Abstract
The human organic cation transporter 1 (OCT1) is a polyspecific transporter involved in the uptake of positively charged and neutral small molecules in the liver. To date, few endogenous compounds have been identified as OCT1 substrates; more importantly, the effect of drugs on endogenous substrate transport has not been examined. In this study, we established monoamine neurotransmitters as substrates for OCT1, specifically characterizing serotonin transport in human embryonic kidney 293 cells. Kinetic analysis yielded a Km of 197 micomolar and a Vmax of 561 pmol/mg protein/minute for serotonin. Furthermore, we demonstrated that serotonin uptake was inhibited by diphenhydramine, fluoxetine, imatinib, and verapamil, with IC50 values in the low micromolar range. These results were recapitulated in primary human hepatocytes, suggesting that OCT1 plays a significant role in hepatic elimination of serotonin and that xenobiotics may alter the elimination of endogenous compounds as a result of interactions at the transporter level.
Introduction
It is widely accepted that the liver plays an essential role in removing drugs, toxins, and other xenobiotics from circulation in the human body. The liver is also involved in the clearance of several endogenous compounds, including circulating monoamine neurotransmitters (Chu et al., 1999; Eisenhofer et al., 2004). As early as 1967, it was demonstrated that the liver is capable of removing more than 70% of the serotonin in portal blood by filtration and metabolism (Thomas and Vane, 1967; Tyce, 1990). Several endogenous compounds, particularly the monoamines, are positively charged at physiologic pH and therefore require transport proteins to facilitate crossing the plasma membrane into hepatocytes. However, the high-affinity dopamine, norepinephrine, and serotonin (SERT) transporters are not expressed in the liver (Ramamoorthy et al., 1993; Eisenhofer, 2001), leaving open to question the transporter(s) responsible for monoamine clearance in the human liver.
Organic cation transporters (OCTs), a subset of the solute carrier SLC22 superfamily of transporters, are polyspecific transporters that mediate the uptake of a wide variety of positively and neutrally charged compounds (Koepsell, 2013). Recently, broad substrate specificity combined with tissue localization, primarily in detoxifying organs, has coupled OCTs to the elimination of several drugs and toxins as well as endogenous compounds (Koepsell et al., 2007; Nies et al., 2011). Expressed primarily in the liver, OCT1 is involved in the hepatic elimination of numerous small molecules and has been linked to the transport of biogenic amines. Previously, the rat organic cation transporter, rOCT1, was shown to transport catecholamines (Busch et al., 1996; Breidert et al., 1998; Jonker and Schinkel, 2004). Additionally, human OCT1 has been associated with neurotransmitter transport; however, there is some controversy in the literature as to substrate specificity and transport efficiency (Kerb et al., 2002; Lips et al., 2005; Amphoux et al., 2006).
Moreover, the interference of drugs with endogenous neurotransmitter clearance, particularly at the transporter level, has not been investigated. Although it is becoming increasingly necessary to identify transporter-mediated drug-drug interactions in the modern age of polypharmacy, little is currently known about the effect therapeutics have on the transport and elimination of endogenous substrates. To elucidate the interactions of common medications and other xenobiotics with endogenous substrates of human OCT1, transport and inhibition of the biogenic amines dopamine, norepinephrine, and serotonin were characterized in both human embryonic kidney 293 (HEK293) cells and primary human hepatocytes.
Materials and Methods
Radiolabeled [3H]-1-methyl-4-phenylpyridinium iodide (MPP+, 85.0 Ci/mmol) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Radiolabeled [3H]-dihydroxyphenylethylamine (dopamine, 46.0 Ci/mmol), [3H]-norepinephrine hydrochloride (14.9 Ci/mmol), and [3H]-hydroxytryptamine creatinine sulfate (serotonin, 28.3 Ci/mmol) were purchased from Perkin Elmer (Boston, MA). Sodium chloride was purchased from Amresco (Solon, OH). HEPES sodium salt (NaCl) and potassium chloride (KCl) were purchased from Fisher Scientific (Fair Lawn, NJ). Imatinib (Gleevec) was purchased from Toronto Research Chemicals (North York, ON, Canada). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture.
