid leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 19: 582–584. [PubMed] [Google Scholar]
- 44. Qian J, Yao DM, Lin J, Chen Q, Li Y, et al. (2010) [Alteration of methylation status of death-associated protein kinase (dapk) gene promoter in patients with acute myeloid leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 18: 1390–1394. [PubMed] [Google Scholar]
- 45. Seedhouse CH, Das-Gupta EP, Russell NH (2003) Methylation of the hMLH1 promoter and its association with microsatellite instability in acute myeloid leukemia. Leukemia 17: 83–88. [DOI] [PubMed] [Google Scholar]
- 46. Jost E, do ON, Wilop S, Herman JG, Osieka R, et al. (2009) Aberrant DNA methylation of the transcription factor C/EBPalpha in acute myelogenous leukemia. Leuk Res 33: 443–449. [DOI] [PubMed] [Google Scholar]
- 47. Iwai M, Kiyoi H, Ozeki K, Kinoshita T, Emi N, et al. (2005) Expression and methylation status of the FHIT gene in acute myeloid leukemia and myelodysplastic syndrome. Leukemia 19: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 48. Wang YK, Zhou JH, Zhou SQ, Fang GA, Li YW, et al. (2011) [Promoter methylation status of hPer3 gene in AML patients and the in vitro effect of decitabine on the status]. Zhonghua Xue Ye Xue Za Zhi 32: 317–321. [PubMed] [Google Scholar]
- 49. Gao F, Li Y, Liu W, Lu XL, Li X, et al. (2006) [Studies on gene expression and the 5′ CpG islands methylation status of E-cadherin in acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi 27: 25–27. [PubMed] [Google Scholar]
- 50. Qian J, Qian Z, Lin J, Yao DM, Chen Q, et al. (2011) Abnormal methylation of GRAF promoter Chinese patients with acute myeloid leukemia. Leuk Res 35: 783–786. [DOI] [PubMed] [Google Scholar]
- 51. Benetatos L, Hatzimichael E, Dasoula A, Dranitsaris G, Tsiara S, et al. (2010) CpG methylation analysis of the MEG3 and SNRPN imprinted genes in acute myeloid leukemia and myelodysplastic syndromes. Leuk Res 34: 148–153. [DOI] [PubMed] [Google Scholar]
- 52. Benetatos L, Dasoula A, Hatzimichael E, Syed N, Voukelatou M, et al. (2011) Polo-like kinase 2 (SNK/PLK2) is a novel epigenetically regulated gene in acute myeloid leukemia and myelodysplastic syndromes: genetic and epigenetic interactions. Ann Hematol 90: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 53. Otto N, Manukjan G, Gohring G, Hofmann W, Scherer R, et al. (2011) ICSBP promoter methylation in myelodysplastic syndromes and acute myeloid leukaemia. Leukemia 25: 1202–1207. [DOI] [PubMed] [Google Scholar]
- 54. Agrawal-Singh S, Isken F, Agelopoulos K, Klein HU, Thoennissen NH, et al. (2012) Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood 119: 2346–2357. [DOI] [PubMed] [Google Scholar]
- 55. Yao DM, Qian J, Lin J, Wang YL, Chen Q, et al. (2011) Aberrant methylation of CCAAT/enhancer binding protein zeta promoter in acute myeloid leukemia. Leuk Res 35: 957–960. [DOI] [PubMed] [Google Scholar]
- 56. Wang XJ, Li J, Fu BJ, Guo LL, Zhang JH, et al. (2009) [Methylation status of JunB and CDH13 gene promoter in CD34(+)CD38(−) chronic myelogenous leukemia cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 17: 1405–1408. [PubMed] [Google Scholar]
- 57. Pehlivan M, Sercan Z, Sercan HO (2009) sFRP1 promoter methylation is associated with persistent Philadelphia chromosome in chronic myeloid leukemia. Leuk Res 33: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 58. Song JJ, Liu Q, Li Y, Yang ZS, Yang L, et al. (2012) Epigenetic inactivation of PLCD1 in chronic myeloid leukemia. Int J Mol Med 30: 179–184. [DOI] [PubMed] [Google Scholar]
- 59. Nakatsuka S, Takakuwa T, Tomita Y, Hoshida Y, Nishiu M, et al. (2003) Hypermethylation of death-associated protein (DAP) kinase CpG island is frequent not only in B-cell but also in T- and natural killer (NK)/T-cell malignancies. Cancer Sci 94: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dou LP, Liu JH, Wang C, Zhao Y, Wang QS, et al. (2009) [Study on the involvement of ZO-1 gene in leukemogenesis]. Zhonghua Xue Ye Xue Za Zhi 30: 473–476. [PubMed] [Google Scholar]
- 61. Deng G, Li ZQ, Zhao C, Yuan Y, Niu CC, et al. (2011) WNT5A expression is regulated by the status of its promoter methylation in leukaemia and can inhibit leukemic cell malignant proliferation. Oncol Rep 25: 367–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)