Introduction
Lung cancer is one of the leading causes of cancer-related death in many parts of the world, including India.[A] According to Globocan 2008 data, the estimated yearly incidence of lung cancer in India is 58567 cases along with mortality of 52269 cases.[1] Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer[2] with 75% cases presenting as locally advanced or metastatic disease at diagnosis.[3] The survival of NSCLC patients in the metastatic or advanced setting (stage IIIB/IV) is improving significantly.[4,5]
Today, we know that NSCLC is a heterogeneous disease with the majority of patients having specific driver mutation abnormalities, such as EGFR, PIK3CA, BRAF and KRAS. For example, activating mutations in the epidermal growth factor receptor (EGFR) gene and sensitivity to anti-EGFR tyrosine kinase inhibitors (e.g. erlotinib and gefitinib) correlate with an improvement in the response rates, progression-free survival and quality of life in the first- or second-line setting,[6,7,8] when treated with an oral tyrosine kinase inhibitor (TKI) like gefitinib. In 2007, a gene rearrangement between the EML4 (echinoderm microtubule-associated protein-like 4) and ALK (anaplastic lymphoma kinase) genes were identified as driver genetic abnormalities in a small but significant fraction of patients with NSCLCs,[9] EML4-ALK rearrangements having been reported in approximately 3–7% of adenocarcinoma NSCLCs.
Crizotinib (Pfizer), an orally bioavailable, small molecule ALK and hepatocyte growth factor receptor (HGF receptor, proto-oncogene c-Met; MET) inhibitor, is an active drug specifically shown to benefit patients with ALK-rearranged NSCLC. Based on data demonstrating a high response rate and good tolerability, the U.S. Food and Drug Administration (FDA) have approved this drug for the treatment of patients with locally advanced or metastatic ALK-rearranged NSCLC when tested with the FDA approved companion test.[9]
This is the report of our first patient treated with crizotinib procured on Pfizer's compassionate program. Subsequently, the drug that has obtained marketing approval in India is available freely.
Patient History
A 44-year-old female never smoker presented in May 2011 with complaint of swelling in the left neck, shortness of breath during mild exertion, persistent low grade fever, intermittent chest pain, cough and expectorant of 10 weeks duration. She had no significant past illnesses, and was not on any medication and had no family history of malignancy. She had been treated elsewhere with antibiotics as well as with seven days of empirical anti-tubercular treatment before presenting to our institution. The given treatment did not provide any relief in the symptoms.
Clinical examination revealed that she was in fair general condition with the Eastern Cooperative Oncology Group (ECOG) performance status score of 2. Chest auscultation showed decreased air entry in the base of the right lung. The baseline workup of complete blood count, renal function test, liver function test, serum electrolytes and blood sugar was normal. Electrocardiography (ECG) and echocardiography (ECHO) did not show any abnormality. positron emission tomography-computed tomography (PET-CT) scan suggested a moderate right side pleural effusion with a large, fluorodeoxyglucose (FDG) avid heterogeneously enhancing, soft tissue mass lesion (5.7 × 7.6 × 4.7) in the middle lobe of right lung (likely primary) abutting and compressing the superior vena cava and right interior chest wall. Mild FDG avid diffuses interstitial thickening. multiple enlarged, FDG avid cervical and mediastinal lymph nodes, liver metastasis (Segment 4A) and right adrenal and aorto caval lymph nodes were also seen [Figure 1]. Biopsy of left supraclavicular lymph node was reported as metastatic poorly differentiated carcinoma. Immunohistochemistry (IHC) suggested adenocarcinoma (TTF and CK7 positive) without any EGFR mutation.
Figure 1.