HEK293 cells were grown at 37°C under humidified 5% CO2 in Dulbeccos’s modified Eagle’s medium (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA). Cells were seeded on 24-well plates coated with 0.1 mg/ml poly-d-lysine (Invitrogen) at a density of 175,000 cells/well. Twenty-four hours after plating, cells were transiently transfected with pcDNA5/FRT (“empty vector,” Invitrogen) or pcDNA5/FRT-hOCT1 (OCT1) using the FuGENE HD transfection reagent (Promega, Madison, WI). Transfection mixtures contained 0.25 µg plasmid cDNA, 0.75 µl FuGENE HD, and Opti-MEM I + GlutaMAX–I (Invitrogen) to a final volume of 25 µl/well. Transfected cells were incubated at 37°C as already described for 24 hours before use.
Freshly isolated human hepatocytes seeded on collagen-coated 24-well plates at a density of 350,000 cells/well were obtained from the KUMC Department of Pharmacology, Toxicology and Therapeutics Cell Isolation Core Laboratory. Hepatocytes were isolated from livers of male and female patients, aged 19–57.
Western Blotting.
Cultured cells and hepatocytes were lysed in hypotonic homogenization solution (1 mM NaCl, 5 mM Tris-HCl, pH 7.5) with protease inhibitor cocktail (Roche, Indianapolis, IN) using a tissue homogenizer on ice. Lysates were subjected to centrifugation at 900 g for 10 minutes, after which supernatant was collected and subjected to further centrifugation at 10,000 g for 20 minutes. The resulting pellets containing protein-enriched plasma membrane were resuspended in hypotonic homogenization solution including protease inhibitor, and protein concentration was determined by BCA Protein Assay (Pierce, Rockford, IL). Proteins (0.5 µg for HEK lysates, 50 µg for hepatocyte lysates) were resolved by SDS-PAGE on 4%–15% Mini-PROTEAN TGX polyacrylamide gels (Bio-Rad, Hercules, CA). Subsequently, proteins were transferred to nitrocellulose membrane. Immunoblotting was performed using standard procedures, with anti-OCT1 primary antibody (Novus Biologicals, Littleton, CO) at a concentration of 1:2000, and HRP-conjugated secondary antibody at 1:10,000. Proteins were detected with ECL substrate (Pierce).
Transport Assays.
HEK293 uptake assays were performed 24 hours post-transfection at 37°C. Media were aspirated, and cells were washed three times with warm (37°C) uptake buffer (116 mM NaCl, 5.3 mM KCl, 1 mM NaH2PO4, 0.8 mM MgSO4, 5.5 mM d-glucose, and 20 mM HEPES sodium salt, pH 7.4). After washing, cells were incubated with 200 µl of uptake buffer containing radiolabeled substrate and sufficient unlabeled substrate to reach specified substrate concentration, as well as putative inhibitors (drugs) where indicated, for the specified amount of time. Transport for all substrates was measured within the initial linear time range (at 30 seconds for MPP+ and at 5 minutes for all other substrates). Uptake was terminated by washing four times with ice-cold uptake buffer. To quantify uptake, cells were lysed with 300 µl/well 1% TX-100 in phosphate-buffered saline, of which 200 µl was transferred to 24-well scintillation plates (Perkin Elmer, Waltham, MA) and mixed with 750 µl of Optiphase Supermix scintillation cocktail (Perkin Elmer). Radioactivity was measured using a MicroBeta Trilux liquid scintillation counter (Perkin Elmer). The remaining cell lysate was used for protein determination for normalization by BCA Protein Assay with bovine serum albumin standards (Pierce). Transporter-specific uptake (net uptake) was determined by subtracting uptake into empty vector cells from the uptake into OCT1-expressing cells. Each data point represents the average of three independent experiments, in which each condition was performed in triplicate.
Transport assays were performed with hepatocytes between 20 and 24 hours after plating according to the method described above for HEK293 cells, using uptake buffer modified from Jigorel et al. (2005) (136 mM NaCl, 5.3 mM KCl, 1.1 mM KH2PO4, 0.8 mM MgSO4, 1.8 mM CaCl2, 11 mM d-glucose, 10 mM HEPES, pH 7.4). Each data point represents the average of three independent experiments, in which each condition was performed in duplicate.
Kinetic Analysis.
For kinetic analysis of serotonin transport, net uptake values from each individual experiment (n = 3) were averaged, and the mean was analyzed by nonlinear regression and fit to the Michaelis-Menten equation to obtain Vmax and Km using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA).
IC50 Determination.
For determination of IC50 values, the net uptake from each individual experiment (n = 3) was converted to percent of control. These values were averaged, and the mean values were plotted in GraphPad Prism 6 and subjected to nonlinear regression.
Statistical Analysis.
Statistical significance was calculated using two-tailed unpaired t tests. A P value < 0.05 was considered significant.
