Abstract
The connection between the heart and the brain has long been anecdotally recognized but systematically studied only relatively recently. Cardiac arrhythmias, especially sudden cardiac death, remain a major public health concern and there is mounting evidence that psychological distress plays a critical role as both a predictor of high-risk cardiac substrate and as an inciting trigger. The transient, unpredictable nature of emotions and cardiac arrhythmias have made their study challenging, but evolving technologies in monitoring and imaging along with larger epidemiological data sets have encouraged more sophisticated studies examining this relationship. Here we review the research on psychological distress including anger, depression and anxiety on cardiac arrhythmias, insights into proposed mechanisms, and potential avenues for future research.
Keywords: arrhythmia, depression, psychological, sudden cardiac death
“Alas, my liege, my wife is dead tonight; Grief of my son’s exile hath stopp’d her breath”
-Romeo and Juliet Act 5 Scene 3 Line 218–219
Montague over his wife’s sudden death.
A systematic review of Shakespeare’s works cites ten deaths due to strong emotions with three of them occurring on stage,1 so the impact of psychological distress on mortality has long been intuited. Similarly, sudden cardiac death (SCD) and ventricular arrhythmias have been observed in response to disasters associated with intense emotion2–5. Recognition of the importance of psychological factors in the development of cardiac arrhythmias in cardiovascular research has grown with increased understanding about the brain-heart connection. In this review we seek to address some of the challenges in investigating emotional factors in arrhythmia, discuss the recent work in this field, and outline potential avenues for future research.
Research challenges
Initiation of an arrhythmic event often requires both a susceptible myocardial substrate and an inciting trigger. For instance, in the case of ischemic ventricular tachycardia (VT), the scar from prior myocardial infarction (MI) demonstrates intermingling of fibrous tissue and surviving myocardial fibers that results in delayed impulse conduction6, as well as reduced cell-to-cell coupling particularly in the infarct border zone7. This substrate may remain clinically silent until an inciting trigger such as a ventricular premature complex initiates sustained re-entry, thereby exposing the vulnerable substrate.
Emotional factors may exhibit both substrate and triggering relationships with arrhythmia (Figure 1), and one useful categorization differentiates stable traits from more transient states. Someone may have a tendency toward anger, but nonetheless may not actually experience the state of anger in a given period as much as another person who repeatedly faces anger-inducing stressors. Psychological traits and states have different conceptual constructs and measurement instruments. For instance, Type D personality is a stable personality type that manifests as a tendency toward negative emotions and against sharing these emotions with others due to fear of rejection8. Stable traits may reinforce the triggering phenomenon associated with a transient emotional state9.
Figure 1.
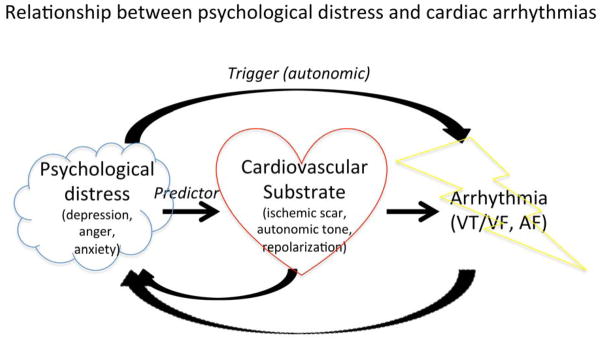
Schematic representation of the proposed relationship between psychological distress and cardiac arrhythmias. Emotions can predict cardiovascular substrate at risk for arrhythmias and can trigger arrhythmias. Arrhythmias and high-risk heart conditions can precipitate psychological distress.
The fact that emotional states and arrhythmia can each occur unexpectedly and transiently poses difficulties in assessing the relationships between the two. In the cardiac literature, measurement of symptoms of psychological distress has often relied on self-report questionnaires or on structured interviews performed at one point in time. Clearly this is not as comprehensive as a repeated assessment, and the unreliability of a single assessment may account for some of the variability in estimated associations. More recently, electronic diaries have been used to perform ecological momentary assessment, where real-time symptoms are prospectively captured over the course of the day10. Arrhythmia measurement has also improved in the past decade through the adoption of technologies such as intracardiac recordings from implanted devices, and through mobile cardiac telemetry monitoring in patients without such devices. However, even with continuous measurement, arrhythmia over the course of one week or even one month is likely to be rare, unless a high risk sample is studied. Implantable cardioverter-defibrillator (ICD) patients have frequently been studied for emotional correlates with arrhythmia, but studies in the general population require many more observations over long periods of time.
