Abstract
Background
Major depressive disorder (MDD) is the leading cause of disability worldwide, and has significant genetic predisposition. Mitochondria may have a role in MDD and so mitochondrial DNA (mtDNA) has been suggested as a possible biomarker for this disease. We aimed to test whether the mtDNA copy number of peripheral blood leukocytes is related to MDD in young adults.
Methods
A case-control study was conducted with 210 MDD patients and 217 healthy controls (HC). The mtDNA copy number was measured by quantitative polymerase chain reaction (qPCR) method. Depression severity was assessed by the Hamilton-17 Depression Rating Scale (HDRS-17).
Results
We found no significant differences in mtDNA copy number between MDD patients and HC, though the power analysis showed that our sample size has enough power to detect the difference. There were also no significant correlations between mtDNA copy number and the clinical characteristics (such as age, age of onset, episodes, Hamilton Depression Rating Scale (HDRS) score and Global Assessment of Function Scale (GAF) score) in MDD patients.
Conclusion
Our study suggests that leukocyte mtDNA copy number is unlikely to contribute to MDD, but it doesn’t mean that we can exclude the possibility of involvement of mitochondria in the disease. Further studies are required to elucidate whether mtDNA can be a biomarker of MDD.
Introduction
Major depressive disorder (MDD) is a severe and complicated mental illness that causes considerable impairment, which has a 12-month prevalence of 6.6% and a lifetime prevalence of 16.2% [1]. The World Health Organization has predicted MDD to be the second leading cause of disability worldwide by the year 2020 [2].
Though the etiology of the disease remains unclear, even environmental circumstances have proven to influence the aetiology of the disease, genetic factor is still believed to have an important role in its pathogenesis. Twin studies suggest a heritability of 40% to 50%, and family studies indicate a twofold to threefold increase in lifetime risk of developing MDD among first-degree relatives [3]. Due to the complex phenotype and the unrepeatable results, the progress to find common genetic variant in MDD has been frustrated. But there were some intriguing findings as described below.
MDD is a brain disease characterized by persistent depressed mood or loss of interest or pleasure from daily activities, and brain is highly dependent on mitochondria’s energy production. That’s why a "mitochondrial psychiatry" model of depression has been proposed [4], so the relationship between MDD and mitochondria has been explored in several studies as follows. In the first place, depression symptoms have been described in patients with kinds of mitochondrial disorders such as external ophthalmoplegia [5], MELAS (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes) [6], [7] and oxidative phosphorylation disease [8]. The comorbidity facts which are most likely, at least in part, a consequence of overlapping neural mechanisms, gave us a clue that mitochondrial dysfunction might be a possible etiopathogenesis of MDD. Second of all, post-mortem studies found reduction of products linked to mitochondrial gene expression in frontal cortex [9] and cerebellum [10] samples from depressed patients. While not only human, rats subjected to depression model were found the inhibition of mitochondrial respiratory chain in cerebral cortex and cerebellum [11]. Furthermore, using the magnetic resonance sounding (MRS) [12], [13] and positron emission tomography (PET) [14] technique, abnormalities of high-energy phosphate metabolism were found in the brain of subjects with MDD. Besides the muscle biopsy took a more direct approach, showing the decrease of mitochondrial adenosine triphosphate production rate (MAPR) and enzyme ratio in the MDD patient in comparison with controls [15]. In addition, patients with depressive disorder were reported to have more mitochondrial DNA (mtDNA) deletion mutations than controls [15], [16] or share the same mtDNA mutation with mitochondrial diseases [5], [17]–[19]. Last but not least, there were some studies that show “matrilineal inheritance” in some depression affected families. For example, depression was reported at very high prevalence in the probable maternal inheritance mothers [18] and a modest maternal bias in the susceptibility towards the development of depression was found [20]. Combined with the fact that mtDNA is inherited exactly through the maternal lineage, we hypothesized that mtDNA may be involved in the pathogenesis of MDD.