Results
Functional Characterization of Human OCT1 in Transiently Transfected HEK293 Cells.
To establish that our model of transient expression of OCT1 in HEK293 cells was functional, transport of the model cation [3H]-1-methyl-4-phenylpyridinium (MPP+) was characterized. Initial time dependencies at low (0.5 µM) and high (100 µM) concentrations demonstrated uptake of MPP+ to be linear through 1 minute, and kinetics experiments yielded a Km of 35 ± 7 µM and a Vmax of 500 ± 36 pmol/mg of protein/min (data not shown). This compares well with previously published values (Gründemann et al., 2003; Umehara et al., 2007) and suggested that our model of OCT1 transport was functional and suitable for further experiments.
OCT1-Mediated Neurotransmitter Transport.
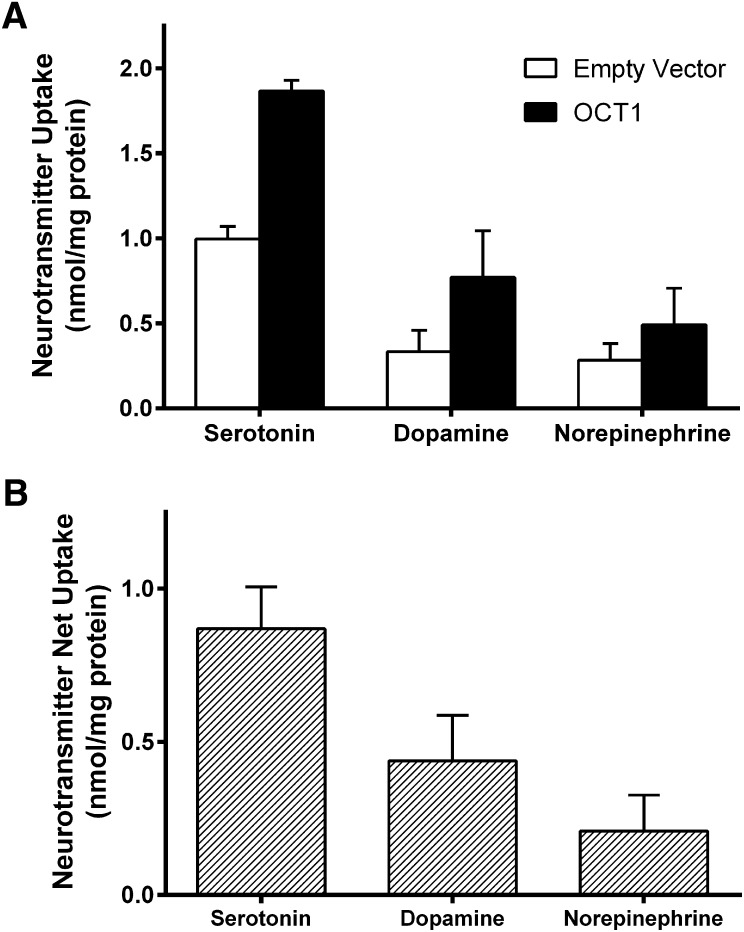
To study the effect of drugs on OCT1-mediated uptake of endogenous substrates, it was first necessary to identify suitable endogenous substrates. To establish monoamine neurotransmitters as substrates of OCT1, transport of 100 µM (0.8 µCi/ml) [3H]-dopamine, [3H]-norepinephrine, and [3H]-serotonin was measured in pcDNA5/FRT- (empty vector) and OCT1-transfected HEK293 cells at 37°C for 5 minutes. Uptake of these monoamines was significantly higher in OCT1-expressing cells than in empty vector cells for all three neurotransmitters (Fig. 1A). OCT1-mediated transport (net uptake) was obtained by subtracting the uptake into empty vector cells from that of OCT1-expressing cells (Fig. 1B). At a time point of 5 minutes, serotonin appeared to be the most efficiently transported substrate. Dopamine uptake was approximately 50% that of serotonin, and norepinephrine uptake was approximately 20% compared with serotonin. The observed norepinephrine uptake was minimal and therefore unlikely to be physiologically relevant. Dopamine uptake by OCT1, although significant, is again unlikely to be germane to the liver because of the low circulating levels of this neurotransmitter. Serotonin, however, is found at high concentrations in the gut; consequently, portal blood levels are significantly higher than arterial blood, reportedly as much as 3-fold (Toh 1954; Gershon and Tack, 2007), making it the best candidate of the three neurotransmitters to be transported by OCT1 in vivo. Because of this, and the data suggesting that serotonin was the superior neurotransmitter substrate for OCT1, serotonin was selected as the model endogenous substrate for further study.
