Abstract
Hypermutation can be defined as an enhancement of the spontaneous mutation rate which the organism uses in certain types of differentiated cells where a high mutation rate is advantageous. At the immunoglobulin loci this process increases the mutation rate > 10(5)-fold over the normal, spontaneous rate. Its proximate cause is called the immunoglobulin mutator system. The most important function of this system is to improve antibody affinity in an ongoing response; it is turned on and off during the differentiation of B lymphocytes. We have established an in vitro system to study hypermutation by transfecting a rearranged mu gene into a cell line in which an immunoglobulin mutator has been demonstrated. A construct containing the mu gene and the 3' kappa enhancer has all the cis-acting elements necessary for hypermutation of the endogenous gene segments encoding the variable region. The activity of the mutator does not seem to depend strongly on the position of the transfected gene in the genome. The mutator is not active in transformed cells of a later differentiation stage. It is also not active on a transfected lacZ gene. These results are consistent with the specificity of the mutator system being maintained and make it possible to delineate cis and trans mutator elements in vitro. Surprisingly, the mutator preferentially targets G-C base pairs. Two hypotheses are discussed: (i) the immunoglobulin mutator system in mammals consists of several mutators, of which the mutator described here is only one; or (ii) the primary specificity of the system is biased toward mutation of G-C base pairs, but this specificity is obscured by antigenic selection.
Full text
PDF

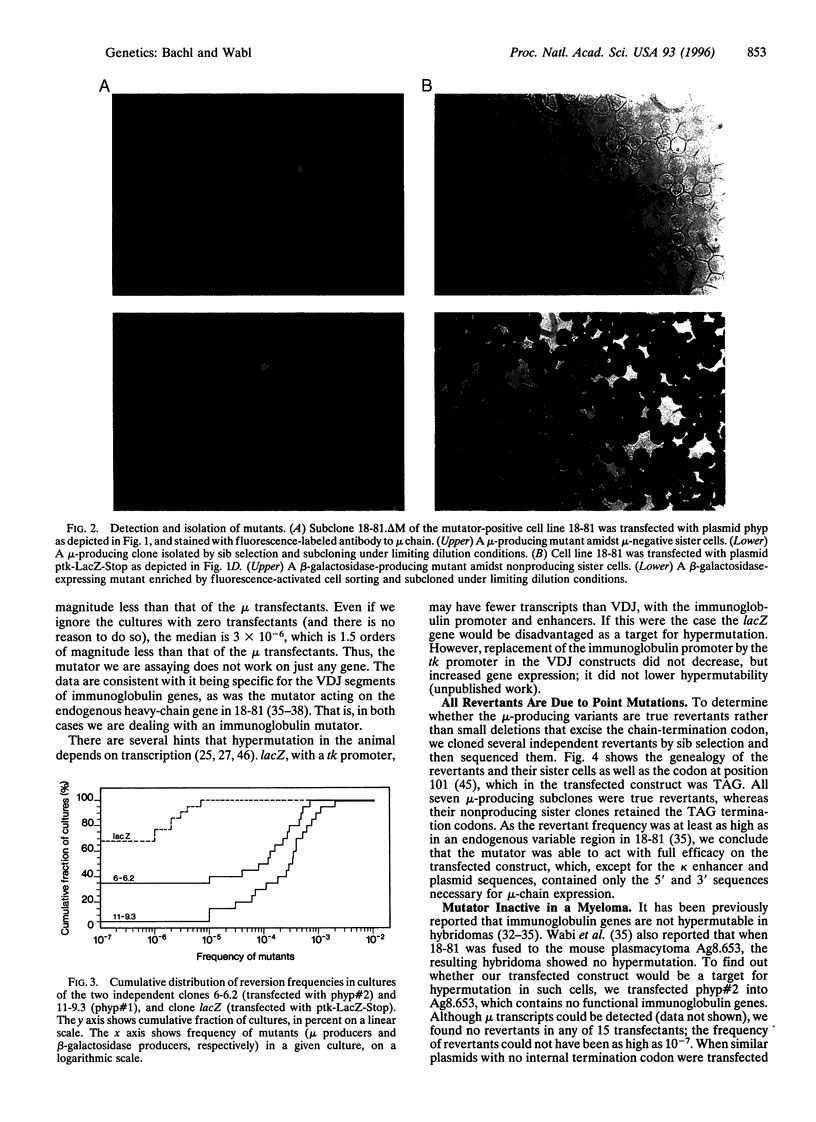
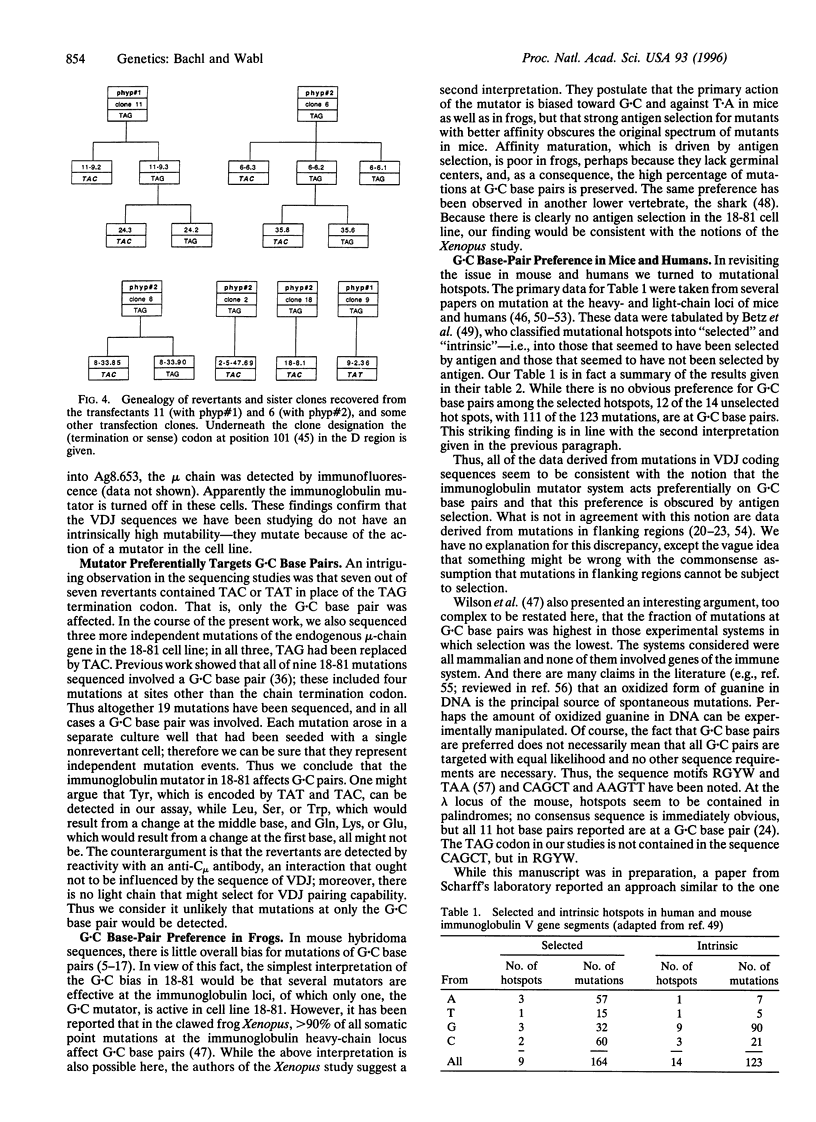

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adetugbo K., Milstein C., Secher D. S. Molecular analysis of spontaneous somatic mutants. Nature. 1977 Jan 27;265(5592):299–304. doi: 10.1038/265299a0. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumal R., Birshtein B. K., Coffino P., Scharff M. D. Mutations in immunoglobulin-producing mouse myeloma cells. Science. 1973 Oct 12;182(4108):164–166. doi: 10.1126/science.182.4108.164. [DOI] [PubMed] [Google Scholar]
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Berek C., Jarvis J. M., Milstein C. Activation of memory and virgin B cell clones in hyperimmune animals. Eur J Immunol. 1987 Aug;17(8):1121–1129. doi: 10.1002/eji.1830170808. [DOI] [PubMed] [Google Scholar]
