Abstract
The RpoS/σS sigma subunit of RNA polymerase (RNAP) controls a global adaptive response that allows many Gram-negative bacteria to survive starvation and various stresses. σS also contributes to biofilm formation and virulence of the food-borne pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium). In this study, we used directional RNA-sequencing and complementary assays to explore the σS-dependent transcriptome of S. Typhimurium during late stationary phase in rich medium. This study confirms the large regulatory scope of σS and provides insights into the physiological functions of σS in Salmonella. Extensive regulation by σS of genes involved in metabolism and membrane composition, and down-regulation of the respiratory chain functions, were important features of the σS effects on gene transcription that might confer fitness advantages to bacterial cells and/or populations under starving conditions. As an example, we show that arginine catabolism confers a competitive fitness advantage in stationary phase. This study also provides a firm basis for future studies to address molecular mechanisms of indirect regulation of gene expression by σS. Importantly, the σS-controlled downstream network includes small RNAs that might endow σS with post-transcriptional regulatory functions. Of these, four (RyhB-1/RyhB-2, SdsR, SraL) were known to be controlled by σS and deletion of the sdsR locus had a competitive fitness cost in stationary phase. The σS-dependent control of seven additional sRNAs was confirmed in Northern experiments. These findings will inspire future studies to investigate molecular mechanisms and the physiological impact of post-transcriptional regulation by σS.
Introduction
In eubacteria, a single multi-subunit RNA polymerase (RNAP) is responsible for transcription. Although the core RNAP (E, α2ββ’ω) is capable of transcript elongation and termination, it cannot specifically initiate transcription from a promoter site. Promoter recognition relies on an additional subunit, σ, which associates with E to form the holoenzyme Eσ [1]. σ directs RNAP to specific promoters, is involved in promoter melting, and dissociates stochastically once sequence-specific promoter DNA contacts are no longer required. All bacteria have a primary house-keeping sigma factor, known as σ70 (RpoD) in Escherichia coli (E. coli) and Salmonella, which promotes the transcription of genes required for the essential functions in the cell. Most bacteria also have one or more alternative σ factors that direct transcription of specific subsets of genes [1]. The alternative sigma factor σS/38 (RpoS) controls a global adaptive response allowing many Gram-negative bacteria to survive nutrient deprivation and environmental stresses [1]–[3]. σS also contributes to virulence and biofilm formation of Salmonella enterica serovar Typhimurium (S. Typhimurium) [3]–[5], a wide host-range pathogen and a major cause of human gastroenteritis and foodborne disease.
Previous works have focused on the complex regulation of rpoS in E. coli K-12 and on σS promoter specificity [2], [3]. In contrast to σ70, σS is almost undetectable in early exponential phase and is induced in stationary phase or in response to various stresses by a fine-tuned combination of transcriptional, translational and proteolytic controls [2], [3]. σS and σ70 bind to almost identical –35 and –10 promoter elements, a finding consistent with the high degree of sequence similarity between these two sigmas in their DNA binding regions [3], [6]. The activity of EσS and Eσ70 holoenzymes can be modulated by additional regulatory proteins that bind to the promoter regions and can also contribute to σ factor selectivity at a given promoter [2], [3].
σS regulons have been characterized using microarrays in E. coli and occasionally in other bacterial species [3], [7], [8], [9], but not in Salmonella. Indeed, previous transcriptional profiling using a S. Typhimurium rpoS mutant only focussed on σS-activated genes requiring σE for maximal expression [10]. More than 10% of the E. coli genes were found to be under positive control by σS [3]. In addition, negative effects of σS on gene expression is an important but poorly understood aspect of σS-dependent control in E. coli [2], [3], [7]. Elimination of these negative effects in rpoS mutants likely contributes to the growth advantage of these mutants in some environments in the absence of stress [11], [12]. Our previous studies suggest that σS exerts negative effects on gene expression and growth capabilities in Salmonella as well [13]–[15], although it is not known to which extent.
In this study, we used directional RNA-sequencing and complementary assays to explore the σS-dependent transcriptome of S. Typhimurium. Our data confirm the large impact of σS on gene transcription in stationary phase bacteria, including gene repression by σS, and provide insights into the main physiological functions of σS in S. Typhimurium. Further, we show that the σS-controlled downstream network includes small RNAs that might endow σS with post-transcriptional regulatory functions and might be intermediate regulators in the down-regulation of gene expression by σS. This study provides a firm basis for future studies to address molecular mechanisms used by σS to control gene expression indirectly and to assess the physiological impact of negative regulation by σS.
Materials and Methods
Bacterial Strains, Bacteriophage and Growth Conditions
Strains are listed in Table S1. Bacteriophage P22HT105/1int was used to transfer mutations and lacZ fusions between Salmonella strains by transduction [16]. Green plates, for screening for P22-infected cells or lysogens, were prepared as described previously [17]. Bacteria were routinely grown in Luria-Bertani medium (LB) [18] at 37°C under aeration. Antibiotics were used at the following concentrations (in µg per ml): carbenicillin (Cb), 100; kanamycin, (Km) 50; and tetracycline (Tet) 20.
DNA Manipulations and Inactivation of Chromosomal Genes
Standard molecular biology techniques were used [4], [18]. Oligonucleotides were obtained from Sigma-Aldrich and are listed in Table S2. DNA sequencing was performed by Beckman Coulter Genomics. Chromosomal deletions in the sdsR, sraL and csrC loci of Salmonella ATCC14028 were generated using tetAR PCR-generated linear DNA fragments (Table S2) and the λ-Red recombination method [19], [20]. The scarless in frame deletion of rpoS in strain VFC331 was achieved with a two-step Red-recombinase-based recombineering procedure [20]. The procedure involves 1) replacement of the rpoS coding sequence by a tetAR module (produced by PCR, Table S2) and 2) replacement of the tetRA module by a DNA fragment obtained by PCR (Table S2) and carrying the desired deletion through positive selection of tetracycline-sensitive recombinants [21]. All strains were confirmed to contain the expected mutation by DNA sequencing. Transcriptional lacZ fusions in the astA, katE and katN genes were previously described [14], [22].
Isolation of Total RNA from S. Typhimurium
Total RNA was isolated from cells grown aerobically until late stationary phase (18 h growth) in LB at 37°C, using TRIzol. Pellets of cells were resuspended in 12.5 mM Tris-HCl (pH 7.6), 10 mM EDTA, 10% glucose. After addition of 1/5 volume of 0.5 M EDTA, disruption of cells was performed by vigorous shaking using glass beads (G1277, Sigma-Aldrich) in acid phenol pH 4.5 (Interchim). After centrifugation, the aqueous phase was carefully mixed with 2 volumes of TRIzol (Invitrogen), and five minutes later with a chloroform-isoamyl alcohol mixture (24∶1). After centrifugation, chloroform-isoamyl alcohol was added to the aqueous phase and the mixture was allowed to stand for five minutes before centrifugation. Total RNA present in the aqueous phase was precipitated with isopropanol. After centrifugation, the pellet was washed in 70% Ethanol, air-dried and resuspended in RNAse-free water. The RNA was subsequently treated with DNaseI (Ambion) and its quality was analyzed using an Agilent BioAnalyzer.
cDNA Library Preparation, Sequencing and Analysis of Sequences
Total RNA from three biological replicates of each strain was isolated from late stationary phase cultures as described above and its quality checked with an Agilent BioAnalyzer. Starting from 10 µg of total RNA, rRNA content was depleted using MicrobExpress kit (Ambion). The rRNA depleted fraction was used for construction of strand specific single end cDNA libraries according to manufacturer’s instructions (using Truseq Small RNA sample prep kit, Illumina). Libraries were sequenced using an Illumina Hiseq2000 sequencer (multiplexing 3 samples per lane) according to manufacturer’s instructions (Illumina). Sequences were demultiplexed using the Illumina pipeline (Gerald, included in CASAVA version 1.7) giving FASTQ formatted reads. Those reads were cleaned from adapter sequences and sequences of low quality using an in-house program. Only sequences with a minimum length of 30 nucleotides were considered for further analysis. Bowtie [23] (version 0.12.7, –chunkmbs 200, -m 50, -e50, -a –best, –solexa1.3-quals) was used to align to the reference genome (CP001363.1 and CP001362.1). HTseq-count (Simon Anders, www-huber.embl.de/users/anders/HTSeq/doc/count.html, parameters: -m intersection-nonempty, -s yes, -t gene) was used for counting genes. Statistical analyses were performed using R version 2.15.1 [24] and Bioconductor packages (http://www.bioconductor.org/). Genes with nul raw counts in all samples were excluded from the data table. Normalization and differential analysis were performed using DESeq version 1.8.3 [25]. The whole dataset was first normalized using the normalization function of DESeq and dispersion was estimated with default parameters. The statistical test was then applied on pairs of strains. Resulting p-values were adjusted for multiple comparisons according to the BH method [26]. Two significance thresholds (0.05 and 0.001) were applied on adjusted p-values in order to declare genes as differentially expressed. The mapped reads were formatted into graph files for visualization using COV2HTML [27] (https://mmonot.eu/COV2HTML) and GBrowse (http://genopole.pasteur.fr/gbrowse/).