Fig. 1.
Transport of serotonin, dopamine, and norepinephrine by human OCT1. (A) HEK293 cells transiently transfected with empty vector and OCT1 plasmid were incubated with 100 µM serotonin, dopamine, or norepinephrine (0.8 µCi/ml) at 37°C for 5 minutes. (B) OCT-mediated uptake (net uptake) was determined by subtracting uptake into empty vector cells from that of OCT1-expressing cells. Mean + S.D. of three independent experiments is shown.
Kinetic Characterization of Serotonin Uptake by OCT1.
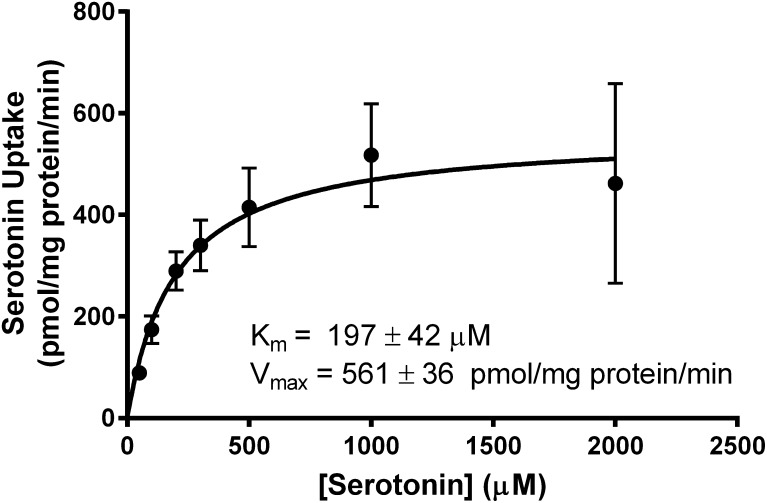
To further characterize serotonin transport, uptake kinetics assessment were performed. Serotonin influx was assessed in empty vector- and OCT1-expressing cells after incubation with increasing concentrations of [3H]-serotonin, from 50 µM to 2 mM (0.8–2.0 µCi/ml), for 5 minutes at 37°C. Net uptake was fit to the Michaelis-Menten equation to yield a Km of 197 ± 42 µM and Vmax of 561 ± 36 pmol/mg protein/min (Fig. 2). These data suggest that serotonin is transported by OCT1 with affinity and capacity comparable to other OCT1 substrates.
Fig. 2.
Kinetics of OCT1-mediated serotonin transport. Empty vector- and OCT1-transfected HEK293 cells were incubated with increasing concentrations of serotonin, ranging from 50 µM (0.8 µCi/ml) to 2 mM (2.0 µCi/ml), for 5 minutes at 37°C. Net uptake was fit to the Michaelis-Menten equation to obtain the affinity constant, Km = 197 ± 42 µM, and maximum transport velocity, Vmax = 561 ± 36 pmol/mg of protein/min. Values ± S.E.M. are the result of three independent experiments.
Inhibition of Serotonin Transport.
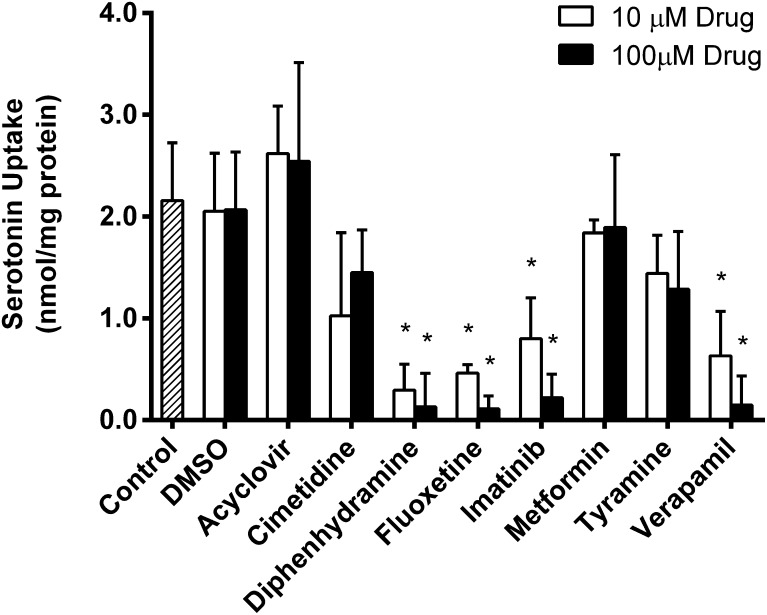
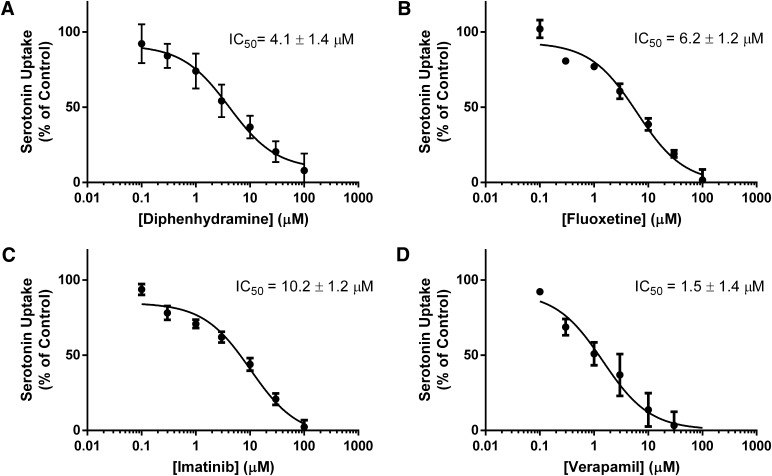
Because OCT1 has moderate affinity for serotonin, it is feasible to hypothesize that OCT1-mediated uptake of serotonin could be inhibited by drugs or other xenobiotics. To evaluate this possibility, the following eight compounds were selected: acyclovir, cimetidine, diphenhydramine, fluoxetine, imatinib, metformin, tyramine, and verapamil. These potential inhibitors were selected based on previous reports that showed