Even with emerging evidence demonstrating associations between psychosocial factors and cardiac arrhythmias, the causal direction is difficult to assess (Figure 1). Individuals who have frequent arrhythmias are potentially more likely to develop depressive symptoms and anxiety due to the somatic symptoms associated with the arrhythmia, or from the treatments – such as defibrillator therapy - necessitated by their arrhythmia. An association between the putative psychosocial risk factor and the arrhythmic outcome may partly reflect this ‘reverse causality.’
Medications that are used to treat individuals with psychiatric disorders may themselves affect the risk of cardiac arrhythmia. For instance, antipsychotic medications11 and certain antidepressant medications12 carry a known risk of affecting repolarization and causing torsades de pointes. Separating the effects of psychiatric disorders versus treatment is often impossible in observational studies13.
Human studies of emotion and arrhythmia
There is a wealth of epidemiologic evidence involving psychosocial factors and ventricular arrhythmia; for an excellent summary of the literature through 1999 please see the review by Hemingway and colleagues14. Here we review the more recent observational and clinical research data, from a symptom-specific perspective, and also discuss data involving atrial fibrillation (AF).
Anger
Among emotional factors, anger has been relatively well-characterized particularly within the conceptual framework of its triggering effect on ventricular arrhythmia. Lampert et al.15 used a case-crossover study design to evaluate emotional triggers of shock for ventricular arrhythmia among 277 ICD patients. Patients were instructed to record their mood states in structured diaries both at the time of an ICD shock and during a later control period. Anger was more frequent in the 15 minutes preceding shock, compared with control periods (odds ratio 1.8, 95% CI 1.04–3.16; p<0.04), although other mood states such as anxiety, worry, sadness, and happiness did not differ. Burg et al.9 studied the same sample of patients further, and found in multivariable analyses that the 17 patients who reported at least moderate anger in the 15 minutes before shock had significantly higher scores on the Speilberger Trait Anger measure, suggesting that stable traits may identify patients at risk for emotion-triggered arrhythmias. VT episodes triggered by anger in ICD patients were more likely to be pause-dependent and polymorphic16.
Depression
As opposed to anger, depression and depressed mood have been studied more for their longer term relationships with arrhythmia risk. This focus may reflect the well-known impact of depression on long-term prognosis in patients with coronary artery disease17,18. Also, depression has been studied more frequently for its association with endpoints such as sudden cardiac death (SCD). Although SCD is often due to VT or ventricular fibrillation (VF), the proportion of SCD that is due to pulseless electrical activity, which typically does not have an arrhythmic component, has been increasing especially among patients without coronary artery disease19.
In a case-control study, Empana et al.20 examined data from enrollees of a health maintenance organization in Washington State. Cases of out-of-hospital cardiac arrest (n=2228) among patients aged 40–79 years were identified from emergency medical service incident reports, and ambulatory medical records were examined for diagnoses of clinical depression. Compared with non-depressed subjects, the multivariable odds of cardiac arrest were increased both among the less severely depressed (OR 1.30, 95% CI 1.04–1.63) and among those with more severe depression (OR 1.77, 95% CI 1.28–2.45). The association between depression and cardiac arrest remained even after exclusion of patients who were taking antidepressant medications, indicating that depression itself was specifically related to cardiac arrest risk. In a population-based cohort analysis, Luukinen et al.21 studied 915 individuals aged 70 years or older in a defined area of northern Finland. In multivariable analyses that included hypertension, diabetes, and congestive heart failure, during 8 year follow-up, depressive symptoms according to a baseline questionnaire were associated with increased risk of SCD (HR 2.74, 95% CI 1.37–5.50), whereas the risk of non-sudden death was not significantly increased. Exclusion of antidepressant users did not change the main results. In an analysis from the Nurses’ Health Study, among 63,000 females without known cardiovascular disease, a proxy variable for clinical depression that consisted of Mental Health Inventory-5 score<53 or antidepressant medication use was associated with SCD in multivariable models that included hypertension, diabetes, and hypercholesterolemia (HR 2.33, 95% CI 1.47 to 3.70). The relationship of depression to subsequent SCD appeared to be related to a specific association with antidepressant use, although confounding by indication could not be ruled out13.