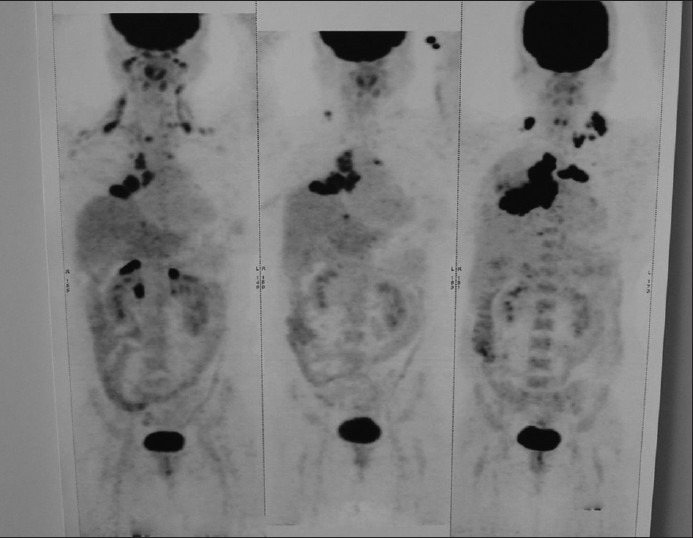
Baseline PET Scan of the patient showing extensive disease
Brain magnetic resonance imaging (MRI) revealed no metastases. The patient was diagnosed to have stage IV (T3N3M1B) adenocarcinoma of the lung. She was commenced with six cycles of pemetrexed and cisplatin based doublet chemotherapy from July 2011 up to February 2012.
At that time, a PET-CT scan showed stable disease by Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Thereafter the patient was given seven cycles of pemetrexed maintenance therapy, which continued till August 2012. The patient became symptomatic with dyspnea and chest pain. Chest X-ray showed re-appearance of pleural effusion. A follow up of PET-CT scan was indicative of disease progression with increase in the size of the lung mass as well as size and number of supraclavicular and mediastinal lymph nodes [Figure 2].
Figure 2.
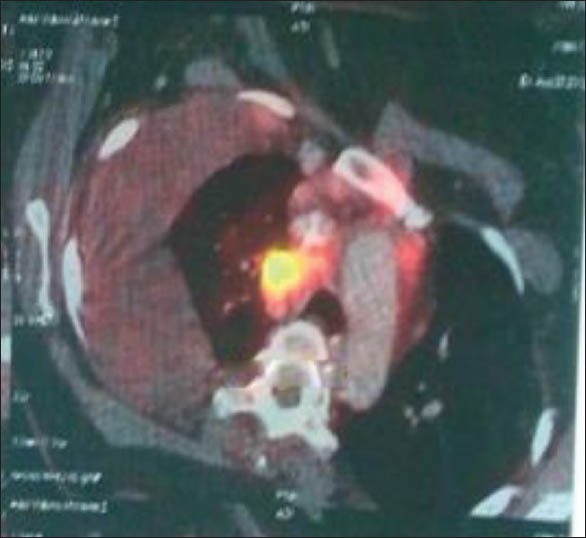
PET CT before treatment with Crizotinib
Subsequently, her original lung biopsy specimen was tested for ALK mutation. The Vysis Break apart test confirmed the patient to be positive for ALK mutation. She was therefore prescribed crizotinib and enrolled in Pfizer's compassionate program. After four months of crizotinib therapy her symptoms improved and PET-CT scan showed reduction in the size of lung mass as well its FDG avidity. The mediastinal lymph nodes, parenchyma opacity in left upper lobe and the right pleural deposits had regressed completely. The right adnexal lesion and the supraclavicular lymph nodes have decreased in size as well as FDG avidity. The sternal lesions were no longer found to be FDG avid and indicated sclerosis [Figure 3].
Figure 3.

PET CT after response to Crizotinib
Crizotinib was very well tolerated. Side effects noted were mild weakness, mild fatigue and visual disturbances that lasted for two days and did not impair her routine activities.
Discussion
This is the first report of efficacy of crizotinib in an Indian patient, the response being obtained with minimal side effects.