So far, most depression studies focus on the point mutation of mtDNA as well as the mitochondrial function, but there have been few studies about the relationship between depression and mtDNA copy number which represents the mtDNA content is important because the mtDNA copy number as a surrogate measure of mitochondrial integrity was supposed to regulate mitochondrial function [21], [22]. Kim et al. had reported that low leukocyte mtDNA copy number is related to depression in community dwelling old women [23]. However, in a recent study, de Sousa et al. found no difference in mtDNA copy number between young adults with bipolar depression and healthy controls [24]. Furthermore, though MDD can develop at any age, the peak ages at onset of MDD are between 20 and 25 years as well as between 40 and 45 years, yet those late onset cases are often associated with structured brain abnormalities like cerebral vascular lesions. Given this, we designed the following case-control study to determine whether leukocyte mtDNA copy number is associated with MDD in young adults.
Materials and Methods
Subjects
The complete details of the entire study design and procedures involved were in accordance with the Declaration of Helsinki. Risks and benefits of the study were presented in detail, and all participants gave written informed consent. If participants were mentally or legally incapable of giving consent, such as minors, their guardians were contacted to fill out the written consent form on the patients' behalf. The study was approved by the ethics committee of the Second Xiangya Hospital of Central South University.
This study included 210 MDD patients which were sought through Outpatient Department, the Second Xiangya Hospital (100 male and 110 female, aged 17–45 years, mean age = 30.2±8.10 years) and 217 matched healthy controls (HC) (109 male and 108 female, aged 18–45, mean age = 30.8±7.05 years). The patients and HC recruited in this study were biologically unrelated and all were of the Han Chinese ethnicity. All patients were 17–45 years old and diagnosed strictly according to the DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision) [25] criteria by a board-certified psychiatrist. Inclusion criteria for HC were: male or female 17–45 years of age, not meeting DSM-IV-TR criteria for an axis I disorder and not having a family history of psychiatric disorder. Exclusion criteria for all subjects included: active substance abuse; serious organic disorders (such as stroke, heart failure and uremia) and other hereditary diseases.
Depression and function assessment
MDD patients were questioned on general parameters, including age, gender, age of onset, marital status, profession, level of education, medication and number of previous episodes. Depression severity was assessed with the Hamilton-17 Depression Rating Scale (HDRS-17) [26]. Psychological, social, and occupational function was assessed with Global Assessment of Function Scale (GAF), which is a 100-point scale described in the DSM-IV-TR [25] on page 34.
Measurement of leukocyte mtDNA copy number
Peripheral leukocyte DNA from 500 µL of whole blood sample was extracted using the TIANamp Blood DNA Kit DP318 (Tiangen Biotech, Beijing, China) and stored at −80°C avoiding repeated freeze-thaw cycles. DNA was checked for purity and concentration, using the NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and all had OD260/OD280 values of 1.8–2.0. The relative mtDNA copy number was normalized to a single-copy nuclear β-globin gene and simultaneously measured by quantitative polymerase chain reaction (qPCR) and the 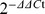 method [27], [28].
method [27], [28].
Forward and reverse primers of β-globin were: 5’-GCTTCTGACACAACTGTGTTCACTAGC-3’ and 5’-CACCAACTTCATCCACGTTCACC-3’, respectively and forward and reverse primers of mtDNA were: 5’-CACCAGCCTAACCAGATTTC-3’ and 5’-GGGTTGTATTGATGAGATTAGT-3’, respectively [29], [30]. Amplification and detection were conducted in 96-well plates with CFX96 Touch real-time PCR detection system (BioRad Laboratories, Hercules, CA, USA). The PCR reaction mix consisted of 10 ng of DNA mixed with 5 µL Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) and 4 pmol of each primer in a final volume of 10 µL. The PCR condition were as follows: initial denaturation at 95°C for 10 minutes; amplification by using 35 cycles including denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. All samples were run in triplicate and only the average Ct values were determined. The mtDNA copy number was calculated with the following equation: relative copy number = 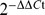 (
(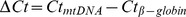 )
)
Statistical analysis
Statistical analyses were conducted with SPSS 17.0. Group results are presented as mean ± S.D. The usual threshold of significance (p) was fixed at 0.05 for two-tailed test. Levene’s test was used to confirm the homogeneity of variances between the groups. Relationships between variables were evaluated with the Spearman’s rank correlation test. Power analysis was performed using the G*Power (version 3.1.7) (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3)
Results
Both groups were well matched for age and gender (for general characteristics of the recruited subjects see Table 1). Patients had mean age of onset of 28.8 (±8.31) and 122 (58.1%) had medication history.