RNA-seq Transcriptome Accession Number
The RNA-seq data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE46380 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46380).
Analysis of sRNAs Expression from RNAseq Data
Normalization and differential analysis of expression of sRNAs previously annotated in the genome of S. Typhimurium SL1344 [28] were performed using DESeq2 version 1.2.5 [25] then integrated in COV2HTML [27] for data analysis with a cut off ratio of 2.
Northern Analysis
Total RNA from Salmonella strains grown for 18 H in LB at 37°C was fractionated on an 8% polyacrylamide–7 M urea gel and transferred to Hybond-N+membranes (RPN1520B GE Healthcare). Blots were hybridized to DNA oligonucleotides (Table S2) labeled at the 5′ends with T4 polynucleotide kinase using the UltraHyb-OLIGO buffer (AM8663, Ambion).
Quantitative Real-time PCR
Quantitative real-time PCR was performed to verify the transcriptomic data using Applied Biosystems 7300 Real-Time PCR system. Total RNA was extracted from cells grown to stationary phase in LB as described above. The RNA (1 µg) was reverse-transcribed 2 hours at 37°C in 50 µl of reverse transcriptase buffer in the presence of 2 mM dNTPs, 1 µl of random hexamers (1 µg/µl p(dN)6, Roche) and 10 U of avian myeloblastosis virus (AMV) reverse transcriptase (Promega) and 40 U of recombinant ribonuclease inhibitor (Rnasin, Promega). The relative amounts of target mRNA were determined by real-time PCR using the Fast Start Universal SYBR Green Master following the manufacturer’s instructions (Roche). A final dissociation curve analysis step from 60°C to 95°C was performed to confirm the amplification specificity. To check whether contaminating chromosomal DNA was present, each sample was tested in control reactions that did not contain reverse transcriptase. The real-time PCR was performed using 200 nM gene-specific primer pairs (Table S2) designed in silico using Primer3 software (http://primer3.ut.ee) to generate amplicons in the 100–150 bp range. A relative standard curve experiment using a ten-fold dilution series of genomic DNA was performed for each primer pair to determine the amplification efficiency. The efficiency of the amplification for all the genes tested was higher than 1.8. Three biological replicates were analysed in duplicate each. rpoZ was used as reference gene as it displays little variation in the transcriptional studies performed in our lab using wild-type and ΔrpoS strains. Gene expression levels were calculated using the comparative Ct method (2−ΔΔCT) as previously described [29]. P values were calculated using a two-tailed t test.
Enzymatic Assays
β-galactosidase activity was measured as described by Miller [30] and is expressed in Miller units.
Sequence Analyses
DNA and amino acid sequence analyses were conducted using the BLAST programs at the NCBI (National Center for Biotechnology Information). Functional annotations were obtained from the MicroScope Microbial Genome Annotation & Analysis Platform (www.genoscope.cns.fr/agc/microscope/home/index.php) [31] and the KEGG server (www.genome.jp/kegg/kegg2.html). Functional analysis of genes with significant changes in expression was done using clusters of orthologous groups (COG) functional categories described for Salmonella enterica serovar Typhimurium ATCC14028 genes (www.genoscope.cns.fr/agc/microscope/genomic/classifCOG.php).
Motility Assay
Three independent stationary phase cultures of strains grown in LB (18 h, 37°C, 200 rpm) were used. 1 µl of culture was inoculated into 0.3% agar LB plates that were incubated at 37°C for 5 h.
Competition Assays
Overnight LB cultures were washed and resuspended in phosphate- buffered saline (NaCl 137 mM, KCl 2.7 mM, Na2HPO4 10 mM, KH2PO4 1.76 mM) to an OD600 of 1.0. Equal numbers of cells of the wild-type strain ATCC14028 and the mutant strain were then mixed in fresh LB medium to give a total of about 3000 cells ml-1 and the mixture was incubated at 37°C with shaking. Aliquots of bacteria were removed at timed intervals and numbers of viable cells of each strain were determined on LB plates containing the appropriate antibiotics. P values were calculated using a two-tailed t test.
Results and Discussion
Global Gene Expression in Wild-type and ΔrpoS Salmonella Strains
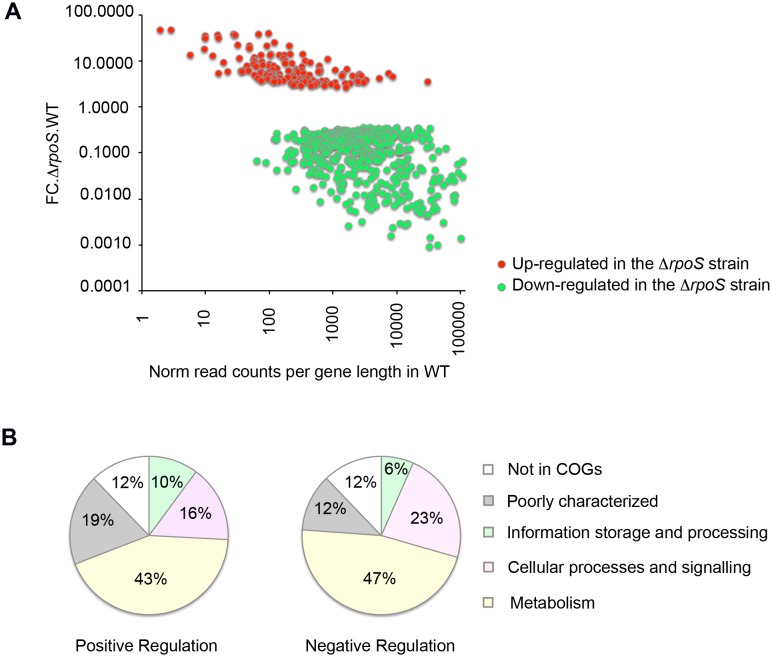
To assess the relative impact of σS at a global level, transcript levels of wild-type and ΔrpoS strains of Salmonella were measured by directional RNA-sequencing using three biological replicates of strains grown to stationary phase in LB (GEO GSE46380). σS is known to accumulate during entry to stationary phase in rich medium and to reach its maximum level of production in late stationary phase [14]. Consistently, in a previous study using RNA sequencing and chromatin immunoprecipitation methods to evaluate transcription in S. Typhimurium in rich medium, σ70 was the main σ factor at early stationary phase [28], suggesting that most σS-regulated genes are expressed in late stationary phase. We thus isolated total RNA from cells in late stationary phase. We identified a total of 1071 genes differentially expressed in the wild-type strain and the ΔrpoS mutant (p<0.05), of which 607 were highly significant (p<0.001) (Dataset S1 and Figure 1). In general, genes up-regulated in the ΔrpoS mutant (145 genes, p<0.001) exhibited lower expression levels and fold-change values than down-regulated genes (462 genes, p<0.001) (Figure 1A). Some σS-dependent genes are likely directly regulated through binding of σS to promoters while others are likely regulated indirectly by σS.
Figure 1. Directional RNAseq data analyses.
(A) Relative expression level and σS-dependency of σS-dependent genes (p<0.001). The x axis shows reads counts in the wild-type strain VF7969 normalized to the lenght of the gene. The y axis shows the fold change in the expression levels of the gene in the ΔrpoS strain VF9356 compared to the wild-type strain (as reported in Dataset S2). Red and green dots represent genes negatively and positively controlled by σS respectively. (B) Functional categories of σS-controlled genes (p<0.001). Genes controlled by σS are grouped according to their functional categories in the COG database (detailed COG assignments are given in Dataset S2). The relative occurrence of genes belonging to each category in the set of genes positively controlled by σS (left pie chart) and negatively controlled by σS (right pie chart) is shown. Some of the genes do not currently have a COG functional category assignment (here represented as not in COGs). Note that some genes have multiple COG category assignments (Dataset S2).
Physiological Functions of the σS Network in S. Typhimurium
Among ATCC14028 genes that have been assigned to a category of orthologous genes (COG), the most prominent categories associated with σS regulation were metabolism, transcription, signal transduction mechanisms and membrane biogenesis and unknown functions (see detailed COG assignments in Dataset S2 and an overview in Figure 1B).
σS had a substantial effect on expression of metabolic genes, primarily for energy production/conversion and transport/metabolism of carbohydrates, amino acids and inorganic ions (Dataset S2). In some cases, most genes in a given pathway are controlled by σS, suggesting a role for this pathway in stationary phase physiology (see for instance pathways shown schematically in Figure 2 and Figures S1–S2, with genes activated and down-regulated by σS in green and red, respectively). A number of genes involved in central energy metabolism exhibited positive σS control (phosphotransferase systems, glycolysis, the pentose phosphate pathway, mixed acid fermentation, and acetate metabolism) whereas genes encoding enzymes in the tricarboxylic acid (TCA) cycle and the operons encoding NADH dehydrogenase-I (nuo) and ATP synthase (atp) were down-regulated by σS (Dataset S2 and Figure S1). σS might thus play a role in transition from aerobic respiration towards more fermentative and/or anaerobic respiratory energy metabolism in stationary phase Salmonella.