In contrast to the above population-based studies, Irvine et al.22 focused on a high-risk group of 671 post-myocardial infarction patients with frequent ventricular premature beats by ambulatory ECG monitoring who were enrolled in the multicenter Canadian Amiodarone Myocardial Infarction Arrhythmia Trial randomized trial of amiodarone versus placebo. In analyses that adjusted for prior myocardial infarction and congestive heart failure, Beck Depression Inventory (BDI) score ≥10 was associated with 2-year SCD (RR 2.45, 95% CI 1.14–5.35) in the placebo group, but not in the amiodarone group. When a measure of dyspnea and fatigue was added to the model, the relationship between depressive symptoms and SCD was attenuated by about 30% and was no longer statistically significant (RR 1.73, 95% CI 0.75–3.98), raising the question of whether somatic symptoms may confound the association between depression and SCD. In another cohort analysis of high-risk patients, among 645 ICD patients in the Triggers of Ventricular Arrhythmias study, depressive symptoms assessed by the Center for Epidemiologic Studies-Depression (CES-D) scale were associated with time to first shock for VT/VF (hazard ratio 3.2, 95% CI 1.1 to 9.9) in multivariable models that included left ventricular ejection fraction, heart failure class, and number of prior ICD shocks23. Even in patients who are among the highest risk for mortality, those with the combination of atrial fibrillation and heart failure, depressive symptoms predict arrhythmic death, as demonstrated in a substudy of the Atrial Fibrillation-Congestive Heart Failure (AF-CHF) Trial among 974 patients24.
Anxiety
Although an overall triggering relationship between anxiety and ICD shocks was not seen in the study by Lampert et al.15, Burg et al.9 found in the same sample that the subgroup of patients reported anxiety prior to their shock had significantly higher levels of trait anxiety. In a separate study of 1012 patients with new ICD implant, those in the highest tertile of anxiety measured by the State-Trait Anxiety Inventory were at increased risk for ventricular arrhythmias during 1-year follow-up (HR 1.91, 95% CI 1.33–2.75) with adjustment for variables including coronary artery disease, diabetes, and beta blocker use25. Lampert et al.26 demonstrated destabilizing effects of mental stress on ventricular arrhythmias using noninvasive-programmed stimulation to induce ventricular tachycardia with and without mental stress in 18 patients with ICDs. Ventricular tachycardia had faster onset and was more difficult to terminate in patients under mental stress.
Phobic anxiety describes anxiety that is triggered by a specific stimulus and is the largest category of anxiety disorders affecting between 5%–12% of the population worldwide27. Kawachi et al.28 used the Crown-Crisp index (CCI) to study the relationship between phobic anxiety and mortality in the Health Professionals Follow-up Study of 33,999 male health professionals. During 2 years of follow-up, an elevated CCI score of 3 or more was associated with elevated risk of cardiac mortality, and this excess risk was due to increased risk of sudden cardiac death (relative risk 6.08, 95% CI 2.35–15.73) in multivariable models. In the Nurses’ Health Study, among 72 359 women with no history of cardiovascular disease, during 12 years of follow-up, women who scored 4 or greater on the CCI were at higher risk of SCD (HR 1.59, 95% CI 0.97 to 2.60). After adjustment for possible intermediaries (hypertension, diabetes, and elevated cholesterol), a trend toward increased risk persisted for SCD (P=0.06)29.
Depression and anxiety often coexist, and the Very Anxious Group Under Scrutiny (VAGUS) study investigated this issue in 940 patients with history of coronary artery disease who were undergoing diagnostic cardiac catheterization. This study was unique in using a ventricular arrhythmia outcome, ascertained from medical records and defined as ventricular tachycardia (non-sustained and sustained) or ventricular fibrillation. Also, this study measured both depression according to the BDI and phobic anxiety using the CCI. During 3 year follow-up, 97 (10.3%) patients had documented ventricular arrhythmia. BDI score ≥10 was associated with ventricular arrhythmia in multivariable analyses that included left ventricular ejection fraction and history of arrhythmia (odds ratio 1.4, 95% CI 1.1 to 1.9). With simultaneous inclusion of BDI score and CCI, depressive symptoms (odds ratio 1.3, 95% CI 0.99–1.7) and phobic anxiety (odds ratio 1.3, 95% CI 0.98–1.8) were each associated with a trend toward increased arrhythmia. A composite index of depression score and phobic anxiety, however, predicted ventricular arrhythmias with a larger effect size than either score alone (odds ratio 1.6, 95% CI 1.2–2.1). The VAGUS study suggested that depression and anxiety may act via common pathways to increase the risk of arrhythmia, and that arrhythmia may explain the mortality risk associated with these factors in patients with coronary artery disease30.
Atrial fibrillation
As opposed to ventricular arrhythmia and SCD, there have been fewer studies published regarding emotional factors and the most common arrhythmia, AF, and the evidence is not as consistent. In a triggering analysis that used event monitoring and electronic diaries, Lampert and colleagues observed in 75 patients with paroxysmal or persistent AF that arrhythmia episodes were more often preceded by negative emotions and less often by happiness31. In a case-cohort study of a population-based sample from Taiwan consisting of 3888 patients with diagnosis of panic disorder (PD) and 38,880 controls, during follow up as long as 7 years, crude AF risk was 1.2% in the PD group and 0.9% in the control group. Multivariable analyses that included age, gender, hypertension, CHF, valvular heart disease, and chronic obstructive pulmonary disease showed elevated AF risk in PD (HR 1.73, 95% CI 1.27–2.37)32. However, in a prospective cohort analysis of 30,746 female health professionals without known heart disease, global psychological distress according to a 5-item questionnaire was not associated with AF risk, and neither were specific items regarding depression and anxiety33. This may be consistent with gender differences in the association of emotion and AF that has previously been noted in the Framingham Offspring Study, where baseline levels of tension, anger, and hostility predicted increased 10-year risk of AF in men, but not in women34,35.