- Betz A. G., Milstein C., González-Fernández A., Pannell R., Larson T., Neuberger M. S. Elements regulating somatic hypermutation of an immunoglobulin kappa gene: critical role for the intron enhancer/matrix attachment region. Cell. 1994 Apr 22;77(2):239–248. doi: 10.1016/0092-8674(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Betz A. G., Neuberger M. S., Milstein C. Discriminating intrinsic and antigen-selected mutational hotspots in immunoglobulin V genes. Immunol Today. 1993 Aug;14(8):405–411. doi: 10.1016/0167-5699(93)90144-a. [DOI] [PubMed] [Google Scholar]
- Betz A. G., Rada C., Pannell R., Milstein C., Neuberger M. S. Passenger transgenes reveal intrinsic specificity of the antibody hypermutation mechanism: clustering, polarity, and specific hot spots. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2385–2388. doi: 10.1073/pnas.90.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Taylor L., Pollard J. W., Steele E. J. Distribution of mutations around rearranged heavy-chain antibody variable-region genes. Mol Cell Biol. 1990 Oct;10(10):5187–5196. doi: 10.1128/mcb.10.10.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brenner S., Milstein C. Origin of antibody variation. Nature. 1966 Jul 16;211(5046):242–243. doi: 10.1038/211242a0. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- González-Fernández A., Gupta S. K., Pannell R., Neuberger M. S., Milstein C. Somatic mutation of immunoglobulin lambda chains: a segment of the major intron hypermutates as much as the complementarity-determining regions. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12614–12618. doi: 10.1073/pnas.91.26.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hinds-Frey K. R., Nishikata H., Litman R. T., Litman G. W. Somatic variation precedes extensive diversification of germline sequences and combinatorial joining in the evolution of immunoglobulin heavy chain diversity. J Exp Med. 1993 Sep 1;178(3):815–824. doi: 10.1084/jem.178.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., McDowell M., Steinberg C. M., Wabl M. Looping out and deletion mechanism for the immunoglobulin heavy-chain class switch. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1581–1585. doi: 10.1073/pnas.85.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. High rates of deletions in the constant region segment of the immunoglobulin mu gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4934–4938. doi: 10.1073/pnas.84.14.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Bignami M. DNA damage tolerance, mismatch repair and genome instability. Bioessays. 1994 Nov;16(11):833–839. doi: 10.1002/bies.950161110. [DOI] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Genes and antibodies. Science. 