Table 1. General characteristics of the recruited subjects.
| Variable | MDD (n = 210) | HC (n = 217) |
| Age, years (mean ± S.D.) | 30.2±8.10 | 30.8±7.05 |
| Gender (male, female) | 100M, 110F | 109M, 108F |
| Age of onset, years (mean ± S.D.) | 28.8±8.31 | |
| Medication, n (%) | 122 (58.1) | |
| First-episode, n (%) | 127 (60.5) | |
| HDRS score (mean ± S.D.) * | 21.0±7.76 | |
| GAF score (mean ± S.D.) ** | 58.0±10.03 |
MDD: major depressive disorder; HC: healthy controls; M: male; F: female; HDRS: Hamilton Depression Rating Scale; GAF: Global Assessment of Function Scale.
* HDRS were to rate the severity of depression in MDD patients, the higher the score, the more severe the depression.
** GAF assigns a clinical judgment in numerical fashion to the MDD patients’ overall functioning level. The scale ranges from 0 (inadequate information) to 100 (superior functioning).
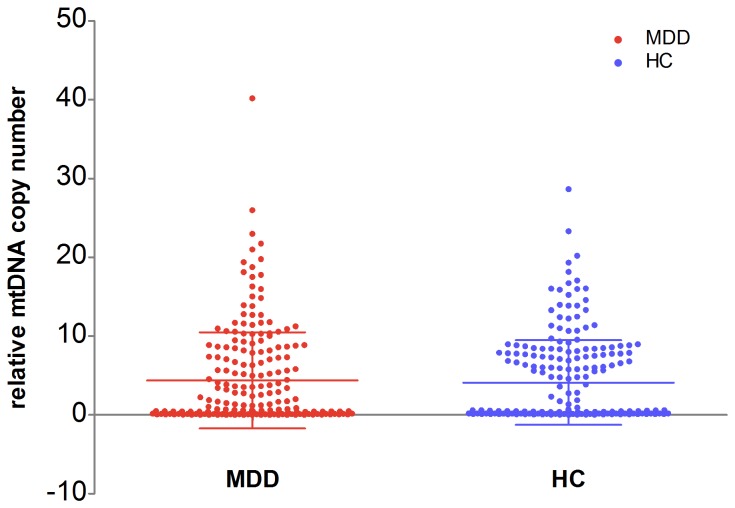
The case-control analyses did not show any significant differences in mtDNA copy number between MDD and HC (MDD patients = 4.4±6.10; healthy controls = 4.1±5.38) (p = 0.650) (see Figure 1), even when gender were considered (p = 0.228 and p = 0.621 for males and females respectively). Then we separated the MDD patients into two groups respectively according to episodes, medication and HDRS score, still did not see any statistically significant differences amongst two groups for either of the variables concerned (see Table 2).
Figure 1. Relative mtDNA copy number of MDD patients and HC.
There is no significant difference in mtDNA copy number between MDD and HC.
Table 2. Comparison of mtDNA copy number between the MDD group and the HC group, and comparison of mtDMA copy number within the MDD group.
| Variable | mtDNA copy number (mean ± S.D.) | P-value | |
| MDD (n = 210) | HC (n = 217) | ||
| Sex | |||
| Male | 4.9±6.07 | 4.0±5.24 | 0.228 |
| Female | 3.9±6.11 | 4.3±5.55 | 0.621 |
| P-value | 0.218 | 0.684 | |
| Episodes | |||
| First episode | 3.8±5.38 | ||
| Recurrence | 5.2±7.01 | ||
| P-value | 0.116 | ||
| Medication | |||
| Take medicine | 4.9±6.63 | ||
| Did not take medicine | 3.6±5.21 | ||
| P-value | 0.120 | ||
| HDRS score * | |||
| score≥18 | 3.9±5.37 | ||
| score<18 | 5.5±7.39 | ||
| P-value | 0.107 | ||
MDD: major depressive disorder; HC: healthy controls; HDRS: Hamilton Depression Rating Scale.
p calculated by the t-test.