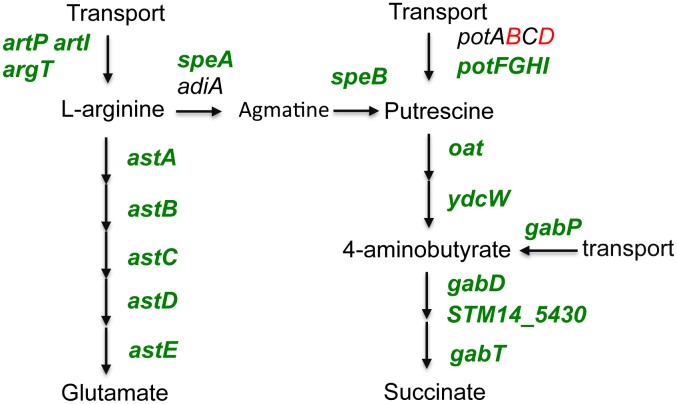
Figure 2. Role of σS in the transport and catabolism of L-arginine.
Schematic representation of σS-dependent pathways involved in metabolism of L-Arginine, putrescine and 4-aminobutyrate. To assess the contribution of σS in the expression of the metabolic pathways indicated, genes differentially expressed with a p value of less than 0.05 in the wild-type and ΔrpoS strains of Salmonella were considered (Dataset S2). Genes showing differential expression with p<0.001 are indicated in bold face. Genes in red and green were negatively and positively controlled by σS respectively. Genes in black did not show differential expression in the wild-type and ΔrpoS strains.
Since σS controls, either positively or negatively, a large number of genes, and it is required for complex phenotypes such as multiple stress resistance and biofilm formation, it is difficult to pinpoint specific genes directly involved in a particular physiological function of σS. However, it is likely that several σS-controlled genes contribute to prevent or repair oxidative damage. First, σS controls antioxydant pathways involving catalases, superoxide dismutase, glutaredoxins and glutathione-S-transferases (Dataset S2). In addition, σS activates genes encoding ferritins/ferrochelatase and Fe-S repair proteins (including dps, bfr, hemH, sufABCDSE, Dataset S2) and controls genes coding for manganese/iron acquisition functions (including activation of mntH, sitABCD, iroBCN and down-regulation of feoB, fepEC, Dataset S2). These findings suggest that σS controls iron use and the concentration of free iron in the cell. This strategy would be consistent with the role of σS in preventing oxidative stress since iron can promote the formation of reactive oxygen species (ROS) during aerobic metabolism [32]. In addition to activating the suf genes encoding an alternative system for iron-sulfur clusters assembly, σS down-regulated the hscBA genes (Dataset S2) encoding chaperones with central roles in assembly of iron-sulfur clusters mediated by the housekeeping Isc system [33]. This suggests that stationary phase cells relay upon the alternative Suf machinery, rather than the housekeeping Isc system, for Fe-S cluster assembly, an hypothesis consistent with the findings that Suf is more resistant to oxidation than Isc and is functional under iron-limiting conditions [33].
σS appears to trigger switching between certain isozymes (see differential regulation of talA/talB, tktA/tktB, acnA/acnB, pykF/pykA, fumA/fumB/fumB, sodA/sodB and nrdAB/nrdEF Figure S1 and Dataset S2). σS can modulate expression of isoenzymes more functional during stationary phase and/or that display key features increasing their activity or stability in these conditions. For instance, selection of isozymes resistant to oxidation and iron depletion [34] and replacement of iron-containing enzymes with iron-independent isoenzymes [35] may be key characteristics of the stationary phase physiology, consistent with the effect of σS in metal acquisition function mentioned above.
σS controlled carbon storage genes for production and degradation of glycogen and the osmoprotectant trehalose (Figure S2) [3] and seems to play an important role in the transport and utilization of amino acids such as L-arginine (Figure 2). The LB medium is rich in amino acids [36] and arginine is a nitrogen reservoir and the precursor for polyamine biosynthesis (Figure 2). These small cationic amines are involved in a variety of functions including resistance to oxidative stress and antibiotics, stabilisation and condensation of DNA during senescence, RNA and protein synthesis and virulence [37]. σS activated genes for transport, synthesis and degradation of putrescine in Salmonella (Figure 2) and E. coli K-12 [6] and might control its intracellular concentration.
Some genes up-regulated in the ΔrpoS strain are probably induced in response to cellular damages derived by the lack of a functional σS protein. For instance, the heat shock protein encoding genes ibpAB and groEL/groES were up-regulated in the ΔrpoS strain possibly because the level of damaged proteins increased in the absence of σS. Indeed, the levels of carbonylated proteins increased in stationary phase E. coli strains lacking σS likely as a result of increased endogeneous oxidative stress [38]. In addition, whereas the σS- mediated induction of genes encoding proteases (including htrA, clpX, ptrB, yggB, tldD, hslV, Dataset S2) may favor the recycling in stationary phase of mis-folded proteins as nutrients, a low level of expression of these genes in the ΔrpoS mutant may contribute to accumulation of damaged proteins.
Altogether, these data on the σS-dependent Salmonella transcription are in general agreement with previous studies in E. coli K-12 [3], [6]–[9]. One major exception concerns the expression of genes associated with motility. Genes encoding the flagellar sigma factor FliA, flagellar proteins and motor components are down-regulated by σS in E. coli K-12 [3], [7]. In contrast, the flagellin genes fliC, and to a lesser extent fljB, were positively controlled by σS, even though transcription of the flhDC genes encoding the master regulator of flagellar synthesis was slightly up-regulated in the Salmonella ΔrpoS strain. In addition, the Salmonella ΔrpoS mutant showed a decrease in motility compared to the wild-type strain (Dataset S2, Figure 3). Given the complexity of regulatory controls affecting motility [3], it is not clear whether the positive regulation of fliC accounts for the effect of σS on motility or whether σS acts through other ways as well. σS also positively regulated flagellar gene expression in another strain of S. Typhimurium, SL1344, and in other pathogens such as Vibrio, Legionella and Pseudomonas [39].
Figure 3. Motility of SalmonellaΔrpoS mutant.
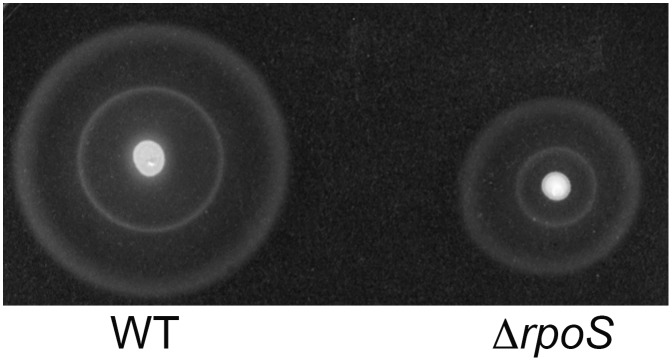
Motility on 0.3% agar LB plates of the wild-type strain ATCC14028 (WT) and its ΔrpoS mutant VFC331 after 5 h at 37°C.
Negative Regulation by σS and Bacterial Fitness
Whereas σS has a positive effect on a large number of genes that likely contribute to stress resistance, it has also a negative effect on the expression of several genes under the control of other sigma factors (Dataset S2) [3], [7], [12], [40]. These negative effects of σS on gene expression likely drive the selection of non-functional rpoS alleles in environments with no stress, where reduced σS activity confers a growth advantage [3], . These observations have led to the proposal that the acquisition of stress resistance mediated by σS comes at the expense of growth capabilities as a consequence of a regulatory antagonism between σS and other σ, mainly σ70 [3], [41], [42]. The implications of negative regulation by σS in the stationary phase physiology have not been studied in detail.
We believe that an important issue to be addressed in future experiments is whether and how the negative effects of σS on gene expression confer any fitness advantage to the bacteria. Genes down-regulated by σS may show antagonistic phenotypic pleiotropy (i.e. their expression is advantageous in some environmental conditions and detrimental in others). Optimizing bacterial fitness in a defined constant environment would require selection for and against these genes, and this evolutionary force would drive gene loss or inactivation, in line with the selection of rpoS mutants in some environmental conditions [11], [12]. However, in fluctuating environments, fine-tuning regulatory processes by σS might be used to adapt bacterial fitness to a variety of natural habitats, including host niches.
Negative effects of σS on the respiratory chain might contribute to the antioxidant defenses by reducing the production of ROS as toxic by-products of aerobic metabolism [3], [32] and might redirect NADH usage to fuel the activity of antioxidant enzymes. Indeed, accumulation of NADH following the inhibition of Salmonella’s electron transport chain by nitric oxide has been identified as an antioxidant strategy [43]. Interestingly also, inhibition of ATP synthase-promoted proton translocation and ATP synthesis is a strategy utilized by Salmonella during infection to control ATP levels and maintain physiological cytoplasmic pH, and membrane potential [44]. Therefore, σS-mediated reduction in the synthesis of the ATP synthase might enable Salmonella to maintain a physiological cytosolic pH and modulate its membrane potential for optimal survival under starvation conditions. Furthermore, down-regulation by σS of the respiratory complexes I (NADH dehydrogenase Nuo) and II (succinate dehydrogenase Sdh) and the σS-dependent switch from the Isc to the Suf Fe-S cluster biosynthesis machinery might reduce the uptake of antibiotics. Indeed, it has been recently shown that, during iron limitation, E. coli cells become intrinsically resistant to aminoglycosides by switching the Fe-S cluster biosynthesis machinery from Isc to Suf and down-regulating respiratory complexes I and II [45]. The Suf system cannot efficiently mature these respiratory complexes, resulting in impairment of the proton motive force, which is required for bactericidal aminoglycoside uptake [45].