1959 Jun 19;129(3364):1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Huang S. Y., Gefter M. L. Influence of clonal selection on the expression of immunoglobulin variable region genes. Science. 1984 Dec 14;226(4680):1283–1288. doi: 10.1126/science.6334361. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Jäck H. M., Ellis N., Wabl M. High rate of somatic point mutation in vitro in and near the variable-region segment of an immunoglobulin heavy chain gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6950–6953. doi: 10.1073/pnas.83.18.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Sharpe M. J., Surani M. A., Neuberger M. S. The importance of the 3'-enhancer region in immunoglobulin kappa gene expression. Nucleic Acids Res. 1990 Oct 11;18(19):5609–5615. doi: 10.1093/nar/18.19.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Adetugbo K., Cowan N. J., Kohler G., Secher D. S. Expression of antibody genes in tissue culture: structural mutants and hybrid cells. Natl Cancer Inst Monogr. 1978 May;(48):321–330. [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada C., González-Fernández A., Jarvis J. M., Milstein C. The 5' boundary of somatic hypermutation in a V kappa gene is in the leader intron. Eur J Immunol. 1994 Jun;24(6):1453–1457. doi: 10.1002/eji.1830240632. [DOI] [PubMed] [Google Scholar]
- Rogerson B. J. Mapping the upstream boundary of somatic mutations in rearranged immunoglobulin transgenes and endogenous genes. Mol Immunol. 1994 Feb;31(2):83–98. doi: 10.1016/0161-5890(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Rogerson B., Hackett J., Jr, Peters A., Haasch D., Storb U. Mutation pattern of immunoglobulin transgenes is compatible with a model of somatic hypermutation in which targeting of the mutator is linked to the direction of DNA replication. EMBO J. 1991 Dec;10(13):4331–4341. doi: 10.1002/j.1460-2075.1991.tb05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozin I. B., Kolchanov N. A. Somatic hypermutagenesis in immunoglobulin genes. II. Influence of neighbouring base sequences on mutagenesis. Biochim Biophys Acta. 1992 Nov 15;1171(1):11–18. doi: 10.1016/0167-4781(92)90134-l. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablitzky F., Wildner G., Rajewsky K. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J. 1985 Feb;4(2):345–350. doi: 10.1002/j.1460-2075.1985.tb03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Sharpe M. J., Milstein C., Jarvis J. M., Neuberger M. S. Somatic hypermutation of immunoglobulin kappa may depend on sequences 3' of C kappa and occurs on passenger transgenes. EMBO J. 1991 Aug;10(8):2139–2145. doi: 10.1002/j.1460-2075.1991.tb07748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. U., DePinho R., Zack D. J., Rudikoff S., Scharff M. D. Instability of immunoglobulin genes in S107 cell line. Somat Cell Mol Genet. 1991 May;17(3):259–276. doi: 10.1007/BF01232821. [DOI] [PubMed] [Google Scholar]