interactions with OCT1 or serotonin-transporting proteins (Sitte et al., 1998; Dresser et al., 2001; Nies et al., 2011). Uptake of 200 µM (1.2 µCi/ml) serotonin was measured for 5 minutes at 37°C in the absence and presence of 10 µM and 100 µM of each compound (Fig. 3). Although there appeared to be a trend of inhibition for almost all the drugs screened, serotonin uptake was significantly (P < 0.05) inhibited by diphenhydramine, fluoxetine, imatinib, and verapamil at both concentrations (Fig. 3). Inhibition by these four drugs was characterized further by determining IC50 values for serotonin transport (Fig. 4). Transfected HEK293 cells were incubated with 100 µM serotonin and increasing concentrations of each drug for 5 minutes at 37°C. Net uptake was converted to the percent of control and was analyzed by nonlinear regression to obtain IC50 values. IC50 values for diphenhydramine, fluoxetine, imatinib and verapamil were determined to be 4.1 ± 1.4 µM, 6.2 ± 1.2 µM, 10.2 ± 1.2 µM, and 1.5 ± 1.4 µM respectively. With IC50 values in the low micromolar range, all four drugs appear to be fairly potent inhibitors of OCT1-mediated serotonin transport.
Fig. 3.
Inhibition of serotonin transport by common drugs. Transport of 200 µM serotonin (1.2 µCi/ml) was measured in HEK293 cells transfected with empty vector or OCT1 plasmid cDNA in the presence of 10 µM or 100 µM drug for 5 minutes at 37°C. Dimethylsulfoxide controls were included at concentrations equivalent to those of imatinib preparations. Mean + S.D. of net uptake is shown. *P < 0.05.
Fig. 4.
Concentration-dependent inhibition of serotonin uptake by OCT1. In HEK293 cells transfected with empty vector or OCT1, uptake of 100 µM serotonin (0.4 µCi/ml) was measured in the presence of increasing concentrations of (A) diphenhydramine, (B) fluoxetine, (C) imatinib, or (D) verapamil. Uptake values are expressed as percent of control and the mean + S.D. of three independent experiments were subjected to nonlinear regression analysis for IC50 determination.
Serotonin Transport and Inhibition in Primary Human Hepatocytes.