Besides effects of σS in metabolism and the respiratory chain functions, additional negative effects of σS on gene expression might contribute to bacterial fitness. σS controls mutagenesis induced by subinhibitory concentrations of antibiotics via the down-regulation of mutS, a gene involved in mismatch-repair [46]. It is tempting to speculate that negative regulation of mutS expression by σS (Dataset S2) might contribute to the appearance of antibiotic resistant mutants, and consequently the survival of bacterial populations in environments containing antibiotics. Down-regulation of porins (for example encoded by ompC, ompF, ompD/nmpC and ompW, Dataset S2) might also confer resistance to antibiotics and other toxic compounds and bacteriophages [47]. More generally, σS controls genes encoding membrane proteins and transporters, especially those belonging to the ATP-Binding Cassette transporter family, suggesting altered membrane composition and traffic in stationary phase (Dataset S2). This membrane remodeling may be directed towards nutrients scavenging and increased resistance against toxic compounds and physical assaults, an hypothesis consistent with the observed positive effect of σS in cell envelope resilience in E. coli [48]. Negative control by σS of surface determinants that are targets for a protective antibody response, such as OmpD [49], may also contribute in the escape of immune response during host infection.
Hierarchical Regulation and Regulatory Loops in the σS-Network and Interplay with other Global Regulators
Because the number of sigma factors exceeds that of the core RNAP, sigma factors compete for binding to the core RNAP available in the cell [2], [3]. Many genes down-regulated by σS (Dataset S2) are transcribed in Salmonella from promoters showing characteristics of σ70-dependent promoters [28], [50]. Negative control by σS is likely in part an indirect effect. According to the current model of negative regulation by σS invoking σ competition [3], [40], [42], σ70-dependent genes are up-regulated in the absence of σS because they are expressed from promoters that are sensitive to the increase in the cellular concentration of Eσ70 that might result from a lack of competition between σS and σ70 for E binding. However, global regulation by σS may also involve intermediate regulators in the σS network, including repressor molecules.
As a first step to explore indirect regulation by σS, the possible regulatory functions of the σS-controlled downstream network were examined. σS affected the transcript levels of numerous genes encoding known or putative signal transducing and/or DNA-binding proteins (Figure 1B and COGs T and K, Dataset S2), suggesting that σS controls the transcription of many secondary transcription factors. Genes for global regulators (csrA, soxS, arcA and to a lesser extent ompR), abundant nucleoid-associated proteins (ihfAB, cbpA, hupAB and to a lesser extent stpA), and modulators of RNAP activity (dksA and to a lesser extent greA, nusG) appeared differentially expressed in the wild-type and ΔrpoS strains (Dataset S2). σS-dependent transcription of cbpA, ifhAB and csrA has been reported in E. coli K-12 [51]–[54] and arcA appeared slightly down-regulated in a E. coli ΔrpoS mutant [8]. Consistent with the RNA-seq data, quantitative reverse transcriptase-polymerase chain reaction showed that transcription of the regulatory genes csrA, dksA, ihfA and ihfB is positively controlled by σS whereas the hupA and hupB genes are down-regulated by σS (Figure 4, Dataset S2). Also, the clpX gene, encoding a subunit of the ATP-dependent complex ClpXP protease, involved in proteolysis of many proteins including σS [2], [3] is activated by σS (Figure 4, Dataset S2). Although these data showed that transcripts levels for csrA, dksA, ihfAB, hupAB and clpX are modulated by σS in late stationary phase in Salmonella, additional experiments are required to determine whether wild-type and ΔrpoS cells differ in the global activity of the corresponding gene products. Indeed, other factors capable of differentially influencing protein levels and activity of these regulators might compensate for the observed variations in their transcript levels in the absence of σS.
Figure 4. σS-dependent transcriptomic expression of pleitropic regulators.
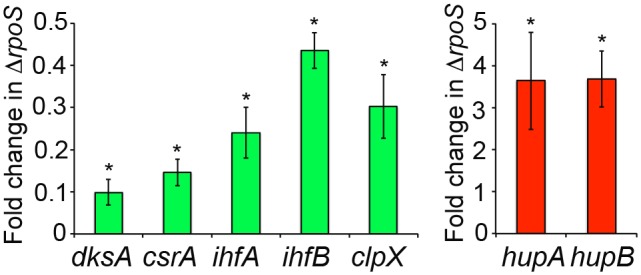
Verification of the σS-dependent transcriptomic expression of csrA, dksA, ihfA, ihfB, hupA, hupB and clpX by quantitative RT-PCR. RNA extracted from stationary phase LB cultures (18 h) of Salmonella wild-type and ΔrpoS strains (VF7969 and VF9356) was reverse transcribed to cDNA and used as a template for qRT-PCR. rpoZ was used for normalization. Red and green bars correspond to genes negatively and positively controlled by σS, respectively. Three biological replicates were analysed in duplicate each and error bars display the standard error of the mean. *, expression levels in the ΔrpoS mutant significantly different from that in the wild-type strain (p<0.05).
As an example, interesting regulatory antagonisms were observed. csrA, encoding a post-transcriptional global regulator, and the small RNA CsrC are both positively controlled by σS (Dataset S2, Figures 4–5 and see paragraph below). CsrA acts mostly negatively by binding and destabilizing mRNAs [53]–[56]. CsrC binds and sequesters CsrA, thereby inhibiting its activity [53], [57]. The other components of the Csr system include the sRNA CsrB, that also binds and sequesters CsrA, and CsrD, a protein that participates in degradation of CsrC and CsrB [53], [56]. In the conditions used, csrB and csrD were detected to low and similar levels in wild-type and ΔrpoS strains (data not shown). The regulation of expression of the Csr system is complex [53], [54]. CsrA indirectly activates its own transcription while repressing its own translation and also controls production of CsrC/CsrB/CsrD. Transcription of csrA in E. coli is controlled by several promoters, two of which are σS-dependent [54]. It is conceivable that σS modulates the fine-tuned balance of the Csr system and uses this system to indirectly regulate target genes at the post-transcriptional level. Also, transcript levels for σE and for its antisigma factor RseA and its coantisigma factor RseB, encoded by the same operon, are all reduced in the ΔrpoS strain, compared to the wild-type (3.5 fold p<0.001 for rseA and about 2 fold p<0.05 for rpoE and rseB, Dataset S2). Since σE has a positive effect on σS expression in stationary phase [10], a possible control of σE expression and/or activity by σS would not be unexpected. σS may have several self-regulatory circuits by controlling the expression of numerous genes that modulate its expression [2], [3], for instance, clpX, rssB/hnr, arcA, hupAB, dksA, and dsrA (p<0.001, Dataset S2). Since these regulators may work either cooperatively or independently, or even have opposing effects on σS expression [2], [3], σS self regulatory control may be important for maintaining a proper level of σS and for integration of signal inputs. Future experiments will assess whether these changes in expression of global regulators at the transcriptome level are transferred at the functional level and whether some of the σS-controlled secondary regulators are intermediate regulators in regulatory cascades and/or contribute to EσS-mediated regulation in feedforward regulatory loops.
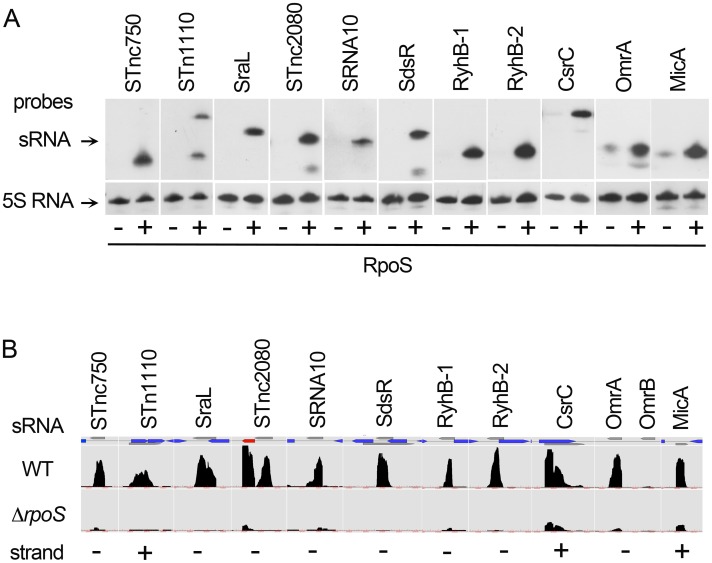
Figure 5. σS-dependent expression of small RNAs in Salmonella.
