Abstract
Background:
Breast cancer is one of the most common cancers worldwide. Alarmingly, the incidence of breast cancer is rising rapidly in India.
Aim:
The present research was focused to assess the role of myricetin; a bioflavonoid in 7,12-dimethylbenzanthracene (DMBA)-induced breast cancer in female Wistar rats.
Materials and Methods:
A total of 36 female Wistar rats (total 6 groups, n = 6 per group) 6 - 8 weeks old, weighing 150 gm were used in the study. DMBA was given at the dose of 7.5 mg/kg subcutaneously in the mammary region once a week for 4 consecutive weeks in group 2. Vincristine was given in the dose of 500 μg/kg intraperitonially every week for 4 consecutive weeks in group 3. Myricetin was given orally in a dose of 50, 100, and 200 mg/kg in group 4, 5, and 6 respectively. The statistical significance of the data was determined using one way analysis of variance and Duncan's multiple range test.
Results:
The result showed that myricetin increased the antioxidant levels in plasma, erythrocyte lysate, and breast tissue and was effective in preventing the oxidative damage induced by the carcinogen DMBA. Myricetin 50, 100, and 200 mg/kg/oral for 120 days treated animal resulted comparable results to that of standard vincristine and control groups.
Conclusions:
Myricetin was found to be either equieffective or more effective than vincristine in all the parameters studied. Myricetin proved the capacity of flavonols to act as antioxidant in cells represents a potential treatment in the field of oncology.
Keywords: Anti oxidants; breast cancer; dimethyl benzanthracene; 7,12-dimethylbenzanthracene; myricetin; vincristine
Introduction
Breast cancer is one of the leading causes of morbidity and mortality among women. Worldwide, it is the second most common type of cancer. The incidence of breast cancer is lowest in less developed countries.[1,2] The higher incidence is attributed to delayed first child birth, shorter duration of breast feeding, family history of breast cancer, early menarche, and late menopause. Westernization of lifestyle, changes in dietary habits, indiscriminate exposures to exogenous estrogens are often considered to be reasons for the higher incidence reported in developing countries. Breast cancer originates from breast tissue most commonly from inner lining of milk ducts or lobules that supply the ducts with milk. The primary route of metastasis is the lymphatic system or blood stream.[3]
Breast cancer can be induced by 7,12-dimethyl benzanthracene (DMBA), a procarcinogen with selectivity for breast cancer in experimental female Wistar rats. It undergoes metabolic activation to carcinogen dihydrodiolepoxide.[4] The carcinogenic and mutagenic activity of DMBA requires metabolic activation by mixed function oxidases located in rat liver microsomes. The dihydrodiolepoxide binds with adenine residues of deoxyribonucleic acid, resulting in mutagenesis and carcinogenesis.[5,6]
The aim of the present study was to evaluate the effect of myricetin on lipid peroxidation in plasma and breast tissue the antioxidant enzymes like thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD) and to compare its efficacy with vincristine a well-known anticancer agent used in the treatment of breast cancer.
Myricetin is a major flavonol distributed ubiquitously in edible plants and is one of the most potent antioxidants of plant origin.[7] The daily intake of myricetin in western diet is estimated to range between 0 and 30 mg with a median of 10 mg.[8] It is abundantly found in Allium cepa, Solanum lycopersicum, Vitis vinifera, Olea europa, Morus alba, Thea sinesis, and Crataegus cuneata. Myricetin is claimed to have antioxidant antiallergic, antiatherogenic, anti-inflammatory, and antiangiogenic actions.[9,10,11,12,13]
Materials and Methods
Chemicals and carcinogen
DMBA was purchased from Sigma chemical company (St. Louis, MO, USA) and myricetin from allergic research group USA. All other chemicals and reagents used were of analytical grade.
Preparation of the drug
Myricetin powder was used as suspension in 0.1% carboxymethyl cellulose and each rat received a daily dose of 50, 100, and 200 mg/kg body weight of myricetin in 2 mL of suspension. DMBA was dissolved in an emulsion of sunflower oil (0.75 mL) and physiological saline (0.25 mL) just prior to use.