Crizotinib was granted accelerated approval by US FDA based on the response rates demonstrated in the phase I and II studies, along with its favorable safety profile for the treatment of ALK mutated advanced lung cancer.[9] Crizotinib is therefore unique in obtaining approval in conjunction with a companion diagnostic assay, the Vysis ALK Break-Apart FISH Probe Kit (Abbott Molecular, Des Plaines, IL). It is likely that this expensive test will be replaced by the cheaper ALK immunohistochemistry (IHC) in the near future.[10]
The recently published phase III study showed that crizotinib is superior to standard chemotherapy in patients with previously treated, advanced non-small-cell lung cancer having ALK rearrangement. The median progression-free survival was 7.7 months in the crizotinib group and 3.0 months in the chemotherapy group (hazard ratio for progression or death with crizotinib, 0.49; 95% confidence interval [CI], 0.37 to 0.64; P < 0.001). The response rates were 65% (95% CI, 58 to 72) with crizotinib, as compared to 20% (95% CI, 14 to 26) with chemotherapy (P < 0.001). Adverse events reported in this study in the crizotinib arm were visual disorder, gastrointestinal side effects, and elevated liver aminotransferase levels. This compares favorably against the commonly reported adverse events of fatigue, alopecia, and dyspnea in the chemotherapy arm.[11] The response in our patient is consistent with the result of this phase III study data.
Newly diagnosed ALK-positive patients can potentially benefit from crizotinib even in the first-line setting. Hence, the question is whether our patient would have had a better outcome and quality of life if we had commenced crizotinib at the initial presentation? The current guidelines from the National Comprehensive Cancer Network (NCCN) recommend the testing for EGFR as well as ALK mutations in all patients with advanced, non-squamous NSCLC[12] as well as crizotinib as one option in the first-line therapy of advanced, ALK-positive NSCLC. This is based on five randomized studies that have shown the superiority of EGFR inhibitors versus platinum doublets in prolonging the progression free-survival (PFS) in the first-line setting. Whether the choice would be optimal or not will be ascertained once we have the results of the currently ongoing trial comparing the use of crizotinib in the first-line setting versus standard chemotherapy.[10]
Conclusion
The patient we have presented benefitted from crizotinib therapy, the report that is consistent with the ALK mutated lung cancer patients. Besides being an oral drug, effective in patients with ALK-positive NSCLC, its favorable safety profile makes crizotinib an effective and convenient therapeutic option for outpatient treatment of ALK-positive NSCLC.
Acknowledgment
I am thankful to Pfizer for providing Crizotinib on compassionate grounds for this patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- A.A Parikh P, Puri T. Personalized medicine: Lung Cancer leads the way. Indian J Cancer. 2013;50:77–9. doi: 10.4103/0019-509X.117005. [DOI] [PubMed] [Google Scholar]
- 1.Globocan 2008 data. [Last accessed on 2012 Sep 26]. Available from: http://globocan.iarc.fr/factsheet.asp .
- 2.Konopa K, Jassem J. The role of pemetrexed combined with targeted agents for non-small cell lung cancer. Curr Drug Targets. 2010;11:2–11. doi: 10.2174/138945010790030965. [DOI] [PubMed] [Google Scholar]
- 3.Reade CA, Ganti AK. EGFR targeted therapy in non-small cell lung cancer: Potential role of cetuximab. Biologics. 2009;3:215–24. [PMC free article] [PubMed] [Google Scholar]
- 4.American cancer society. Detailed guide: Lung cancer - non-small cell. Non-small cell lung cancer survival rates by stage. [Last accessed on 2012 Mar 22]. Available from http://www.cancer.org/Cancer/LungCancer-Non-SmallCell/DetailedGuide/index .
- 5.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi L, Jänne PA. Crizotinib for ALK-rearranged non-small cell lung cancer: A new targeted therapy for a new target. Clin Cancer Res. 2012;18:3737–42. doi: 10.1158/1078-0432.CCR-11-2393. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Engelmann JA. ALK in lung cancer: Past, present, and future. J Clin Oncol. 2013;31:1105–11. doi: 10.1200/JCO.2012.44.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New 12 National comprehensive cancer network clinical practice guidelines in oncology: Non Small cell lung cancer. Version. 2.2013. [Last accessed on 2013 Mar 12]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf .
- 12.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]