(A) The indicated sRNAs were detected in Northern experiments in the wild-type strain ATCC14028 (+) and its ΔrpoS derivative VFC331 (−). The positions of bands were in agreement with the expected transcript lengths [28], [64], [65] (Table S3) except for STnc1110 (195 nt, detected with an additional band of 120 nt) that might be processed in late stationary phase. Blots were stripped and re-probed with 5S RNA probe to confirm loading of equal quantities of wild type and ΔrpoS RNA. (B) Mapped reads, in the wild-type and ΔrpoS strains, VF7969 and VF9356 respectively, of the σS-dependent RNAs assessed in Northern experiments. The mapped reads were formatted into graph files for visualization at a strand-specific manner using COV2HTML. The annotated sRNA genes are indicated as grey arrows and open-reading frames annotated in ATCC14028 are shown as blue arrows. Open-reading frames overlapping the sRNAs are small putative CDS of unknown function annotated in ATCC14028. STnc2080 is located upstream of STM14_3200, indicated in red, a gene directing the synthesis of a tRNA-Arg and activated by σS (Dataset S2). SdsR is complementary to a portion of the sRNA transcribed from the opposite strand, SraC. The scale for read counts on the y axis was 1–250 for STnc1110 and sRNA10, 1–1000 for OmrA and MicA, 1–2500 for STnc750 and RyhB-1, 1–5000 for STnc2080 and RyhB-2, 1–10000 for SraL, SdsR and CsrC.
Besides the control of regulatory proteins, control of metabolic/signaling enzymes by σS might lead to variations in levels of signalling molecules and affect protein modifications and/or gene expression at different levels. For instance, genes involved in the metabolism of the second messenger C-di-GMP [3], [55], [58] showed differential transcript levels in the wild-type and ΔrpoS strains (yaiC, ydiV, yegE, STM14_2408, STM14_2209, STM14_5555, STM14_4086, STM14_2047, Dataset S2). Also, putrescine affects global gene expression [37] and the control of its intracellular levels (Figure 2) might be a mechanism of indirect gene regulation by σS. Since σS plays a central role in metabolism, it may affect the levels of intermediate metabolites with signalling functions such as CoA derivatives, NADH/NADPH, glutamate, acetate and acetyl-phosphate.
σS-dependent sRNAs
sRNAs are important pleiotropic regulatory elements [59]–[61]. Some sRNAs are positive regulators but the majority of sRNAs negatively regulate their targets by translational repression and/or destabilization of the mRNA [59]–[61]. Thus, sRNAs might be important contributors to negative regulation of gene expression by σS, as recently shown for σE [62]. More than one hundred sRNAs have been recently annotated in S. Typhimurium SL1344 [28]. Corresponding coordinates of these sRNAs in the genome of ATCC14028 are listed in Table S3. The RNA-seq data offered the possibility to assess whether some of these annotated sRNAs are differentially expressed in the wild-type strain and ΔrpoS mutant. Fourty-two sRNA showed differential expression levels (>2 fold, p<0.001) in the wild-type and ΔrpoS strains (Table 1) and the σS-dependent control of eleven of them was confirmed by Northern experiments (Figure 5). Of these, only four (SraL, SdsR and RyhB-1/-2) were known to be controlled by σS [63]–[65] whereas the other sRNAs are novel σS-targets. Some sRNAs differentially expressed in wild-type and ΔrpoS strains were detected to low levels in the growth condition used (Table 1). Control of their expression by σS might be amplified or abolished in other growth conditions. Indeed, the σS control of gene expression depends on growth conditions and many genes are fully expressed under the control of σS under a specific condition [3], [8].
Table 1. sRNAs differentially expressed in wild-type and ΔrpoS strains.
| sRNA | Mean rpoS | Mean WT | Fold Changea | Start | End | Strand |
| CsrC | 4333 | 19747 | 0.26 | 4223741 | 4223984 | + |
| CyaR | 345 | 56 | 4.99 | 2282684 | 2282769 | + |
| DsrA | 694 | 217 | 2.83 | 2080053 | 2080139 | − |
| GcvB | 2 | 17 | 0.24 | 3155126 | 3155326 | + |
| GlmY | 7111 | 17770 | 0.43 | 2759217 | 2759400 | − |
| IsrI | 7 | 248 | 0.05 | 2812865 | 2813112 | − |
| IstR-1,2 | 67 | 283 | 0.29 | 4011708 | 4011839 | − |
| MicA | 99 | 494 | 0.24 | 2987088 | 2987161 | + |
| OmrA | 28 | 621 | 0.07 | 3189931 | 3190017 | − |
| OxyS | 9 | 41 | 0.29 | 4356452 | 4356570 | − |
| RybA | 104 | 334 | 0.36 | 903092 | 903188 | − |
| RybB | 59 | 229 | 0.3 | 943606 | 943684 | − |
| RybD | 90 | 10 | 5.26 | 808431 | 808515 | + |
| RydC | 2789 | 402 | 5.33 | 1739650 | 1739715 | + |
| RyeF | 72 | 419 | 0.21 | 2012350 | 2012656 | − |
| RygC | 466 | 2565 | 0.22 | 3242088 | 3242232 | + |
| RygD | 913 | 3695 | 0.28 | 3380548 | 3380692 | − |
| RyhB-1 | 124 | 1238 | 0.14 | 3729100 | 3729194 | − |
| RyhB-2 | 165 | 4480 | 0.06 | 1362850 | 1362950 | − |
| SdsR | 3 | 4823 | <0.001 | 1979458 | 1979560 | − |
| SraC | 122 | 17 | 5.37 | 1979380 | 1979690 | + |
| SraL | 38 | 23180 | <0.001 | 4518412 | 4518552 | − |
| sRNA10 | 6 | 117 | 0.09 | 680323 | 680422 | − |
| SroC | 13056 | 49251 | 0.32 | 729258 | 729410 | − |
| STnc1060 | 32 | 109 | 0.35 | 467925 | 467990 | − |
| STnc1080 | 133 | 42 | 2.76 | 1064537 | 1064598 | − |
| STnc1110 | 13 | 204 | 0.1 | 1696589 | 1696782 | + |
| STnc1200 | 12 | 2 | 3.79 | 926560 | 926629 | − |
| STnc1220 | 1 | 9 | 0.26 | 1501842 | 1501914 | − |
| STnc1280 | 763 | 290 | 2.41 | 2093821 | 2093893 | + |
| STnc1300 | 373 | 129 | 2.57 | 2125107 | 2125245 | + |
| STnc1330 | 6 | 1246 | 0.01 | 2322111 | 2322325 | + |
| STnc1380 | 84 | 14 | 4.47 | 2783476 | 2783543 | − |
| STnc1390 | 41 | 137 | 0.35 | 1294132 | 1294195 | − |
| STnc150 | 4 | 30 | 0.2 | 1335643 | 1335799 | − |
| STnc1560 | 97 | 312 | 0.36 | 2502911 | 2503019 | + |
| STnc2080 | 45 | 3081 | 0.03 | 2813257 | 2813365 | − |
| STnc290 | 144 | 26 | 3.81 | 3214058 | 3214136 | − |
| STnc540 | 46 | 181 | 0.3 | 1429394 | 1429487 | + |
| STnc570 | 461 | 2600 | 0.22 | 1603700 | 1604389 | − |
| STnc580 | 13 | 1 | 4.66 | 1759908 | 1760029 | − |
| STnc750 | 36 | 1258 | 0.05 | 3259604 | 3259692 | − |
Fold change estimated by DESeq2 using normalized means.
(p<0.001).
The σS-control of sRNAs might be direct (for instance in the case of SdsR and SraL) [63], [64] or indirect. In particular, basal expression levels in stationary phase of some sRNAs such as OmrA and MicA were detected in the ΔrpoS mutant suggesting the existence of alternative mechanisms of expression. Some of the σS-dependent sRNAs are regulated by other regulators such as Fur, σE and OmpR [59]–[61], and regulatory interactions linking these regulators and σS in stationary phase might result in the control of the sRNA by σS. In contrast to OmrA, the highly-similar and OmpR-regulated sRNA OmrB [28], [59] was detected in very low levels in the conditions used in this study (Figure 5B), suggesting that these two sRNAs are differentially regulated in late stationary phase.
Since sRNAs act by direct pairing with multiple mRNA targets [59]–[61], they might have pleiotropic effects and significantly expand the regulatory role of σS at the post transcriptional level. The targets of most of these sRNA are unknown so far but some of them have pleiotropic effects in outer membrane protein synthesis, metabolism remodeling, motility and biofilm formation [28], [59]–[61]. For instance, RyhB-1/RyhB-2, OmrA, MicA and SdsR down-regulate expression of many genes [59]–[61], [64]–[68] including genes negatively controlled by σS (sodB, sdh, acnB, ompD, mutS, Dataset S2). In E. coli RyhB inhibits the production of iron-storage and iron-using proteins during growth under iron-limiting conditions and it has been proposed that this regulation enables iron sparing for essential pathways [68]. Experiments are underway to assess to which extent RyhB sRNAs might play a role in σS-dependent modulation of iron use in late stationary phase. During the preparation of this manuscript, SraL was shown to be controlled by σS and to down-regulate the expression of a chaperone encoded by the tig gene and involved in protein folding [63]. Under the conditions used in our study, the σS-dependent control of SraL (Figure 5) did not significantly affect tig transcripts levels or this effect was masked by compensatory regulations in the network. A few sRNAs appeared down-regulated by σS (Table 1). Among those displaying the highest fold change in their expression levels between wild-type and ΔrpoS strains, RydC activates translation of the cfa mRNA produced from a σ70-dependent promoter [69]. cfa encodes a cyclopropane fatty acid synthase which modifies phospholipids and contributes to the stability of the bacterial membrane and acid resistance [3], [69]. In stationary phase, σS activates expression of the cfa gene (Dataset S2) [3] and the σS-dependent promoter yields a shorter isoform of the cfa mRNA, insensitive to RydC regulation [69]. Thus, in stationary phase, when cfa transcription relies on σS, RydC might be dispensible and possibly detrimental for expression of other stationary phase genes and its expression might be downregulated accordingly by σS. Alternatively, RydC might be up-regulated in the ΔrpoS strain to activate synthesis of cyclopropane fatty acid synthase and compensate for the absence of σS-induction of cfa transcription (i.e. RydC-mediated activation of cfa might be a backup mechanism).