Animals and experimental design
A total of 36 female Wistar rats (total 6 groups, n = 6 per group) 6-8 weeks old, weighing 150 gm were randomised into control and experimental group. Group 1: Normal control, given distilled water orally daily. Group 2: Cancer control, DMBA was given at the dose of 7.5 mg/kg subcutaneously in the mammary region once a week for 4 consecutive weeks to induce breast cancer. Group 3: Standard drug (DMBA + vincristine), vincristine was given in the dose of 500 μg/kg intraperitoneal every week for 4 consecutive weeks. Group 4: Test drug low dose (DMBA + myricitin), myricitin 50 mg/kg/wt given orally every day for 16 weeks. Group 5: Test drug medium dose (DMBA + myricitin), Myricitin 100 mg/kg/wt given orally every day for 16 weeks. Group 6: Test drug high dose (DMBA + myricitin), myricitin 200 mg/kg/wt given orally every day for 16 weeks. Myricitin was given along with DMBA on the same day in respective groups. We used vincristine as standard drug as it does not act through estrogen receptors unlike other drugs. Cancer was induced by a chemical carcinogen and staging of breast cancer was not done. The study was started after getting approval from the institutional animal ethical committee. The rats were housed in the animal house, Rajah Muthiah Medical College, Annamalai University in an air conditioned room with a 12 h light and dark cycle. The animals were provided with vitamin enriched pellet diet consisting of 23% wheat flour, 60% roasted Bengal gram powder, 5% skimmed milk powder, 4% casein, 4% refined oil, salt mixture with 4% starch, and choline. The rats were maintained in accordance with the Indian National Law on animal care and use (Reg No 190/2007/CPCSEA). The total period of study was 16 weeks. The weights of the rats were recorded at the beginning of the experiment, at weekly intervals and at the end of the study period. The animals were sacrificed after overnight fasting at the end of 16 weeks.
Blood collection and preparation of erythrocyte lysate
After sacrificing the rats, blood was collected into 5.0 mL heparinized tubes and plasma was separated by centrifugation at 800 × g for 5 min at 4°C. After separation of plasma, the buffy coat was removed and packed cells were washed thrice with ice cold physiological saline containing glucose (5.5 mM). Erythrocyte lysate was prepared by lysing a known volume of erythrocytes by addition of two volumes of distilled water to packed erythrocytes and were centrifuged at 3000 × g for 10 min at 4°C to separate the erythrocyte lysate. Mammalian erythrocytes have a limited life span and great sensitivity to oxidative damage. The presence of high concentration of iron in erythrocytes makes it vulnerable to oxidative damage.[14] Reactive oxygen species interacts with ferric ion to form ferrous ion which generates a very highly reactive hydroxyl ion that is critical in causing molecular damage.[15]
Preparation of tissue homogenate
The breast tissue was excised immediately and washed with chilled isotonic saline. The tissue homogenate were prepared in ice cold 0.1M Tris-HCL buffer at pH 7.2. The homogenate was centrifuged and the supernatant was used for assays.
Estimation of antioxidant enzyme activity and TBARS
TBARS in tissues and plasma were determined by the method of Ohkwava.[16] The level of superoxide dismutase was estimated by the method of Kakkar et al.[17] The results were compared with vincristine group.
Statistical analysis
The statistical significance of the data was determined using one-way analysis of variance and the significant difference between the treatments groups were evaluated by Duncan's multiple range test. The results were considered as statistically significant at P < 0.05.
Results
General observation
No change in body weight and growth rate was observed in the treated groups. The final body weight (g) body weight gain/loss (g) food intake (g/day), food efficiency (body weight gain (g/day)/food intake) remained the same with no change in all the experimental rats there were no signs of toxicity and cachexia was not observed even in DMBA-treated rats. Generally, cachexia is regulated by a variety of factors involving duration and severity of cancer.
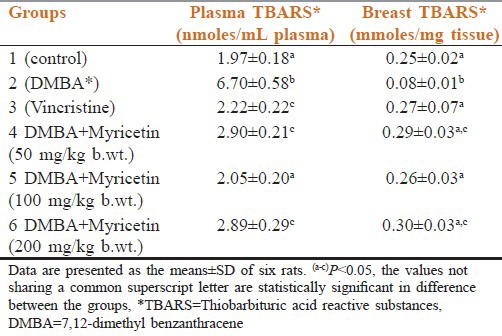
Plasma TBARS
When compared with DMBA group (group 2) the plasma TBARS levels of all the myricetin treated groups that is group 4 myricetin 50 mg/kg body weight, group 5 myricetin 100 mg/kg body weight, group 6 myricetin 200 mg/kg body weight and vincristine (group 3) are statistically significant. But group 4 and group 6 are statistically not significant when compared with vincristine group, but group 5 is statistically significant when compared with vincristine group but not significant when compared with control group.
