Abstract
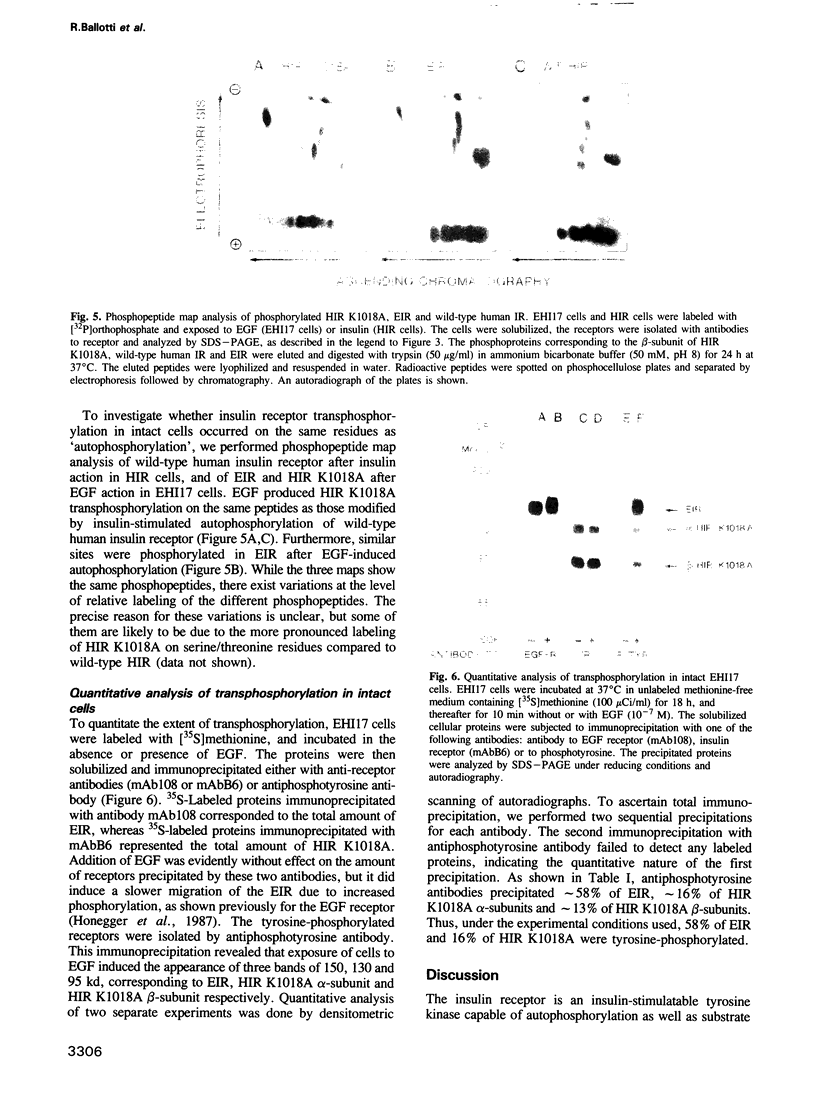
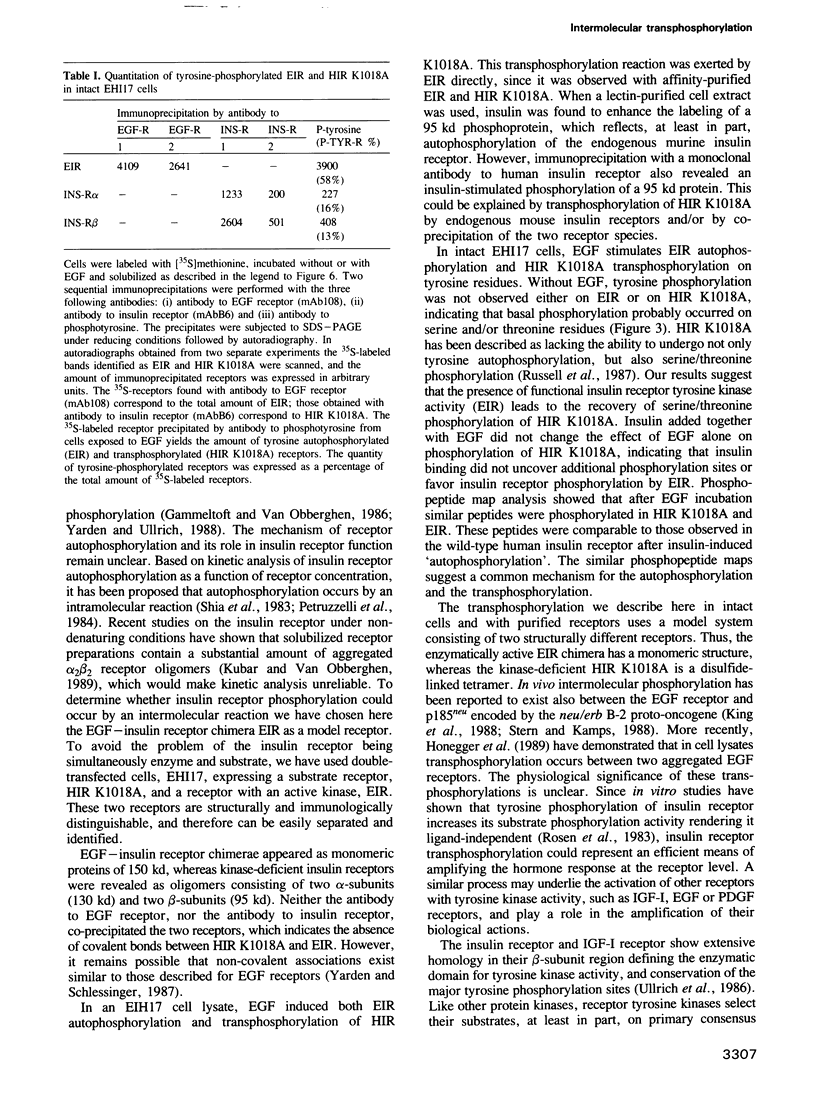
The insulin receptor, a glycoprotein consisting of two extracellular alpha- and two transmembrane beta-subunits, is thought to mediate hormone action by means of its tyrosine-specific protein kinase activity. To explore the mechanism of insulin receptor phosphorylation we have used NIH3T3 cells transfected with two receptor constructs: one encoding a chimeric receptor composed of the extracellular domain of the human EGF receptor and the cytosolic domain of the human insulin receptor beta-subunit, and a second construct encoding a kinase-defiecient human insulin receptor. Stimulation of these cells with EGF induced tyrosine autophosphorylation of the EGF-insulin receptor chimera (150 kd) and tyrosine phosphorylation of the beta-subunit of the kinase-deficient insulin receptor (95 kd). The phosphopeptides of the autophosphorylated cytoplasmic domain of the EGF-insulin receptor chimera were comparable to those of the transphosphorylated beta-subunit of the kinase-deficient insulin receptor and of the wild-type human insulin receptor. When immunoaffinity purified EGF-insulin receptor hybrids and kinase-deficient insulin receptors were used in a cell lysate phosphorylation assay, it was found that addition of EGF produced 32P-labeling of both receptor species. We conclude that EGF acting directly through the EGF-insulin receptor chimera causes transphosphorylation of the kinase-deficient insulin receptor. These data support the notion that autophosphorylation of the insulin receptor may proceed by an intermolecular mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballotti R., Scimeca J. C., Kowalski A., Van Obberghen E. Antiphosphotyrosine antibodies modulate insulin receptor kinase activity and insulin action. Cell Signal. 1989;1(2):195–204. doi: 10.1016/0898-6568(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Bernier M., Laird D. M., Lane M. D. Insulin-activated tyrosine phosphorylation of a 15-kilodalton protein in intact 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1844–1848. doi: 10.1073/pnas.84.7.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Kris R. M., Ullrich A., Schlessinger J. Evidence that autophosphorylation of solubilized receptors for epidermal growth factor is mediated by intermolecular cross-phosphorylation. Proc Natl Acad Sci U S A. 1989 Feb;86(3):925–929. doi: 10.1073/pnas.86.