σS-dependent sRNAs might also target proteins instead of mRNAs [59]. As mentioned above the finding that csrC and csrA were both positively controlled by σS (Figures 4–5, Dataset S2) reveals an interesting regulatory antagonism and suggests that σS modulates the fine-tuned balance of the Csr system in late stationary phase. Whereas expression of the CsrC and CsrB sRNAs is coordinated by positive transcriptional control mediated by the two-component regulatory system BarA/SirA in Salmonella [57], csrC but not csrB was found to be controlled by σS, suggesting that these two sRNAs are differentially regulated in the stationary phase of growth.
The physiological impact of the σS-dependent regulatory RNAs network and its possible connections with the hierarchical σS-dependent transcriptional network will be an exciting issue for future studies.
Inactivation of the astA and sdsR Loci has a Fitness Cost in Stationary Phase
Many σS-dependent genes are of unknown functions (Figure 1B) and physiological roles in starved populations of most of the σS-dependent genes are unexplored. Understanding what genes regulated by σS may do for the cell is an important issue for future studies. Even for genes with known functions, understanding whether and how they help bacteria deal with survival in stationary phase and stress conditions is far from being complete. Our RNAseq data pinpoint to metabolic functions as key characteristics of σS activity in Salmonella, as its was previously suggested in E. coli K-12 [3]. However, the contribution of σS-regulated metabolic functions in the physiology of non/slow growing bacteria needs to be further evaluated through construction of mutations in relevant pathways. For instance, σS might activate the transport and utilization of L-arginine (Figure 2). The astCADBE operon required for the degradation of arginine is transcribed from two promoters, one is dependent on σ54, the other on σS [70], [71]. We previously isolated a mutant of Salmonella carrying a Tn5B21 transposon insertion in the astA gene, creating a astA-lacZ gene fusion [22] (Table S1). Consistent with the RNAseq data, expression of the astA-lacZ fusion in stationary phase was dependent on σS (Figure 6A).
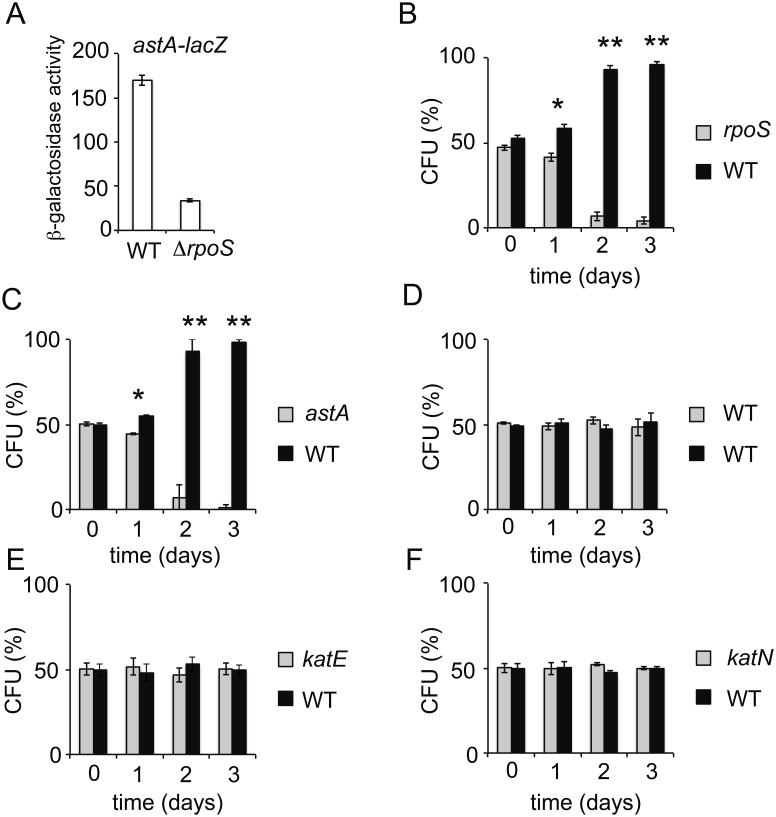
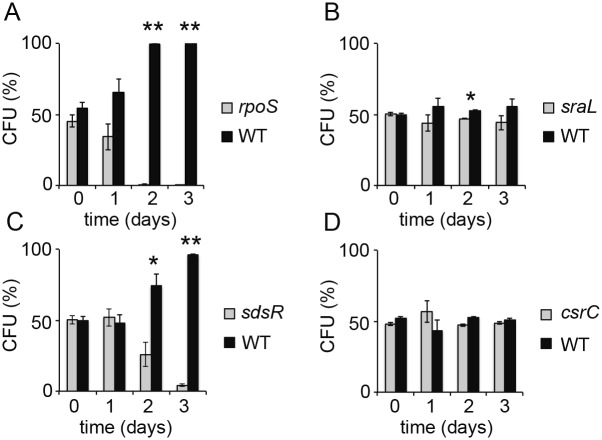
Figure 6. The σS-dependent ast pathway confers a competitive fitness advantage during stationary phase.
(A) Expression of a astA-lacZ gene fusion in Salmonella wild-type strain (VFD793, WT) and ΔrpoS derivative (VFD794) grown to late stationary phase in LB. The error bars represent standard deviations for three independent measurements. (B, C, E, F) Competition assays between the wild-type strain ATCC14028 (WT) and the mutant strain VF9356 (rpoS, panel B), VFD793 (astA, panel C), VF8082 (katE, panel E) and VF8088 (katN, panel F). (D) A control competition assay showing similar fitness of the two strains ATCC14028 and VF7969 is consistent with our previous data [14]. Equal cell numbers of the wild-type strain ATCC14028 and the mutant strain were mixed in LB medium to give a total of about 3000 cells ml-1 (time 0) and the mixtures were incubated at 37°C with shaking. Aliquots of bacteria were removed at timed intervals and numbers of viable cells of each strain were determined. Cells number of each strain is reported as a percentage of the total number of viable cells in the culture. The error bars represent standard deviations for three independent measurements. * and **, statistically significant competitive disadvantage of the mutant compared to the wild-type (*p<0.05, **p<0.0005).
We previously showed that the wild-type strain of Salmonella has a competitive advantage over the ΔrpoS mutant in stationary phase [14] (Figure 6B). To assess the impact of L-arginine degradation in maintenance metabolism, we performed similar competition experiments in which the wild-type strain and astA mutant were mixed in equal cell numbers in LB liquid medium and the numbers of each were followed for several days (Figure 6C). The wild-type strain ATCC14028 showed a competitive advantage during stationary phase over the astA mutant (Figure 6C). Three days after inoculation of the medium, more than 98% of the cells population was wild-type. In similar control experiments, the wild-type strain ATCC14028 showed similar fitness as the wild-type strain 2922 K (Figure 6D) [14]. The fitness disadvantage of the astA mutant was not due to the Tn5B21 insertion since strains carrying Tn5B21 insertions in the katE and katN genes showed similar fitness as the wild-type strain (Figure 6EF). The σS-dependent katE and katN genes encode catalases [72] involved in the destruction of hydrogen peroxide (H2O2). Cellular respiration using oxygen may result in the accumulation of ROS [32]. The inactivation of catalases did not affect Salmonella fitness under the conditions used, possibly due to redundant functions in Salmonella [73]. Alternatively, as discussed above, σS may set up conditions minimizing endogenous oxidative stress and, under these conditions, catalase production might be a preventive σS response. These data showed that inactivation of the ast pathway has a fitness cost and thus arginine degradation may be a key feature for competitive fitness of stationary phase Salmonella in rich medium. Additional experiments are underway to explored the mechanistic basis for this finding.
To investigate whether the competition assay could be a valuable tool to reveal the activity of sRNA in physiological conditions, we constructed Salmonella mutants, in which the sdsR, sraL and csrC genes were deleted and replaced by a tetRA cartridge, and assessed their fitness in competition experiments with the wild-type strain (Figure 7). These sRNAs were chosen because they were among the most highly expressed σS-dependent sRNAs (Table 1). The csrC and sraL mutants showed similar fitness as the parental strain, while the sdsR mutant was outcompeted (Figure 7), indicating that deletion of the sdsR locus has a competitive fitness cost. Deletion of sdsR also inactivates the antisens overlapping gene encoding the SraC sRNA [64]. However, considering the low amount of SraC compared to SdsR (Table 1), the physiological effect of the mutation likely results from one or several of the regulatory functions of SdsR [46], [61], [64], [67] and experiments are underway to investigate this issue further. In addition, a high-throughput screening method based on the competition assay will be used to assess the impact of σS-dependent sRNAs in various physiological growth conditions.