Insights into mechanisms
A frequently postulated mechanism relating the risk of arrhythmia to psychological distress is cardiac autonomic dysfunction, i.e., sympathetic predominance and parasympathetic withdrawal. Grippo and colleagues have studied a depressive phenotype in a rat model through a stress induction, the components of which included exposures such as continuous overnight illumination, paired housing, and white noise. The rats that were randomly exposed to the stress induction developed increased heart rate, reduced heart rate variability, and greater ventricular arrhythmia in response to aconitine infusion36. Carney and colleagues have demonstrated in the post-MI setting that depressed patients have reduced heart rate variability, particularly in the very-low-frequency spectrum according to frequency domain analyses of 24-hour ambulatory electrocardiography (ECG) monitoring37. An analysis of 311 depressed and 367 non-depressed post-MI patients in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) clinical trial suggested that reduced heart rate variability partially mediated the relationship between depression and mortality38.
Emotional factors may also influence arrhythmia risk by affecting the stability of cardiac repolarization. Experimental anger-recall tasks have evoked T wave alternans in ICD patients39,40, and furthermore, anger-induced T wave alternans in the laboratory setting predicted VT/VF episodes in ICD patients41. This work suggests that anger triggers ventricular arrhythmia in the short term by inducing repolarization instability. Depression has also been studied for effects on repolarization; Carney et al.42 compared 20 post-myocardial infarction patients with major depression and 20 post-MI patients without depression and found that the QT variability index, a marker for ventricular arrhythmia, was significantly higher in the depressed group. There may be a gender modification with repolarization abnormalities, as the electrocardiographic QT interval has been observed to be increased in women, but not in men after acute coronary syndrome43.
Specific regions in the brain may be responsible for mediating the pro-arrhythmic effects of emotions. Lane and Jennings proposed the “brain-heart laterality hypothesis” suggesting that lateralization of emotions in the cerebral hemispheres and of autonomic inputs to the surfaces of the heart contributed to repolarization instability and to SCD44. By simultaneously measuring brain and heart activity using H2(15)O positron emission tomography and ECGs during mental and physical stress, Critchley et al.45 demonstrated in a sample of ten cardiology clinic patients that right lateralized midbrain activity corresponded to electrocardiographic repolarization abnormalities.
We can also look to specific cardiac disease processes that involve emotional factors in order to gain insights of possible mechanisms in the larger population. For instance, in stress cardiomyopathy, or Takotsubo syndrome, a primary driver is profound catecholamine surge46,47. Commonalities between the two conditions have prompted speculation that depression may increase the propensity to develop stress cardiomyopathy48. Depression has been observed to be relatively common in patients with stress cardiomyopathy48, and cardiac norepinephrine spillover is elevated in patients with depression and comorbid panic disorder49,50. In long QT syndrome type 2, caused by a loss of function mutation in the KCNH2 gene, SCD has been described to occur more frequently in response to sudden arousal or to emotional stress51. Lane et al.52 observed that among 38 patients with long QT syndrome, psychological stress was elevated and peak happiness was reduced during the 24 hours prior to cardiac events consisting of syncope, aborted cardiac arrest, or defibrillator shock.
The relative lack of evidence for an association between negative emotions and AF in long-term follow-up studies may actually be consistent with the distinct effects of autonomic stimulation on atrial arrhythmias. As opposed to VT/VF, parasympathetic stimulation may play an important role in the development of AF53–55, particularly in younger patients56 and in those with ‘lone’ AF57. Increased heart rate variability, a marker of parasympathetic heart rate modulation, has been associated with higher risk of recurrent AF after cardioversion58. Further work is necessary to clarify whether atrial and ventricular arrhythmias have differing pathophysiologic relationships with emotions.
Potential therapies: the case of depression post-MI
Repeated observational studies continue to validate the relationship between psychological distress and arrhythmias, particularly VT/VF. In addition, animal models have suggested that therapies ‘upstream’ from the heart may reduce cardiac risk associated with psychological distress. In an ischemic pig model, Skinner et al.59 showed that psychological stress was an important factor in inducing VF, and that behavioral adaptation or intracerebral beta blocker injection could prevent VF initiation60. Grippo et al.61 demonstrated in a prairie vole model that intravenous oxytocin ameliorated the negative heart rate and heart rate variability effects, as well as the behavioral responses, to social isolation.