* Patients are considered to be currently suffered from depression with a score of ≥18 on the HDRS, while are defined as being in remission if they had an HDRS score less than 18.
As mtDNA copy number did not show a normal distribution, the relationships between variables were evaluated with Spearman’s rank correlation test and were considered as very weak to negligible correlations when the absolute values of the correlation coefficients (rs) were less than 0.2. We found some very weak positive correlations between mtDNA copy number, episodes and GAF score, also some very weak negative correlations between mtDNA copy number, age, age of onset, education and HDRS scores. But none of these correlations met statistical significance (for details see Table 3).
Table 3. Correlations between mtDNA copy number and other variables in MDD patients.
| Variables | rs | P-value |
| Age | −0.080 | 0.251 |
| Age of onset | −0.098 | 0.157 |
| Episodes | 0.095 | 0.168 |
| Education | −0.042 | 0.548 |
| HDRS score | −0.145 | 0.035* |
| GAF score | 0.004 | 0.956 |
rs: Spearman's rank correlation coefficient; HDRS: Hamilton Depression Rating Scale; GAF: Global Assessment of Function Scale.
p calculated by the Spearman’s rank correlation test.
* p = 0.035<0.05, but the correlation coefficient (rs) = −0.145>−0.2, so the correlation between mtDNA copy number and HDRS score are still not significant.
Based on the data of the previously published study in depression [23], we could calculate out the effect size is 0.41. Then, we used this effect size to calculate the power of the current study. The results show that our sample has reasonable power under various effect size value (99% for exactly 0.41, 92% for 0.41*80%, 72% for 0.41*60%), which means our sample size is enough to detect the difference in leukocyte mtDNA copy number between these two groups.
Discussion
Mitochondria are cytoplasmic organelles of the eukaryotic system that play central roles in energy metabolism, free radical production, calcium homeostasis and apoptosis [31]. mtDNA is vital for maintaining normal mitochondrial function and energy production for the body [32]. mtDNA copy number which appears to have high heritability is very important for normal mitochondrial function [33]. Altered mtDNA copy number regulation can result in diseases such as multiple sclerosis [34], renal cell carcinoma [33], type 2 diabetes [35], cardiomyopathy [36], breast cancer [37] and also suggested to be associated with aging [38]. So we wondered that when depression occurred in brain which is an energy intensive organ, how leukocyte mtDNA copy number would change.
The results of our study suggest that no significant leukocyte mtDNA copy number variation distinguishes the MDD and HC. Moreover, leukocyte mtDNA copy number also did not show a significant difference related age, age of onset, education, medication situation, episodes, HDRS score and GAF score. That means, to some extent, no matter how old is the patient, no matter if he/she is taking antidepressant and whether he/she is currently suffered from depression or being in remission, leukocyte mtDNA copy number is not notably affected.
Our finding is inconsistent with Kim et al.’s finding of a positive association between low leukocyte mtDNA copy number and depression in old women [23], but is consistent with the results of a recent study in young adults with bipolar depression [24]. This may due to the different age span that both our subjects are much younger than the old women evaluated by Kim et al. And there is a potential explanation of our results is the “threshold hypothesis of mtDNA copy number control” which means there are some thresholds which work to push the mtDNA copy number towards a middle-range [39]. Thus, we postulate that the depression symptoms might not be severe enough to break the threshold and cause mtDNA copy number decline. Combining with the positive result of mtDNA copy number in old depressive women and our negative result in young adults, we postulate that as people grew older the compensation ability weakened, threshold down-regulated, finally lead to a decreasing in mtDNA copy number.
However the result from this study should be carefully interpreted because it has a numbers of limitations. Relative mtDNA copy number is not accurate enough, could have missed some information. We did not evaluate mitochondrial function to test whether it has a relationship with mtDNA copy number and MDD. Furthermore the mtDNA copy number varies among different tissues [40], but we don’t know the standard leukocyte mtDNA copy number and have no idea about whether leukocyte mtDNA can represent the whole body’s mitochondrial activity or the brain’s metabolic level.