Figure 7. Competitive fitness of sRNA mutants during stationary phase.
Competition assays between the wild-type strain ATCC14028 (WT) and the mutant strain VFC326 (rpoS, panel A), VFD164 (sraL, panel B), VFD197 (sdsR, panel C) and VFD510 (csrC, panel D). See also legend in Figure 6.
Conclusion
This study provides insights into the positive and negative effects of σS on global gene transcription in Salmonella and suggests that metabolism, membrane composition, iron use and oxidative stress resistance are keys features of σS activity. This study also provides a firm basis for future studies to address molecular mechanisms of indirect regulation of gene expression by σS. Our results pinpoint regulation by sRNAs as one possible mechanism mediating indirect control of gene expression by σS, expanding the regulatory scope of σS at the post transcriptional level. These findings open up new fields of investigation in the regulatory network orchestrated by σS where transcriptional and post-transcriptional control mechanisms might cooperate or work in opposite direction to allow for dynamic and flexible regulatory patterns and additional signal inputs. In particular, molecular mechanisms underlying negative effects of σS on gene expression are not well documented and call for further investigation. Some of the regulatory proteins and small RNAs identified in this study might endow σS with repressor functions. The possibility that negative effects of σS on gene expression confer fitness advantage to the bacteria is also an interesting issue for future studies. Down-regulation of expression by σS might target genes that would otherwise decrease the fitness and persistence of bacterial cells and/or populations when they are fully expressed in stationary phase. Identification of these genes and understanding the mechanisms by which their full expression results in a fitness cost might provide insights into the survival mecanisms of non-actively growing bacterial populations and might have implication for antibacterial strategies. We expect that the data presented here will inspire future studies to address these questions.
Supporting Information
Central metabolic pathways controlled by σS in LB stationary phase cultures of Salmonella. Central metabolic pathways, including glycolysis and gluconeogenesis, the pentose phosphate pathway, the tricarboxylic acid (TCA) cycle, acetate and pyruvate metabolism are shown schematically. To assess the contribution of σS in the expression of the metabolic pathways indicated, genes differentially expressed with a p value of less than 0.05 in the wild-type and ΔrpoS strains of Salmonella were considered (Dataset S2). Genes showing differential expression with p<0.001 are indicated in bold face. Genes in red and green were negatively and positively controlled by σS respectively. Genes in black did not show differential expression in the wild-type and ΔrpoS strains.
(TIF)
Metabolic pathways controlled by σS in LB stationary phase cultures of Salmonella. Schematic representation of pathways controlled by σS. (A) degradation of N-acetylneuraminate, N-acetyl-β-D-mannosamine and N-acetyl-D-glucosamine, (B) 4-hydroxyphenylacetate catabolism, (C) L-arabinose degradation, (D) propionate degradation, (E) Glycogen biosynthesis and degradation, (F) galactose degradation, (G) Ethanolamine utilization, (H) trehalose biosynthesis and degradation. (I) Glycine metabolism, (J) Glutamine transport and metabolism, (K) Glutathione metabolism, (L) Aspartate degradation, (M) L-serine degradation, (N) L-cysteine degradation and hydrogen sulfite biosynthesis. See also legend of Figure S1.
(TIF)
Bacterial strains used in this study.
(DOC)
Oligonucleotides used in this study.
(DOC)
Coordinates in the ATCC14028 genome of the sRNAs annotated in SL1344.
(XLS)
Differential gene expression in wild-type and ΔrpoS strains.
(XLS)
Annotation of σS-controlled genes (from Dataset S1, p<0.05). Genes differentially expressed in the wild-type strain and the ΔrpoS mutant with p<0.001 are indicated in bold face.
(XLS)
Acknowledgments
We thank Anthony Pugsley and all members of the laboratory for their kind support. We are very grateful to Stephen Lory and Bertil Gummesson for helpful comments and to Emilie Camiade and Nara Figueroa-Bossi for their advices on q-PCR and handling sRNAs, respectively. We acknowledge persons from the Microscope platform for the equipment and analyses made available and Emmanuel Quevillon for his help in RNAseq analyses using GBrowse.
Funding Statement
This work was supported by the French National Research Agency (ANR- 11-BSV3-009 to FN) and by grants from the Institut Pasteur and the Centre National de la Recherche Scientifique. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Österberg S, del Peso-Santos T, Shingler V (2011) Regulation of alternative sigma factor use. Annu Rev Microbiol 65: 37–55. [DOI] [PubMed] [Google Scholar]
- 2. Battesti A, Majdalani N, Gottesman S (2011) The RpoS-mediated general stress response in Escherichia coli . Annu Rev Microbiol 65: 189–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hengge R (2011) The general stress response in Gram-negative bacteria. In: Storz G, Hengge R, editors. Bacterial Stress Responses. Washington, DC: ASM Press. 251–289.
- 4. Robbe-Saule V, Jaumouillé V, Prévost M-C, Guadagnini S, Talhouarne C, et al. (2006) Crl activates transcription initiation of RpoS-regulated genes involved in the multicellular behavior of Salmonella enterica serovar Typhimurium. J Bacteriol 188: 3983–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dong T, Schellhorn HE (2010) Role of RpoS in virulence of pathogens. Infect Immun 78: 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maciag A, Peano C, Pietrelli A, Egli T, De Bellis G, et al. (2011) In vitro transcription profiling of the σS subunit of bacterial RNA polymerase: re-definition of the σS regulon and identification of σS-specific promoter sequence elements. Nucl Acids Res 39: 5338–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patten CL, Kirchhof MG, Schertzberg MR, Morton RA, Schellhorn HE (2004) Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol Gen Genomics 272: 580–591. [DOI] [PubMed] [Google Scholar]
- 8. Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R (2005) Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J Bacteriol 187: 1591–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lacour S, Landini P (2004) SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of sigmaS-dependent genes and identification of their promoter sequences. J Bacteriol 186: 7186–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bang IS, Frye JG, McClelland M, Velayudhan J, Fang FC (2005) Alternative sigma factor interactions in Salmonella: sigmaE and sigmaH promote antioxidant defences by enhancing sigmaS levels. Mol Microbiol 56: 811–823. [DOI] [PubMed] [Google Scholar]
- 11. Zambrano MM, Siegele DA, Almiron M, Tormo A, Kolter R (1993) Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259: 1757–1760. [DOI] [PubMed] [Google Scholar]
- 12. Notley-McRobb L, King T, Ferenci T (2002) rpoS mutations and loss of general stress resistance in Escherichia coli populations as a consequence of conflict between competing stress responses. J Bacteriol 184: 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robbe-Saule V, Algorta G, Rouilhac I, Norel F (2003) Characterization of the RpoS status of clinical isolates of Salmonella enterica . Appl Environ Microbiol 69: 4352–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robbe-Saule V, Dias Lopes M, Kolb A, Norel F (2007) Physiological effects of Crl in Salmonella are modulated by σS level and promoter specificity. J Bacteriol 189: 2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monteil V, Kolb A, Mayer C, Hoos S, England P, et al. (2010) Crl binds to domain 2 of σS and confers a competitive advantage to a natural rpoS mutant of Salmonella enterica serovar Typhi. J Bacteriol 192: 6401–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmieger H (1972) Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet 119: 75–88. [DOI] [PubMed] [Google Scholar]
- 17. Sternberg NL, Maurer R (1991) Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium . Methods Enzymol 204: 18–43. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn.). New York : Cold Spring Harbor Laboratory Press. 1,626 p. [Google Scholar]
- 19. Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerlach RG, Jäckel D, Hölzer SU, Hensel M (2009) Rapid Oligonucleotide-Based Recombineering of the Chromosome of Salmonella enterica . Appl Environ Microbiol 75: 1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bochner BR, Huang H-C, Schieven GL, Ames BN (1980) Positive Selection for Loss of Tetracycline Resistance. J Bacteriol 143: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ibanez-Ruiz M, Robbe-Saule V, Hermant D, Labrude S, Norel F (2000) Identification of RpoS (sigmaS)-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol 182: 5749–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
- 25. Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate : A practical and powerful approach to multiple testing. J Royal Stat Soc 57: 289–300. [Google Scholar]
- 27. Monot M, Orgeur M, Camiade E, Brehier C, Dupuy B (2014) COV2HTML: A Visualization and Analysis Tool of Bacterial Next Generation Sequencing (NGS) Data for Postgenomics Life Scientists, OMICS: A Journal of Integrative Biology. 18: 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kröger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, et al. (2012) The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A 109: E1277–E1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 30.Miller JH (1972) Experiments in Molecular Genetics. New York : Cold Spring Harbor Laboratory Press.
