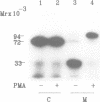
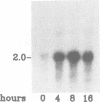
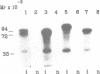
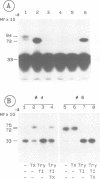
Abstract
Two forms of plasminogen activators inhibitor 2 (PAI-2) are synthesized by human and murine monocytes/macrophages: one accumulates in the cytosol, while the other is translocated into the endoplasmic reticulum, glycosylated and secreted. We show here that a single mRNA encodes both forms of PAI-2. Firstly, a single PIA-2 mRNA was detected by Northern blot hybridization and by RNase protection. Secondly, transfection of a PAI-2 cDNA led to the synthesis of both forms of PAI-2. Finally, in vitro translation of an mRNA transcript of the PAI-2 cDNA in the presence of microsomal membranes generated two topologically distinct forms of PAI-2. The cytosolic and secreted forms of PAI-2 do not result from the use of two translation start sites, since their synthesis initiates at the same AUG, in a sequence context that is conserved between the human and murine genes. Thus, the accumulation of one polypeptide into two topologically distinct cellular compartments can be achieved by facultative translocation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belin D., Godeau F., Vassalli J. D. Tumor promoter PMA stimulates the synthesis and secretion of mouse pro-urokinase in MSV-transformed 3T3 cells: this is mediated by an increase in urokinase mRNA content. EMBO J. 1984 Aug;3(8):1901–1906. doi: 10.1002/j.1460-2075.1984.tb02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Mudd E. A., Prentki P., Yi-Yi Y., Krisch H. M. Sense and antisense transcription of bacteriophage T4 gene 32. Processing and stability of the mRNAs. J Mol Biol. 1987 Mar 20;194(2):231–243. doi: 10.1016/0022-2836(87)90371-8. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Busso N., Belin D., Failly-Crépin C., Vassalli J. D. Plasminogen activators and their inhibitors in a human mammary cell line (HBL-100). Modulation by glucocorticoids. J Biol Chem. 1986 Jul 15;261(20):9309–9315. [PubMed] [Google Scholar]
- Carlson M., Taussig R., Kustu S., Botstein D. The secreted form of invertase in Saccharomyces cerevisiae is synthesized from mRNA encoding a signal sequence. Mol Cell Biol. 1983 Mar;3(3):439–447. doi: 10.1128/mcb.3.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L. Characterization of a macrophage-derived plasminogen-activator inhibitor. Similarities with placental urokinase inhibitor. Biochem J. 1985 Aug 15;230(1):109–116. doi: 10.1042/bj2300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., Vassalli P. Modulations of functional activity in differentiated macrophages are accompanied by early and transient increase or decrease in c-fos gene transcription. J Immunol. 1987 Aug 1;139(3):949–955. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Dreano M., Fouillet X., Brochot J., Vallet J. M., Michel M. L., Rungger D., Bromley P. Heat-regulated expression of the hepatitis B virus surface antigen in the human Wish cell line. Virus Res. 1987 Jul;8(1):43–59. doi: 10.1016/0168-1702(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Garcia P. D., Ou J. H., Rutter W. J., Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988 Apr;106(4):1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton C., Kruithof E. K., Schleuning W. D. Phorbol ester induces the biosynthesis of glycosylated and nonglycosylated plasminogen activator inhibitor 2 in high excess over urokinase-type plasminogen activator in human U-937 lymphoma cells. J Cell Biol. 1987 Mar;104(3):705–712. doi: 10.1083/jcb.104.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Golder J. P., Stephens R. W. Minactivin: a human monocyte product which specifically inactivates urokinase-type plasminogen activators. Eur J Biochem. 1983 Nov 15;136(3):517–522. doi: 10.1111/j.1432-1033.1983.tb07771.x. [DOI] [PubMed] [Google Scholar]