Breast TBARS
When compared with DMBA group (group 2) the breast TBARS levels of all myricetin-treated groups that is group 4 myricetin 50 mg/kg bodyweight, group 5 myricein 100 g/kg bodyweight, group 6 myricetin 200 mg/kg bodyweight, and vincistine group are statistically significant. Group 5 and vincristine (group 3) are not statistically significant when compared with control group and group 4 when compared with group 6 is also statistically not significant [Table 1].
Table 1.
Effect of myricetin and 7,12-dimethyl benzanthracene on thiobarbituric acid reactive substances levels of control and experimental groups
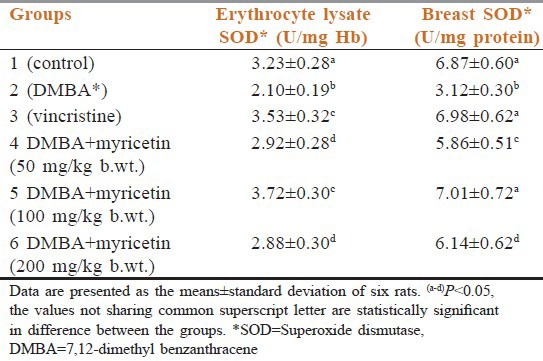
Lysate SOD
When compared with DMBA (group 2) the myricetin-treated groups that is group 4 myricetin 50 mg/kg body weight, group 5 myricetin 100 mg/kg body weight and group 6 myricetin 200 mg/kg bodyweight, and vincristin group 3 are statistically significant. Group 4 when compared with group 6 is not statistically significant. But the vincristine group when compared with group 5 is also statistically is not significant.
Breast SOD
When compared with DMBA (group 2) the myricetin treated groups that is group 4 myricetin 50 mg/kg body weight, group 5 myricetin 100 mg/kg body weight and group 6 myricetin 200 mg/kg bodyweight and vincristin (group 3) are statistically significant. Vincristine group when compared with group 5 is statistically not significant [Table 2].
Table 2.
Effect of myricetin and 7,12-dimethyl benzanthracene on superoxide dismutase activities of control and experimental groups
Discussion
In this present study, the breast cancer efficacy of myricetin was determined by its action on plasma and breast tissues; the parameters such as lipid peroxidation, erythrocyte lysate superoxide dismutase were studied. The results were compared with vincristine, a standard drug used in the management of breast cancer.
When compared with the control group the inhibition of DMBA-induced elevated TBARS in plasma was significantly higher in 100 mg/kg of myricetin than vincristine (P > 0.05). DMBA administered rats resulted in decreased lipid peroxidation in breast tissue. This inverse relationship between concentration of lipid peroxidation and the rate of cellular proliferation and differentiation in a carcinogen treated animal is well-documented.[18]
In breast cells the TBARS levels at myricetin 50 mg/kg dose was more effective than the other two doses. All three dose ranges of myricetin were equieffective to vincristine (P > 0.05) and were comparable to the control value indicating that 100% recovery was achieved by myricetin. This proves that myricetin completely neutralized the toxic effects of DMBA on breast cancer cells and useful indication, can be utilized in the prevention of cancer.[19]
The erythrocyte lysate SOD levels (in plasma) in DMBA-treated rats were reduced when compared with the control group. The SOD levels were replenished significantly by myricetin at all three dose levels and increased erythrocyte lysate SOD by myricetin at 100 mg/kg dose was maximum and were comparable to the experimental group treated with vincristine. Myricetin at 50 mg/kg was almost equieffective to myricetin 100 mg/kg dose.
In breast cells DMBA resulted in significant reduction of breast SOD when compared with the control group. Myricetin-treated groups showed an increase of this antioxidant in breast tissue at 100; 200 mg/kg and was not significant at 50 mg dose, the anticancer effects were comparable to vincristine and control groups. SOD being the primary antioxidant plays a greater role in the protection of cells against the oxidative damage induced by DMBA.[20,21,22]
Myricetin elevated the level of both erythrocyte lysate and breast SOD to near normal values these results proved preventive factor of breast cancer. All the above results were correlated in our macroscopical pictures [Figure 1] and also ulcers, tumors [Figures 2 and 3] produced in rat treated with DMBA which were attenuated in the treatment groups. Thus, myricetin was found to be either equieffective or more effective than vincristine in all the parameters studied. Myricetin proved the capacity of flavonols to act as antioxidant in cells definitely represents a potential in the field of oncology.[23,24,25,26,27]
Figure 1.