Most treatment trials involving psychological distress and heart disease have involved treatment for depression after MI. However, to date there is little or no evidence that treating depression in such individuals improves cardiovascular outcomes or prevents arrhythmia events, possibly due to limited power to detect differences in rare endpoints38,62. It may also be that analogous to the Cardiac Arrhythmia Suppression Trial (CAST) where anti-arrhythmic therapy resulted in elevated mortality in post-MI patients with frequent ventricular premature complexes63, treatment of the risk marker (depression) may not target the relevant mechanism involved in arrhythmia.
Perhaps psychological markers may simply help identify those at increased cardiac risk and who may benefit from treatment to improve arrhythmia burden. One intriguing study that has indicated the importance of psychological factors as a potential risk marker of arrhythmia treatment is an analysis of the AF-CHF trial. This was a multicenter, randomized control trial of 1376 patients that compared rhythm control to rate control in patients with heart failure and atrial fibrillation64. Although there were no differences in cardiovascular mortality between the two groups in the main trial, a sub-group analysis showed that among the 233 patients in the highest quartile of anxiety scores, rhythm control improved cardiac mortality compared with rate control (hazard ratio, 0.54; 95% confidence interval 0.32–0.93; P=0.022) with a statistically significant treatment interaction (P=0.009)65.
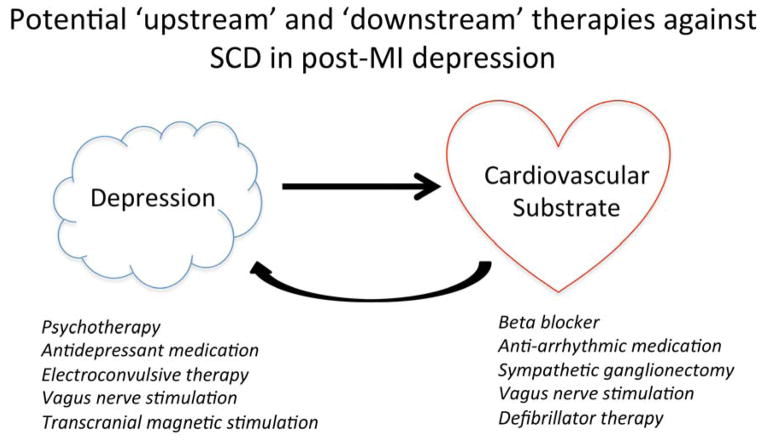
If we consider both the ‘upstream’ and ‘downstream’ therapies that are available for patients with depression post-MI and that could potentially improve arrhythmia risk, many of them involve modulation of the autonomic nervous system (Figure 2). Vagus nerve stimulation is appealing in possibly having simultaneous benefits for both conditions66,67. However, only one of the therapies, the ICD, has been proven to reduce mortality from arrhythmia in heart disease patients, albeit with high device costs, with a number needed to treat of approximately 18 primary prevention devices to save one person’s life, and with continued unaddressed burden of SCD post-MI68. Dealing with the public health problem of SCD may require improved identification of individuals who are at high enough risk for ventricular arrhythmia to benefit from an ICD, and psychological distress is a potential candidate marker of this risk. For instance, ICD therapy is currently indicated for primary prevention of SCD in post-MI patients with reduced left ventricular ejection fraction<0.35, especially with symptomatic heart failure69. Using heart failure symptoms to help identify patients who would benefit from ICDs involves several parallels with potentially using psychological states such as depression. For example, the diagnosis of depression is ultimately a clinical one, and depends to a large extent on subjective assessments taken from the perspective of the patient. In a similar fashion, assigning heart failure functional class can depend on relatively subjective assessments that nonetheless carry prognostic importance. Also, heart failure symptoms and depressive symptoms can wax and wane over time, especially with treatment, yet patients are often characterized within a certain bandwith of clinical status. Ultimately, heart failure is a modifier condition that helps identify patients who would derive mortality benefit from ICD therapy, and is not a critical participant in arrhythmia per se. So too, conditions such as depression may help identify more individuals who have the potential to benefit from ICD therapy, beyond current indications.
Figure 2.
Schematic representation of potential therapies for sudden cardiac death in high-risk post-MI patients suffering from depression.