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- King C. R., Borrello I., Bellot F., Comoglio P., Schlessinger J. Egf binding to its receptor triggers a rapid tyrosine phosphorylation of the erbB-2 protein in the mammary tumor cell line SK-BR-3. EMBO J. 1988 Jun;7(6):1647–1651. doi: 10.1002/j.1460-2075.1988.tb02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Kubar J., Van Obberghen E. Oligomeric states of the insulin receptor: binding and autophosphorylation properties. Biochemistry. 1989 Feb 7;28(3):1086–1093. doi: 10.1021/bi00429a024. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Meckling-Hansen K., Nelson R., Branton P., Pawson T. Enzymatic activation of Fujinami sarcoma virus gag-fps transforming proteins by autophosphorylation at tyrosine. EMBO J. 1987 Mar;6(3):659–666. doi: 10.1002/j.1460-2075.1987.tb04805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Taylor S. I. An endogenous substrate for the insulin receptor-associated tyrosine kinase. J Biol Chem. 1985 Apr 10;260(7):4461–4467. [PubMed] [Google Scholar]
- Riedel H., Dull T. J., Honegger A. M., Schlessinger J., Ullrich A. Cytoplasmic domains determine signal specificity, cellular routing characteristics and influence ligand binding of epidermal growth factor and insulin receptors. EMBO J. 1989 Oct;8(10):2943–2954. doi: 10.1002/j.1460-2075.1989.tb08444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. S., Gherzi R., Johnson E. L., Chou C. K., Rosen O. M. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. J Biol Chem. 1987 Aug 25;262(24):11833–11840. [PubMed] [Google Scholar]
- Sadoul J. L., Peyron J. F., Ballotti R., Debant A., Fehlmann M., Van Obberghen E. Identification of a cellular 110 000-Da protein substrate for the insulin-receptor kinase. Biochem J. 1985 May 1;227(3):887–892. doi: 10.1042/bj2270887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia M. A., Rubin J. B., Pilch P. F. The insulin receptor protein kinase. Physicochemical requirements for activity. J Biol Chem. 1983 Dec 10;258(23):14450–14455. [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988 Apr;7(4):995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987 Mar 10;26(5):1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Yu K. T., Werth D. K., Pastan I. H., Czech M. P. src kinase catalyzes the phosphorylation and activation of the insulin receptor kinase. J Biol Chem. 1985 May 10;260(9):5838–5846. [PubMed] [Google Scholar]