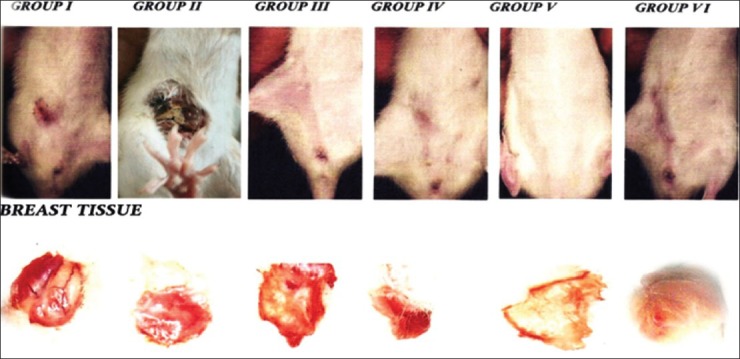
Macroscopic changes
Figure 2.
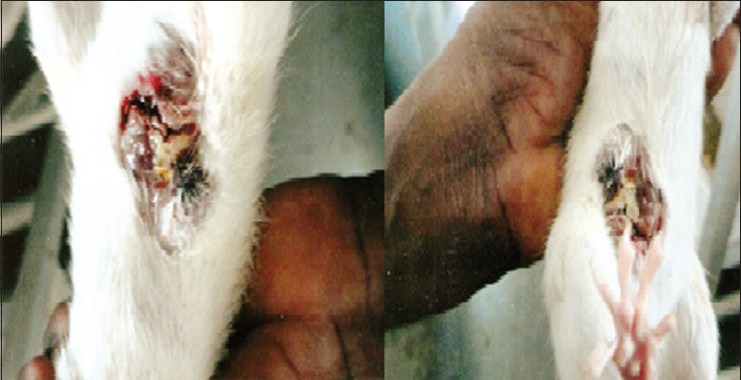
Ulcer produced in the rat treated with 7,12-dimethyl benzanthracene
Figure 3.

Tumors produced in the rat treated with 7,12-dimethyl benzanthracene
Conclusion
The capacity of flavonols to act as antioxidant in cells definitely represents a potential in the field of oncology. Although basic research in cancer biology has provided new targets into a sharp focus, new and novel approaches to cancer prevention and treatment are needed. Myricetin is given orally while vincristine can be given only through parenteral route. In this preclinical study orally administered myricetin on DMBA-induced breast cancer was evaluated and compared with vincristine. Myricetin increased the level of plasma erythrocyte lysate, breast superoxide dismutase, and inhibited the plasma lipid peroxidation. The effect was either comparable or superior to the action of vincristine.
Acknowledgment
My sincere thanks to Dr. Vanitha Samuel, Dr. Swadhin Ranjan Behera, Dr. Uday Kumar.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Reddy NS, Nirmala P, Chidambaram N, Kumar PA. Quercetin in dimethyl benzanthracene induced breast cancer in rats. Am J Pharmacol Toxicol. 2012;7:68–72. [Google Scholar]
- 2.Notani PN. Global variation in cancer incidence and mortality. Curr Sci. 2001;81:465–74. [Google Scholar]
- 3.Sariego J. Breast cancer in the young patient. Am Surg. 2010;76:1397–400. [PubMed] [Google Scholar]
- 4.Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. Mechanism of 7,12-Dimethylbenz [a] anthracene-induced immunotoxicity: Role of metabolic activation at the target organ. Jpn J Pharmacol. 2001;86:302–9. doi: 10.1254/jjp.86.302. [DOI] [PubMed] [Google Scholar]
- 5.Yang SK, Dower WV. Metabolic pathways of 7,12-dimethylbenz [a] anthracene in hepatic microsomes. Proc Natl Acad Sci U S A. 1975;72:2601–5. doi: 10.1073/pnas.72.7.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Segura LM, Bojda-Diolez F, Lenoir V, Naftolin F, Kerdelhue B. Estrogen-like effects of the mammary carcinogen 7,12-dimethylbenz (alpha) anthracene upon hypothalamic neuronal membranes. Brain Res Bull. 1992;28:625–8. doi: 10.1016/0361-9230(92)90113-c. [DOI] [PubMed] [Google Scholar]
- 7.Erdman JW, Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, et al. Flavonoids and heart: Proceedings of the ILSI North America Flavonoids Workshop, May 31-June, 2006, Washington, DC. J Nutr. 2007;137:718–37S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 8.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, et al. Daily quercetin supplementation dose dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–21. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 9.Filipe P, Haigle J, Silva JN, Freitas J, Fernandes A, Mazière JC, et al. Anti-and pro-oxidant effects of quercetin in copper-induced low density lipoprotein oxidation Quercetin as an effective antioxidant against pro-oxidant effects of urate. Eur J Biochem. 2004;271:1991–9. doi: 10.1111/j.1432-1033.2004.04111.x. [DOI] [PubMed] [Google Scholar]
- 10.Marozzi FJ, Jr, Kocialski AB, Malone MH. Studies on the antihistaminic effects of thymoquinone, thymohydroquinone and quercetin. Arzneimittelforschung. 1970;20:1574–7. [PubMed] [Google Scholar]