- Shulman M. J., Heusser C., Filkin C., Köhler G. Mutations affecting the structure and function of immunoglobulin M. Mol Cell Biol. 1982 Sep;2(9):1033–1043. doi: 10.1128/mcb.2.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Wabl M., Burrows P. D., von Gabain A., Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc Natl Acad Sci U S A. 1985 Jan;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M., Jäck H. M., Meyer J., Beck-Engeser G., von Borstel R. C., Steinberg C. M. Measurements of mutation rates in B lymphocytes. Immunol Rev. 1987 Apr;96:91–107. doi: 10.1111/j.1600-065x.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Weber J. S., Berry J., Manser T., Claflin J. L. Position of the rearranged V kappa and its 5' flanking sequences determines the location of somatic mutations in the J kappa locus. J Immunol. 1991 May 15;146(10):3652–3655. [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Weiss U., Zoebelein R., Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992 Feb;22(2):511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- Wilson M., Hsu E., Marcuz A., Courtet M., Du Pasquier L., Steinberg C. What limits affinity maturation of antibodies in Xenopus--the rate of somatic mutation or the ability to select mutants? EMBO J. 1992 Dec;11(12):4337–4347. doi: 10.1002/j.1460-2075.1992.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Rabinowitz J. L., Green N. S., Kobrin B. J., Scharff M. D. A well-differentiated B-cell line is permissive for somatic mutation of a transfected immunoglobulin heavy-chain gene. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2810–2814. doi: 10.1073/pnas.92.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegner M., Steinhauser G., Berek C. Development of antibody diversity in single germinal centers: selective expansion of high-affinity variants. Eur J Immunol. 1994 Oct;24(10):2393–2400. doi: 10.1002/eji.1830241020. [DOI] [PubMed] [Google Scholar]
- van der Stoep N., van der Linden J., Logtenberg T. Molecular evolution of the human immunoglobulin E response: high incidence of shared mutations and clonal relatedness among epsilon VH5 transcripts from three unrelated patients with atopic dermatitis. J Exp Med. 1993 Jan 1;177(1):99–107. doi: 10.1084/jem.177.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]