Future directions and conclusions
It is apparent that psychological distress and negative emotions are important factors in the development of arrhythmia, yet many questions remain. It is not clear which matters more in the development of arrhythmia, a person’s psychological substrate or the time-limited (and more modifiable) emotional states through which he or she passes. This has important implications for the possibility of improving arrhythmia burden through therapies aimed at the emotional condition of patients. Also, other than T wave alternans in the case of anger-induced arrhythmia, there are still relatively few physiologic indicators that might help identify those patients at greatest risk for arrhythmia due to psychological distress. Ideally, relatively simple clinical tests, such as the 12-lead ECG or Holter monitor, could provide some insight when combined with comprehensive assessments of exposure to emotional stressors, but this remains to be clarified. The reasons for observed gender differences in the relationship between arrhythmia and psychological distress, such as in stress cardiomyopathy, are also not resolved. Perhaps the identification of mechanisms for these differences might help the development of therapies.
It is an exciting time in the field of behavioral cardiology, as research that integrates the brain and the heart has opened new lines of investigation. In addition to the proposed mechanisms mentioned here, medication adherence and lifestyle modification are also under more rigorous scrutiny as possible mediators of the cardiac risk of psychological distress70. As cardiologists have learned from prior experience (CAST), reducing the burden of disease from cardiac arrhythmia requires a comprehensive approach that involves the patient, not just the ECG. In a similar fashion, the recognition is spreading that reduction of arrhythmia risk in patients with psychological distress needs to incorporate the heart as well as the mind.
Acknowledgments
Dr Whang is supported by a Scientist Development grant from the American Heart Association Founders Affiliate.
Abbreviations
- SCD
sudden cardiac death
- VT
ventricular tachycardia
- MI
myocardial infarction
- ICD
implantable cardioverter-defibrillator
- AF
atrial fibrillation
- VF
ventricular fibrillation
- BDI
Beck Depression Inventory
- CES-D
Center for Epidemiologic Studies-Depression
- CCI
Crown-Crisp index
- VAGUS
Very Anxious Group Under Scrutiny
- PD
panic disorder
- CHF
congestive heart failure
- ECG
electrocardiogram
References
- 1.Heaton KW. Faints, fits, and fatalities from emotion in Shakespeare’s characters: survey of the canon. BMJ. 2006;333(7582):1335–8. doi: 10.1136/bmj.39045.690556.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213–28. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leor J, Poole WK, Kloner RA. Sudden Cardiac Death Triggered by an Earthquake. N Engl J Med. 1996;334(7):413–9. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 4.Meisel SR, Kutz I, Dayan KI, et al. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet [Internet] 1991;338(8768):660–1. doi: 10.1016/0140-6736(91)91234-l. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med3&AN=1679475. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg JS, Arshad A, Kowalski M, et al. Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. Journal of the American College of Cardiology. 2004;44(6):1261–4. doi: 10.1016/j.jacc.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Kawara T, Derksen R, de Groot JR, et al. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104(25):3069–75. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- 7.Kostin S, Rieger M, Dammer S, et al. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242(1–2):135–44. [PubMed] [Google Scholar]
- 8.Pedersen SS, Tekle FB, Hoogwegt MT, Jordaens L, Theuns DAMJ. Shock and patient preimplantation type D personality are associated with poor health status in patients with implantable cardioverter-defibrillator. Circ Cardiovasc Qual Outcomes. 2012;5(3):373–80. doi: 10.1161/CIRCOUTCOMES.111.964197. [DOI] [PubMed] [Google Scholar]
- 9.Burg MM, Lampert R, Joska T, Batsford W, Jain D. Psychological traits and emotion-triggering of ICD shock-terminated arrhythmias. Psychosom Med. 2004;66(6):898–902. doi: 10.1097/01.psy.0000145822.15967.15. [DOI] [PubMed] [Google Scholar]
- 10.Kamarck TW, Schwartz JE, Shiffman S, Muldoon MF, Sutton-Tyrrell K, Janicki DL. Psychosocial stress and cardiovascular risk: what is the role of daily experience? Journal of personality. 2005;73(6):1749–74. doi: 10.1111/j.0022-3506.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Glassman AH, Bigger JTJ. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. The American journal of psychiatry. 2001;158(11):1774–82. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 12.Vieweg WVR, Hasnain M, Howland RH, et al. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med. 2012;125(9):859–68. doi: 10.1016/j.amjmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Whang W, Kubzansky LD, Kawachi I, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. Journal of the American College of Cardiology. 2009;53(11):950–8. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemingway H, Malik M, Marmot M. Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. Eur Heart J. 2001;22(13):1082–101. doi: 10.1053/euhj.2000.2534. [DOI] [PubMed] [Google Scholar]