- 11.Janisch KM, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Radic Res. 2004;38:877–84. doi: 10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- 12.Wadsworth TL, Koop DR. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol. 1999;57:941–9. doi: 10.1016/s0006-2952(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 13.Chan MM, Mattiacci JA, Hwang HS, Shah A, Fong D. Synergy between ethanol and grape polyphenols, quercetin and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem Pharmacol. 2000;60:1539–48. doi: 10.1016/s0006-2952(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 14.Glass GA, Gershon H, Gershon D. The effect of donor and cell age on several characteristics of rat erythrocytes. Exp Hematol. 1983;11:987–95. [PubMed] [Google Scholar]
- 15.Masotti L, Casali E, Galeotti T. Lipid peroxidation in tumour cells. Free Radic Biol Med. 1988;4:377–86. doi: 10.1016/0891-5849(88)90089-5. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H, Onishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 18.Navarro J, Obsrador E, Carretero J, Petschen I, Avino J, Perez P, et al. Changes in glutathione status and the antioxidant system in blood and in cancer cells associated with tumour growth in vivo. Free Radic Biol Med. 1999;26:410–8. doi: 10.1016/s0891-5849(98)00213-5. [DOI] [PubMed] [Google Scholar]
- 19.Das U. A radical approach to cancer. Med Sci Monit. 2002;8:RA79–92. [PubMed] [Google Scholar]
- 20.Elucheri S, Overley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, et al. CuZn SOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–80. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y. Free radicals antioxidant enzymes and carcinogenesis. Free Radic Biol Med. 1990;8:583–99. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 22.Oberley LW, Oberley TD. Role of antioxidant enzymes in the cancer phenotype. In: Clerch LB, Massaro D, editors. Oxygen, Gene Expression and Cellular Function. Washington: Marcel Dekker; 1997. pp. 279–308. [Google Scholar]
- 23.Laxmi A, Subramanian S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7, 12-dimethylbenz (a) anthracene induced mammary carcinoma in experimental rats. Biochimie. 2013 doi: 10.1016/j.biochi.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Skrajnowska D, Bobrowska-Korczak B, Tokarz A, Bialek S, Jezierska E, Makowska J. Copper and resveratrol attenuates serum catalase, glutathione peroxidase, and element values in rats with DMBA-induced mammary carcinogenesis. Biol Trace Elem Res. 2013;156:271–8. doi: 10.1007/s12011-013-9854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Assis S, Wang M, Jin L, Bouker KB, Hilakivi-Clarke LA. Exposure to excess estradiol or leptin during pregnancy increases mammary cancer risk and prevents parity-induced protective genomic changes in rats. Cancer Prev Res (Phila) 2013;6:1194–211. doi: 10.1158/1940-6207.CAPR-13-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandakumar N, Rengarajan T, Balamurugan A, Balasubramanian M. Modulating effects of hesperidin on key carbohydrate-metabolizing enzymes, lipid profile, and membrane-bound adenosine triphosphatases against 7,12-dimethylbenz (a) anthracene-induced breast carcinogenesis. Hum Exp Toxicol. 2013 doi: 10.1177/0960327113485252. [DOI] [PubMed] [Google Scholar]
- 27.Yang SH, Suh JH, Lee MG, Kim SH. Pharmacokinetic drug interactions between ondansetron and tamoxifen in female Sprague-Dawley rats with DMBA-induced mammary tumor. Anticancer Res. 2013;33:521–8. [PubMed] [Google Scholar]