Conclusion
Based on our analysis in this study, we conclude that leukocyte mtDNA copy number is unlikely to contribute to MDD. Despite the negative findings, it is still meaningful as the first study showing the relationship between leukocyte mtDNA copy number and MDD in young adults. But it also doesn’t mean we can exclude the possibility that mitochondria may be involved in the development of MDD. On the contrary, our study indirectly reflects that mtDNA mutation may play a more important role in mtDNA expression or mitochondrial dysfunction and even in MDD. For this reason, further studies are required to elucidate if mtDNA is associated with MDD, determine how it influence the progress of MDD and whether mtDNA can be a biomarker of MDD.
Acknowledgments
We would like to thank the participants for donating blood samples, and Xi Xiang for statistical advice and two anonymous reviewers for help comments.
Funding Statement
The study was supported by the National Natural Science Foundation of China (Grant Nos. 30900486 and 81371480 to J.T., 81271484 to X.C., 81100996 to Y.L., 81071099 and 81271499 to Y.T.), and the National Key Basic Research and Development Program (973) (Grant No. 2012CB517904 to X.C.). However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kupfer DJ, Frank E, Phillips ML (2012) Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 379: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD (1996) Evidence-based health policy—lessons from the Global Burden of Disease Study. Science 274: 740–743. [DOI] [PubMed] [Google Scholar]
- 3. Lohoff FW (2010) Overview of the genetics of major depressive disorder. Curr Psychiatry Rep 12: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gardner A, Boles RG (2005) Is a "Mitochondrial Psychiatry" in the Future? A Review. Current Psychiatry Reviews 1: 255–271. [Google Scholar]
- 5. Suomalainen A, Majander A, Haltia M, Somer H, Lonnqvist J, et al. (1992) Multiple deletions of mitochondrial DNA in several tissues of a patient with severe retarded depression and familial progressive external ophthalmoplegia. J Clin Invest 90: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shanske AL, Shanske S, Silvestri G, Tanji K, Wertheim D, et al. (1993) MELAS point mutation with unusual clinical presentation. Neuromuscul Disord 3: 191–193. [DOI] [PubMed] [Google Scholar]
- 7. Koene S, Kozicz TL, Rodenburg RJ, Verhaak CM, de Vries MC, et al. (2009) Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord 114: 327–332. [DOI] [PubMed] [Google Scholar]
- 8. Morava E, Gardeitchik T, Kozicz T, de Boer L, Koene S, et al. (2010) Depressive behaviour in children diagnosed with a mitochondrial disorder. Mitochondrion 10: 528–533. [DOI] [PubMed] [Google Scholar]
- 9. Whatley SA, Curti D, Marchbanks RM (1996) Mitochondrial involvement in schizophrenia and other functional psychoses. Neurochem Res 21: 995–1004. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Shachar D, Karry R (2008) Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One 3: e3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rezin GT, Cardoso MR, Goncalves CL, Scaini G, Fraga DB, et al. (2008) Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem Int 53: 395–400. [DOI] [PubMed] [Google Scholar]
- 12. Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF (1997) Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry 154: 116–118. [DOI] [PubMed] [Google Scholar]
- 13. Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, et al. (1998) 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci 248: 289–295. [DOI] [PubMed] [Google Scholar]
- 14. Buchsbaum MS, Wu J, DeLisi LE, Holcomb H, Kessler R, et al. (1986) Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J Affect Disord 10: 137–152. [DOI] [PubMed] [Google Scholar]
- 15. Gardner A, Johansson A, Wibom R, Nennesmo I, von Dobeln U, et al. (2003) Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord 76: 55–68. [DOI] [PubMed] [Google Scholar]
- 16. Kato M, Nakamura M, Ichiba M, Tomiyasu A, Shimo H, et al. (2011) Mitochondrial DNA deletion mutations in patients with neuropsychiatric symptoms. Neurosci Res 69: 331–336. [DOI] [PubMed] [Google Scholar]