- 15.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106(14):1800–5. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- 16.Stopper M, Joska T, Burg MM, et al. Electrophysiologic characteristics of anger-triggered arrhythmias. Heart Rhythm. 2007;4(3):268–73. doi: 10.1016/j.hrthm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 18.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91(4):999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 19.Rea TD, Eisenberg MS, Sinibaldi G, White RD. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004;63(1):17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Empana JP, Jouven X, Lemaitre RN, et al. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med. 2006;166(2):195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 21.Luukinen H, Laippala P, Huikuri HV. Depressive symptoms and the risk of sudden cardiac death among the elderly. Eur Heart J. 2003;24(22):2021–6. doi: 10.1016/j.ehj.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Irvine J, Basinski A, Baker B, et al. Depression and risk of sudden cardiac death after acute myocardial infarction: testing for the confounding effects of fatigue. Psychosom Med. 1999;61(6):729–37. doi: 10.1097/00006842-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Whang W, Albert CM, Sears SFJ, et al. Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. Journal of the American College of Cardiology. 2005;45(7):1090–5. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 24.Frasure-Smith N, Lespérance F, Habra M, et al. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120(2):134–40. doi: 10.1161/CIRCULATIONAHA.109.851675. 3pfollowing140. [DOI] [PubMed] [Google Scholar]
- 25.Habibovic M, Pedersen SS, van den Broek KC, et al. Anxiety and Risk of Ventricular Arrhythmias or Mortality in Patients With an Implantable Cardioverter Defibrillator. Psychosom Med. 2012 doi: 10.1097/PSY.0b013e3182769426. [DOI] [PubMed] [Google Scholar]
- 26.Lampert R, Jain D, Burg MM, Batsford WP, McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000;101(2):158–64. doi: 10.1161/01.cir.101.2.158. [DOI] [PubMed] [Google Scholar]
- 27.Phil Barker PD. Psychiatric and Mental Health Nursing. CRC Press; 2009. [Google Scholar]
- 28.Kawachi I, Colditz GA, Ascherio A, et al. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89(5):1992–7. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 29.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–7. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 30.Watkins LL, Blumenthal JA, Davidson JR, Babyak MA, McCants CBJ, Sketch MHJ. Phobic anxiety, depression, and risk of ventricular arrhythmias in patients with coronary heart disease. Psychosom Med. 2006;68(5):651–6. doi: 10.1097/01.psy.0000228342.53606.b3. [DOI] [PubMed] [Google Scholar]
- 31.Lampert R, Burg M, Brandt C, Dziura J, Liu H, Donovan T, Soufer R, Jamner L. Impact of emotions on triggering of atrial fibrillation. Circulation. 2008;118:S640. Abstract 1036. [Google Scholar]
- 32.Cheng YF, Leu H-B, Su CC, et al. Association Between Panic Disorder and Risk of Atrial Fibrillation:A Nationwide Study. Psychosom Med. 2012 doi: 10.1097/PSY.0b013e318273393a. [DOI] [PubMed] [Google Scholar]
- 33.Whang W, Davidson KW, Conen D, Tedrow UB, Everett BM, Albert CM. Global Psychological Distress and Risk of Atrial Fibrillation Among Women: The Women’s Health Study. J Am Heart Assoc. 2012;1(3):e001107. doi: 10.1161/JAHA.112.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB, Benjamin EJ. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation. 2004;109(10):1267–71. doi: 10.1161/01.CIR.0000118535.15205.8F. [DOI] [PubMed] [Google Scholar]
- 35.Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RBS, Benjamin EJ. Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med. 2005;67(5):692–6. doi: 10.1097/01.psy.0000174050.87193.96. [DOI] [PubMed] [Google Scholar]
- 36.Grippo AJ, Santos CM, Johnson RF, et al. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286(2):H619–26. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- 37.Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005;165(13):1486–91. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 38.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA: the journal of the American Medical Association. 2003;289(23):3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 39.Kop WJ, Krantz DS, Nearing BD, et al. Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation. 2004;109(15):1864–9. doi: 10.1161/01.CIR.0000124726.72615.60. [DOI] [PubMed] [Google Scholar]
- 40.Lampert R, Shusterman V, Burg MM, et al. Effects of psychologic stress on repolarization and relationship to autonomic and hemodynamic factors. J Cardiovasc Electrophysiol. 2005;16(4):372–7. doi: 10.1046/j.1540-8167.2005.40580.x. [DOI] [PubMed] [Google Scholar]
- 41.Lampert R, Shusterman V, Burg M, et al. Anger-induced T-wave alternans predicts future ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Journal of the American College of Cardiology. 2009;53(9):774–8. doi: 10.1016/j.jacc.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carney RM, Freedland KE, Stein PK, et al. Effects of depression on QT interval variability after myocardial infarction. Psychosom Med. 2003;65(2):177–80. doi: 10.1097/01.psy.0000033129.21715.4b. [DOI] [PubMed] [Google Scholar]