- Gupta D. K., Theisen N., von Figura K., Hasilik A. Comparison of biosynthesis and subcellular distribution of lysozyme and lysosomal enzymes in U937 monocytes. Biochim Biophys Acta. 1985 Nov 20;847(2):217–222. doi: 10.1016/0167-4889(85)90023-0. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Traunecker A., Tonegawa S. Somatic mutation creates diversity in the major group of mouse immunoglobulin kappa light chains. J Exp Med. 1984 Feb 1;159(2):417–435. doi: 10.1084/jem.159.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Morimoto K., Uemura Y. Urokinase inhibitor in human placenta. Nature. 1968 Jan 20;217(5125):253–254. doi: 10.1038/217253a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof E. K., Vassalli J. D., Schleuning W. D., Mattaliano R. J., Bachmann F. Purification and characterization of a plasminogen activator inhibitor from the histiocytic lymphoma cell line U-937. J Biol Chem. 1986 Aug 25;261(24):11207–11213. [PubMed] [Google Scholar]
- Kwiatkowski D. J., Mehl R., Yin H. L. Genomic organization and biosynthesis of secreted and cytoplasmic forms of gelsolin. J Cell Biol. 1988 Feb;106(2):375–384. doi: 10.1083/jcb.106.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Kruithof E. K., Schleuning W. D. Plasminogen activator inhibitor 1 and 2 are tumor necrosis factor/cachectin-responsive genes. J Exp Med. 1988 Aug 1;168(2):751–759. doi: 10.1084/jem.168.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Sasavage N. L., Nilson J. H., Horowitz S., Rottman F. M. Nucleotide sequence of bovine prolactin messenger RNA. Evidence for sequence polymorphism. J Biol Chem. 1982 Jan 25;257(2):678–681. [PubMed] [Google Scholar]
- Schleuning W. D., Medcalf R. L., Hession C., Rothenbühler R., Shaw A., Kruithof E. K. Plasminogen activator inhibitor 2: regulation of gene transcription during phorbol ester-mediated differentiation of U-937 human histiocytic lymphoma cells. Mol Cell Biol. 1987 Dec;7(12):4564–4567. doi: 10.1128/mcb.7.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubin M., Long E. O., Mach B. Two forms of the Ia antigen-associated invariant chain result from alternative initiations at two in-phase AUGs. Cell. 1986 Nov 21;47(4):619–625. doi: 10.1016/0092-8674(86)90626-4. [DOI] [PubMed] [Google Scholar]
- Tabe L., Krieg P., Strachan R., Jackson D., Wallis E., Colman A. Segregation of mutant ovalbumins and ovalbumin-globin fusion proteins in Xenopus oocytes. Identification of an ovalbumin signal sequence. J Mol Biol. 1984 Dec 15;180(3):645–666. doi: 10.1016/0022-2836(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Vidal S., Mottet G., Kolakofsky D., Roux L. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol. 1989 Feb;63(2):892–900. doi: 10.1128/jvi.63.2.892-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Webb A. C., Collins K. L., Snyder S. E., Alexander S. J., Rosenwasser L. J., Eddy R. L., Shows T. B., Auron P. E. Human monocyte Arg-Serpin cDNA. Sequence, chromosomal assignment, and homology to plasminogen activator-inhibitor. J Exp Med. 1987 Jul 1;166(1):77–94. doi: 10.1084/jem.166.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlwend A., Belin D., Vassalli J. D. Plasminogen activator-specific inhibitors in mouse macrophages: in vivo and in vitro modulation of their synthesis and secretion. J Immunol. 1987 Aug 15;139(4):1278–1284. [PubMed] [Google Scholar]
- Wohlwend A., Belin D., Vassalli J. D. Plasminogen activator-specific inhibitors produced by human monocytes/macrophages. J Exp Med. 1987 Feb 1;165(2):320–339. doi: 10.1084/jem.165.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R. D., Ahern S. M., Le Beau M. M., Lebo R. V., Sadler J. E. Structure of the gene for human plasminogen activator inhibitor-2. The nearest mammalian homologue of chicken ovalbumin. J Biol Chem. 1989 Apr 5;264(10):5495–5502. [PubMed] [Google Scholar]
- Ye R. D., Wun T. C., Sadler J. E. Mammalian protein secretion without signal peptide removal. Biosynthesis of plasminogen activator inhibitor-2 in U-937 cells. J Biol Chem. 1988 Apr 5;263(10):4869–4875. [PubMed] [Google Scholar]
- Ye R. D., Wun T. C., Sadler J. E. cDNA cloning and expression in Escherichia coli of a plasminogen activator inhibitor from human placenta. J Biol Chem. 1987 Mar 15;262(8):3718–3725. [PubMed] [Google Scholar]
- Young P. R., Hazuda D. J., Simon P. L. Human interleukin 1 beta is not secreted from hamster fibroblasts when expressed constitutively from a transfected cDNA. J Cell Biol. 1988 Aug;107(2):447–456. doi: 10.1083/jcb.107.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Towards a comparative anatomy of N-terminal topogenic protein sequences. J Mol Biol. 1986 May 5;189(1):239–242. doi: 10.1016/0022-2836(86)90394-3. [DOI] [PubMed] [Google Scholar]