- 31. Vallenet D, Engelen S, Mornico D, Cruveiller S, Fleury L, et al. (2009) MicroScope: a platform for microbial genome annotation and comparative genomics. Database (Oxford). 2009: bap021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiang SM, Schellhorn HE (2012) Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys 525: 161–169. [DOI] [PubMed] [Google Scholar]
- 33. Roche B, Aussel L, Ezraty B, Mandin P, Py B, et al. (2013) Iron/sulfur proteins biogenesis in prokaryotes: Formation, regulation and diversity. Bioch Biophys Acta 1827: 455–469. [DOI] [PubMed] [Google Scholar]
- 34. Varghese S, Tang Y, Imlay JA (2003) Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J Bacteriol 185: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andrews SC (2011) Making DNA without iron-induction of a manganese-dependent ribonucleotide reductase in response to iron starvation. Mol Microbiol 80: 286–289. [DOI] [PubMed] [Google Scholar]
- 36. Sezonov G, Joseleau-Petit D, D’Ari R (2007) Escherichia coli physiology in Luria-Bertani broth. J Bacteriol 189: 8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42: 39–51. [DOI] [PubMed] [Google Scholar]
- 38. Dukan S, Nyström T (1999) Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J Biol Chem 274: 26027–26032. [DOI] [PubMed] [Google Scholar]
- 39. Dong T, Schellhorn HE (2009) Global effects of RpoS on gene expression in pathogenic Escherichia coli O157:H7 strain EDL933. BMC Genomics 10: 349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farewell A, Kvint K, Nyström T (1998) Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol 29: 1039–1051. [DOI] [PubMed] [Google Scholar]
- 41. Ferenci T (2005) Maintaining a healthy SPANC balance through regulatory and mutational adaptation. Mol Microbiol 57: 1–8. [DOI] [PubMed] [Google Scholar]
- 42. Nystrom T (2004) Growth versus maintenance: a trade-off dictated by RNA polymerase availability and sigma factor competition? Mol Microbiol 54: 855–62. [DOI] [PubMed] [Google Scholar]
- 43. Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J, et al. (2008) Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem 283: 7682–7689. [DOI] [PubMed] [Google Scholar]
- 44. Lee EJ, Pontes MH, Groisman EA (2013) A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’s own F1Fo ATP synthase. Cell 154: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, et al. (2013) Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340: 1583–1587. [DOI] [PubMed] [Google Scholar]
- 46. Gutierrez A, Laureti L, Crussard S, Abida H, Rodríguez-Rojas A, et al. (2013) β-lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat Commun 4: 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández L, Hancock RE (2012) Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev 25: 661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Charoenwong D, Andrews S, Mackey B (2011) Role of rpoS in the development of cell envelope resilience and pressure resistance in stationary-phase Escherichia coli . Applied Environ Micobiol 77: 5220–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, et al. (2009) The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A 106: 9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramachandran VK, Shearer N, Jacob JJ, Sharma CM, Thompson A (2012) The architecture and ppGpp-dependent expression of the primary transcriptome of Salmonella Typhimurium during invasion gene expression. BMC Genomics 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamashino T, Kakeda M, Ueguchi C, Mizuno T (1994) An analogue of the DnaJ molecular chaperone whose expression is controlled by sigma S during the stationary phase and phosphate starvation in Escherichia coli. . Mol Microbiol 13: 475–483. [DOI] [PubMed] [Google Scholar]
- 52. Aviv M, Giladi H, Schreiber G, Oppenheim AB, Glaser G (1994) Expression of the genes coding for the Escherichia coli integration host factor are controlled by growth phase, rpoS, ppGpp and by autoregulation. Mol Microbiol 14: 1021–1031. [DOI] [PubMed] [Google Scholar]
- 53. Romeo T, Vakulskas CA, Babitzke P (2013) Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ Microbiol 15: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yakhnin H, Yakhnin AV, Baker CS, Sineva E, Berezin I, et al. (2011) Complex regulation of the global regulatory gene csrA: CsrA-mediated translation repression, transcription from five promoters by Eσ70 and EσS, and indirect transcriptional activation by CsrA. Mol Microbiol 81: 689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jonas K, Edwards AN, Ahmad I, Romeo T, Römling U, et al. (2010) Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella Typhimurium. Environ Microbiol 12: 524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Timmermans J, Van Melderen L (2010) Post-transcriptional global regulation by CsrA in bacteria. Cell Mol Life Sci 67: 2897–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martinez LC, Martinez-Flores I, Salgado H, Fernandez-Mora M, Medina-Rivera A, et al. (2014) In silico identification and experimental characterization of regulatory elements controlling the expression of the Salmonella csrB and csrC genes. J Bacteriol 196: 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Römling U, Galperin MY, Gomelsky M (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messager. Microbiol Mol Biol Rev 77: 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gottesman S, Storz G (2011) Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 3: a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hébrard M, Kröger C, Srikumar S, Colgan A, Händler K, et al. (2012) sRNAs and the virulence of Salmonella enterica serovar Typhimurium. RNA Biol 9: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mika F, Hengge R (2013) Small Regulatory RNAs in the control of motility and biofilm formation in E. coli and Salmonella . Int J Mol Sci 14: 4560–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gogol EB, Rhodius VA, Papenfort K, Vogel J, Gross CA (2011) Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci U S A 108: 12875–12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Silva IJ, Ortega AD, Viegas SC, García-Del Portillo F, Arraiano CM (2013) An RpoS-dependent sRNA regulates the expression of a chaperone involved in protein folding. RNA 19: 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fröhlich KS, Papenfort K, Berger AA, Vogel J (2012) A conserved RpoS-dependent small RNA controls the synthesis of major porin OmpD. Nucleic Acids Res 40: 3623–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Padalon-Brauch G, Hershberg R, Elgrably-Weiss M, Baruch K, Rosenshine I, et al. (2008) Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res 36: 1913–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim JN, Kwon YM (2013) Genetic and phenotypic characterization of the RyhB regulon in Salmonella Typhimurium. Microbiol Res 168: 41–49. [DOI] [PubMed] [Google Scholar]
- 67. Monteiro C, Papenfort K, Hentrich K, Ahmad I, Le Guyon S, et al. (2012) Hfq and Hfq-dependent small RNAs are major contributors to multicellular development in Salmonella enterica serovar Typhimurium. RNA Biol 9: 489–502. [DOI] [PubMed] [Google Scholar]
- 68. Massé E, Vanderpool CK, Gottesman S (2005) Effect of RyhB small RNA on global iron use in Escherichia coli . J Bacteriol 187: 6962–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fröhlich KS, Papenfort K, Fekete A, Vogel J (2013) A small RNA activates CFA synthase by isoform-specific mRNA stabilization. EMBO J 32: 2963–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Klupakis AK, Reitzer L (2002) ArgR-independent induction and ArgR-dependent superinduction of the astCABDE operon in Escherichia coli . J Bacteriol 184: 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu C-D, Abdelal AT (1999) Role of ArgR in activation of the ast operon, encoding enzymes of the arginine succinyltransferase pathway in Salmonella typhimurium . J Bacteriol 181: 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Robbe-Saule V, Coynault C, Ibanez-Ruiz M, Hermant D, Norel F (2001) Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigmaS). Mol Microbiol 39: 1533–1545. [DOI] [PubMed] [Google Scholar]
- 73. Hébrard M, Viala JP, Méresse S, Barras F, Aussel L (2009) Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol 191: 4605–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Central metabolic pathways controlled by σS in LB stationary phase cultures of Salmonella. Central metabolic pathways, including glycolysis and gluconeogenesis, the pentose phosphate pathway, the tricarboxylic acid (TCA) cycle, acetate and pyruvate metabolism are shown schematically. To assess the contribution of σS in the expression of the metabolic pathways indicated, genes differentially expressed with a p value of less than 0.05 in the wild-type and ΔrpoS strains of Salmonella were considered (Dataset S2). Genes showing differential expression with p<0.001 are indicated in bold face. Genes in red and green were negatively and positively controlled by σS respectively. Genes in black did not show differential expression in the wild-type and ΔrpoS strains.
(TIF)
Metabolic pathways controlled by σS in LB stationary phase cultures of Salmonella. Schematic representation of pathways controlled by σS. (A) degradation of N-acetylneuraminate, N-acetyl-β-D-mannosamine and N-acetyl-D-glucosamine, (B) 4-hydroxyphenylacetate catabolism, (C) L-arabinose degradation, (D) propionate degradation, (E) Glycogen biosynthesis and degradation, (F) galactose degradation, (G) Ethanolamine utilization, (H) trehalose biosynthesis and degradation. (I) Glycine metabolism, (J) Glutamine transport and metabolism, (K) Glutathione metabolism, (L) Aspartate degradation, (M) L-serine degradation, (N) L-cysteine degradation and hydrogen sulfite biosynthesis. See also legend of Figure S1.
(TIF)
Bacterial strains used in this study.
(DOC)
Oligonucleotides used in this study.
(DOC)
Coordinates in the ATCC14028 genome of the sRNAs annotated in SL1344.
(XLS)
Differential gene expression in wild-type and ΔrpoS strains.
(XLS)
Annotation of σS-controlled genes (from Dataset S1, p<0.05). Genes differentially expressed in the wild-type strain and the ΔrpoS mutant with p<0.001 are indicated in bold face.
(XLS)