- 43.Whang W, Julien HM, Higginbotham L, et al. Women, but not men, have prolonged QT interval if depressed after an acute coronary syndrome. Europace. 2012;14(2):267–71. doi: 10.1093/europace/eur246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane RD, Jennings JR. Hemispheric asymmetry, autonomic asymmetry and the problem of sudden cardiac death. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge (MA): MIT Press; 1995. pp. 271–304. [Google Scholar]
- 45.Critchley HD, Taggart P, Sutton PM, et al. Mental stress and sudden cardiac death: asymmetric midbrain activity as a linking mechanism. Brain. 2005;128(Pt 1):75–85. doi: 10.1093/brain/awh324. [DOI] [PubMed] [Google Scholar]
- 46.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 47.Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. Journal of the American College of Cardiology. 2009;53(15):1320–5. doi: 10.1016/j.jacc.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Ziegelstein RC. Depression and tako-tsubo cardiomyopathy. Am J Cardiol. 2010;105(2):281–2. doi: 10.1016/j.amjcard.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Barton DA, Dawood T, Lambert EA, et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25(10):2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 50.Baumert M, Lambert GW, Dawood T, et al. QT interval variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol Heart Circ Physiol. 2008;295(3):H962–8. doi: 10.1152/ajpheart.00301.2008. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 52.Lane RD, Reis HT, Peterson DR, Zareba W, Moss AJ. Happiness and stress alter susceptibility to cardiac events in Long QT Syndrome. Ann Noninvasive Electrocardiol. 2009;14(2):193–200. doi: 10.1111/j.1542-474X.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105(23):2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 54.Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121(24):2615–23. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YJ, Chen SA, Tai CT, et al. Role of atrial electrophysiology and autonomic nervous system in patients with supraventricular tachycardia and paroxysmal atrial fibrillation. Journal of the American College of Cardiology. 1998;32(3):732–8. doi: 10.1016/s0735-1097(98)00305-2. [DOI] [PubMed] [Google Scholar]
- 56.Herweg B, Dalal P, Nagy B, Schweitzer P. Power spectral analysis of heart period variability of preceding sinus rhythm before initiation of paroxysmal atrial fibrillation. Am J Cardiol. 1998;82(7):869–74. doi: 10.1016/s0002-9149(98)00494-9. [DOI] [PubMed] [Google Scholar]
- 57.Huang JL, Wen ZC, Lee WL, Chang MS, Chen SA. Changes of autonomic tone before the onset of paroxysmal atrial fibrillation. Int J Cardiol. 1998;66(3):275–83. doi: 10.1016/s0167-5273(98)00241-1. [DOI] [PubMed] [Google Scholar]
- 58.Vikman S, Makikallio TH, Yli-Mayry S, Nurmi M, Airaksinen KE, Huikuri HV. Heart rate variability and recurrence of atrial fibrillation after electrical cardioversion. Ann Med. 2003;35(1):36–42. doi: 10.1080/07853890310004110. [DOI] [PubMed] [Google Scholar]
- 59.Skinner JE, Lie JT, Entman ML. Modification of ventricular fibrillation latency following coronary artery occlusion in the conscious pig. Circulation. 1975;51(4):656–67. doi: 10.1161/01.cir.51.4.656. [DOI] [PubMed] [Google Scholar]
- 60.Skinner JE, Reed JC. Blockade of frontocortical-brain stem pathway prevents ventricular fibrillation of ischemic heart. Am J Physiol. 1981;240(2):H156–63. doi: 10.1152/ajpheart.1981.240.2.H156. [DOI] [PubMed] [Google Scholar]
- 61.Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–53. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 63.Thomas SAFE, Wimbush F, Schron E. Psychological factors and survival in the cardiac arrhythmia suppression trial (CAST): a reexamination. Am J Crit Care. 1997;6(2):116–26. [PubMed] [Google Scholar]
- 64.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 65.Frasure-Smith N, Lespérance F, Talajic M, et al. Anxiety sensitivity moderates prognostic importance of rhythm-control versus rate-control strategies in patients with atrial fibrillation and congestive heart failure: insights from the Atrial Fibrillation and Congestive Heart Failure Trial. Circ Heart Fail. 2012;5(3):322–30. doi: 10.1161/CIRCHEARTFAILURE.111.964122. [DOI] [PubMed] [Google Scholar]
- 66.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev. 2011;16(2):195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 67.Martin JLR, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147–55. doi: 10.1016/j.eurpsy.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Solomon SD, Zelenkofske S, McMurray JJV, et al. Sudden Death in Patients with Myocardial Infarction and Left Ventricular Dysfunction, Heart Failure, or Both. N Engl J Med. 2005;352(25):2581–8. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 69.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 70.Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. Journal of the American College of Cardiology. 2006;48(11):2218–22. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]