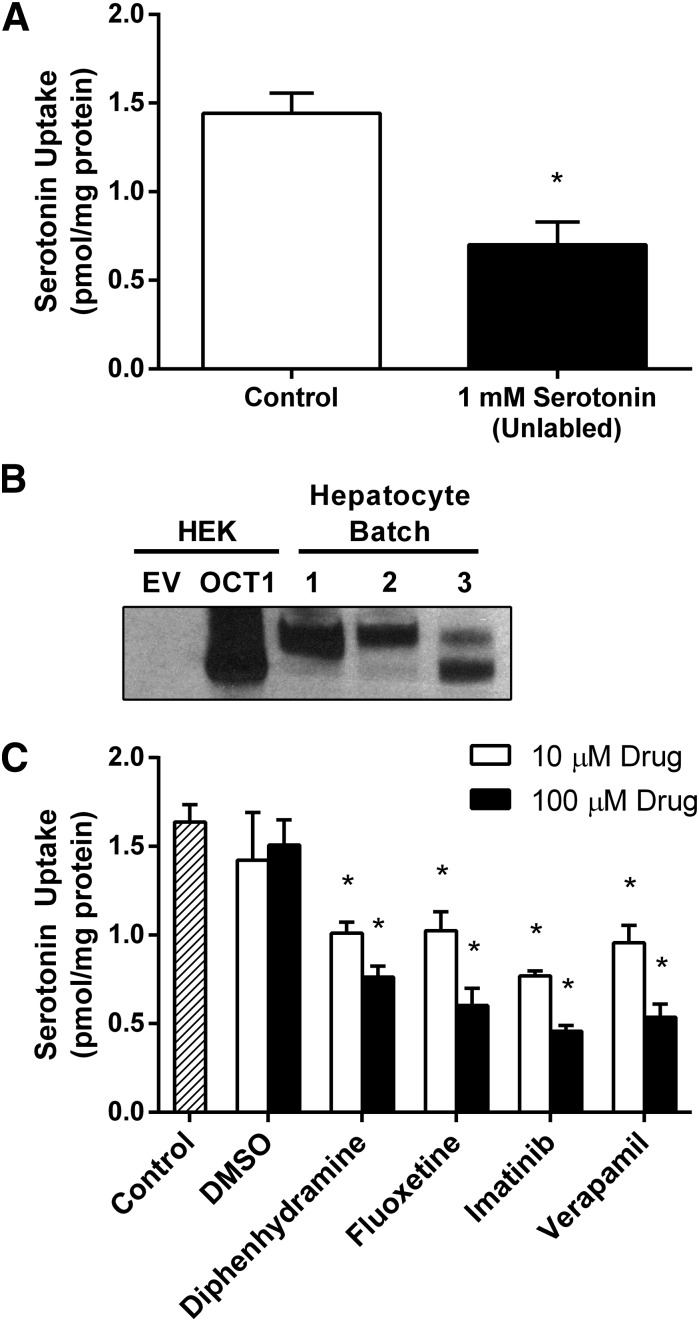
To determine whether the observed transport and inhibition of serotonin is likely to be physiologically relevant, uptake and inhibition studies were conducted with freshly isolated and plated primary human hepatocytes. First, serotonin uptake into hepatocytes was assessed by incubating cells with 1.2 µCi/ml (42 nM) [3H]-serotonin at 37°C for 5 minutes (Fig. 5A). Significant serotonin uptake was observed, even at this very low substrate concentration. To establish that the observed uptake of serotonin was carrier-mediated, hepatocytes were incubated with [3H]-serotonin in the presence of 1 mM unlabeled serotonin intended to inhibit any carrier-mediated transport of radiolabeled serotonin. Addition of 1 mM unlabeled serotonin decreased the uptake of [3H]-serotonin by more than 50% (Fig. 5A), suggesting that most of the serotonin uptake in hepatocytes is carrier-mediated. To confirm expression of OCT1 in the hepatocytes used to measure serotonin transport, Western blotting was performed using a commercially available anti-OCT1 antibody. HEK-EV and HEK-OCT1 lysates were used as controls (Fig. 5B). For each batch of hepatocytes, OCT1 expression levels were high, and total OCT1 expression was consistent between batches. Next, the ability of the four drugs to inhibit serotonin uptake into hepatocytes was investigated. Hepatocytes were incubated with [3H]-serotonin, as described already, in the presence of 10 µM and 100 µM diphenhydramine, fluoxetine, imatinib, or verapamil (Fig. 5C). Each drug significantly (P < 0.05) inhibited serotonin uptake into hepatocytes at both concentrations.
Fig. 5.