- 17. Onishi H, Kawanishi C, Iwasawa T, Osaka H, Hanihara T, et al. (1997) Depressive disorder due to mitochondrial transfer RNALeu(UUR) mutation. Biol Psychiatry 41: 1137–1139. [DOI] [PubMed] [Google Scholar]
- 18. Burnett BB, Gardner A, Boles RG (2005) Mitochondrial inheritance in depression, dysmotility and migraine? J Affect Disord 88: 109–116. [DOI] [PubMed] [Google Scholar]
- 19. Molnar MJ, Perenyi J, Siska E, Nemeth G, Nagy Z (2009) The typical MERRF (A8344G) mutation of the mitochondrial DNA associated with depressive mood disorders. J Neurol 256: 264–265. [DOI] [PubMed] [Google Scholar]
- 20. Bergemann ER, Boles RG (2010) Maternal inheritance in recurrent early-onset depression. Psychiatr Genet 20: 31–34. [DOI] [PubMed] [Google Scholar]
- 21. Jeng JY, Yeh TS, Lee JW, Lin SH, Fong TH, et al. (2008) Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J Cell Biochem 103: 347–357. [DOI] [PubMed] [Google Scholar]
- 22. Malik AN, Czajka A (2013) Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13: 481–492. [DOI] [PubMed] [Google Scholar]
- 23. Kim MY, Lee JW, Kang HC, Kim E, Lee DC (2011) Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Arch Gerontol Geriatr 53: e218–221. [DOI] [PubMed] [Google Scholar]
- 24. de Sousa RT, Uno M, Zanetti MV, Shinjo SM, Busatto GF, et al. (2013) Leukocyte mitochondrial DNA copy number in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 48C: 32–35. [DOI] [PubMed] [Google Scholar]
- 25.Association AP (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Pub.
- 26. Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winer J, Jung CK, Shackel I, Williams PM (1999) Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270: 41–49. [DOI] [PubMed] [Google Scholar]
- 28. Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, et al. (2000) Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 285: 194–204. [DOI] [PubMed] [Google Scholar]
- 29. Bi R, Zhang AM, Zhang W, Kong QP, Wu BL, et al. (2010) The acquisition of an inheritable 50-bp deletion in the human mtDNA control region does not affect the mtDNA copy number in peripheral blood cells. Hum Mutat 31: 538–543. [DOI] [PubMed] [Google Scholar]
- 30. Wang D, Su LY, Zhang AM, Li YY, Li XA, et al. (2012) Mitochondrial DNA copy number, but not haplogroup, confers a genetic susceptibility to leprosy in Han Chinese from Southwest China. PLoS One 7: e38848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallace DC (2010) Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen 51: 440–450. [DOI] [PubMed] [Google Scholar]
- 32. Curran JE, Johnson MP, Dyer TD, Goring HH, Kent JW, et al. (2007) Genetic determinants of mitochondrial content. Hum Mol Genet 16: 1504–1514. [DOI] [PubMed] [Google Scholar]
- 33. Xing J, Chen M, Wood CG, Lin J, Spitz MR, et al. (2008) Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst 100: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blokhin A, Vyshkina T, Komoly S, Kalman B (2008) Variations in mitochondrial DNA copy numbers in MS brains. J Mol Neurosci 35: 283–287. [DOI] [PubMed] [Google Scholar]
- 35. Choi YS, Kim S, Pak YK (2001) Mitochondrial transcription factor A (mtTFA) and diabetes. Diabetes Res Clin Pract 54 Suppl 2S3–9. [DOI] [PubMed] [Google Scholar]
- 36. Bai RK, Wong LJ (2005) Simultaneous detection and quantification of mitochondrial DNA deletion(s), depletion, and over-replication in patients with mitochondrial disease. J Mol Diagn 7: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu M, Zhou Y, Shi Y, Ning L, Yang Y, et al. (2007) Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life 59: 450–457. [DOI] [PubMed] [Google Scholar]
- 38. Laderman KA, Penny JR, Mazzucchelli F, Bresolin N, Scarlato G, et al. (1996) Aging-dependent functional alterations of mitochondrial DNA (mtDNA) from human fibroblasts transferred into mtDNA-less cells. J Biol Chem 271: 15891–15897. [DOI] [PubMed] [Google Scholar]
- 39. Clay MLL, Deng JJ, Bai Y (2009) Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics 36: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dimmock D, Tang LY, Schmitt ES, Wong LJ (2010) Quantitative evaluation of the mitochondrial DNA depletion syndrome. Clin Chem 56: 1119–1127. [DOI] [PubMed] [Google Scholar]