Serotonin transport in primary human hepatocytes. (A) Between 20 and 24 hours after plating, freshly isolated primary human hepatocytes were incubated for 5 minutes with 1.2 µCi/ml (radiolabeled only, 42 nM) serotonin. Carrier-mediated transport was inhibited by the presence of 1 mM unlabeled serotonin (right). (B) OCT1 expression was confirmed in hepatocytes used to measure serotonin uptake. 50 µg of protein isolated from hepatocytes was resolved by SDS-PAGE and blotted with anti-OCT1 antibody; 0.5 µg of protein isolated from HEK293 cells transfected with empty vector (HEK-EV) and OCT1 (HEK-OCT1) was included as control. (C) Drug-mediated inhibition of serotonin transport was conducted as in (A) in the presence of 10 µM and 100 µM diphenhydramine, fluoxetine, imatinib, or verapamil. Dimethylsulfoxide controls were included at sufficient concentrations to match those in imatinib preparations. Net uptake is represented as the mean + S.D. of three independent experiments.*P < 0.05.
Discussion
This study demonstrates that serotonin is a substrate of human OCT1 and, more importantly, that OCT1-mediated serotonin transport can be inhibited by several commonly prescribed drugs. Taken together, these findings suggest that hepatic clearance of endogenous substrates, including biogenic amines, can be affected by small molecule therapeutics at the transporter level. Our results illustrate that diphenhydramine, fluoxetine, imatinib, and verapamil inhibit serotonin uptake in OCT1-expressing HEK293 cells and in primary human hepatocytes.
The liver has been established as a key organ in the elimination of endogenous compounds, including monoamine neurotransmitters, from the body. As previously mentioned, it is unlikely that OCT1 plays a major role in the uptake of dopamine or norepinephrine in the liver because of both low circulating concentrations and relatively low transport, as documented in Fig. 1. However, the same cannot be said for serotonin. Approximately 95% of the body’s serotonin is synthesized and stored in the gut, where it is released to initiate peristalsis and activate secretory reflexes (Gershon and Tack, 2007). While the SERT is expressed in the gut and functions in the reabsorption of released serotonin, a significant portion of serotonin reaches portal circulation; in fact, serotonin concentrations in portal blood can be as much as threefold higher than in arterial blood (Toh, 1954). Additionally, the liver is responsible for the removal of up to 70% of the serotonin from portal blood (Thomas and Vane, 1967; Tyce, 1990). Given that SERT is not expressed in the liver (Ramamoorthy et al., 1993), we hypothesized that OCT1 may be one transporter involved in serotonin uptake in the liver. Previous studies have investigated serotonin transport by OCT1, although with conflicting results. Kerb et al. (2002) demonstrated serotonin transport by human OCT1 as a test probe for comparison of wild-type and polymorphic transporters. Conversely, Amphoux et al. (2006) reported that human OCT1 showed very little specific transport of serotonin, among other neurotransmitters; they also failed to show OCT3-mediated transport of several monoamines known to be OCT3 substrates, and those that were transported yielded Km values much higher than established elsewhere (Duan and Wang, 2010), suggesting a potential flaw in the expression system or other methods used. In any case, we have demonstrated OCT1-mediated serotonin transport in both HEK293 cells and in hepatocytes, confirming that serotonin is indeed a substrate of OCT1, and our results indicate that OCT1 is an important element in the elimination of serotonin from portal blood.
Although this study suggests that OCT1 is a key component in hepatic elimination of serotonin, we cannot completely rule out minor contributions of other cation transporters that have also been reported to transport biogenic amines. Organic cation transporter 3 (OCT3) and plasma membrane monoamine transporter (PMAT) are both high-capacity neurotransmitter transporters and together constitute the “uptake2” mechanism for monoamine clearance in the brain (Wu et al., 1998; Zhou et al., 2007). Studies have shown that, in addition to OCT1, both OCT3 and PMAT are expressed in the liver, though at very low levels. PMAT mRNA levels were nearly undetectable in the liver (Engel et al., 2004), suggesting its function pertains primarily to the brain, and hepatic OCT3 mRNA levels were shown to be between 6% and 30% that of OCT1 (Nies et al., 2009; Chen et al., 2010). Additionally, the affinities of PMAT and OCT3 for serotonin are relatively low (Duan and Wang, 2010) compared with that established in this study for OCT1 (Fig. 2). Combined, the low expression levels and relative transport affinities of OCT3 and PMAT suggest that they are likely minor components of serotonin uptake in hepatocytes, further solidifying the role of OCT1 in serotonin elimination in the liver.
Because hepatic clearance of serotonin may rely heavily on OCT1 transport, it is important to understand the effects that drug interaction with OCT1 has on serotonin uptake in the liver. The inhibition screen performed in this study (Fig. 3) indicates that several drugs are capable of inhibiting serotonin transport by OCT1. Diphenhydramine, fluoxetine, imatinib, and verapamil all significantly inhibited serotonin uptake in both HEK293 cells and human hepatocytes (Figs. 3–5). Furthermore, even though uptake was not significantly inhibited, a trend of inhibition was observed for cimetidine and metformin, as well as the notorious monoamine neurotransmitter transporter inhibitor tyramine. Additionally, IC50 values determined for diphenhydramine, fluoxetine, imatinib, and verapamil were all in the low micromolar range, 4.1 ± 1.4 µM, 6.2 ± 1.2 µM, 10.2 ± 1.2 µM, and 1.5 ± 1.4 µM respectively (Fig. 4). Given that substrate-dependent inhibition has been reported for OCT2 (Belzer et al., 2013) and multidrug and toxin extrusion protein 1 (Martínez-Guerrero and Wright, 2013), comparison of the IC50 values obtained in the present study with previously reported values obtained using other substrates might give some insight into how different substrates are handled by OCT1. Previously, diphenhydramine and fluoxetine inhibited MPP+ uptake with IC50 values of 3.4 µM and 2.8 µM, respectively (Müller et al., 2005; Haenisch et al., 2012), and verapamil inhibited TEA+ transport with an IC50 of 2.9 µM (Zhang et al., 1998). These values are comparable to the IC50 values we obtained for OCT1-mediated uptake of serotonin, which suggests that serotonin and the two model substrates MPP+ and TEA+ are transported in a similar way by OCT1. In contrast, previous reports demonstrated that imatinib inhibited OCT1-mediated metformin uptake with an IC50 value of 1.5 µM (Minematsu and Giacomini, 2011), whereas in our study imatinib inhibited serotonin uptake with an IC50 value of 10.2 µM. This confirms the substrate-dependent inhibition seen with other organic cation transporters (Belzer et al., 2013; Martínez-Guerrero and Wright, 2013) and suggests that these transporters have complex binding pockets, with different interaction sites for different substrates. Regardless, these novel results are strong evidence that xenobiotics may inhibit serotonin uptake in the liver, potentially hindering proper hepatic clearance of serotonin in vivo, and it is plausible that these same effects would be seen with other endogenous substrates as well.
Undoubtedly, drug-mediated inhibition of serotonin transport would be dependent on drug concentrations achieved in vivo. Peak plasma drug concentrations have been shown to reach 66 ng/ml (0.3 µM) for diphenhydramine, 302 ng/ml (1 µM) for fluoxetine, 3380 ng/ml (6.8 µM) for imatinib, and 400 ng/ml (0.9 µM) for verapamil (Blyden et al., 1986; Peng et al., 2004; http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=d534867a-e4ef-46ce-b61d-857387ce450a#section-12.3; http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8834bbd7-c7ae-41e0-9d54-0178f76886ae#nlm34090-1). These plasma levels are not necessarily high enough to elicit significant OCT1 inhibition. However, in this case, because OCT1 is localized to the liver, portal blood drug concentrations are likely more relevant. Although drug concentrations have not been measured in portal blood, it is likely that portal drug concentrations are significantly higher than those measured in plasma, given that all of the drugs tested are dosed orally. This suggests that these drugs may indeed affect serotonin transport in the liver.
Inhibition of serotonin uptake in the liver may bear several implications, including a potential increase in circulating serotonin levels as well as locally increased extracellular serotonin concentrations in the liver. Increased serotonin levels in the circulation have the potential to lead to certain specific toxicities, including alterations in the blood coagulation cascade. When activated, platelets degranulate, releasing a variety of factors, including serotonin, to initiate coagulation (Troxler et al., 2007). Recently, SSRIs have been shown to exert an anticoagulative effect as a result of decreases in serotonin levels in the blood (Bottlender et al., 1998). Conversely, drug-mediated inhibition of serotonin uptake in the liver could increase blood serotonin levels, potentially resulting in hypercoagulopathy. In addition, increases in circulating serotonin have the potential to cause acute changes in blood pressure due to its vasoactive properties (Rapport, 1949; Page and McCubbin, 1953). Furthermore, serotonin has been implicated in changes in renal blood flow (Blackshear et al., 1986), which might suggest a role for increased circulating serotonin levels in kidney dysfunction. Additionally, the importance of serotonin in both liver injury and regeneration has recently been established. Work completed by Pierre-Alain Clavien and others exposed a critical function of serotonin in liver regeneration (Lesurtel et al., 2006; Nocito et al., 2007b). Conversely, serotonin has also been implicated in mitochondrial dysfunction and hepatocellular injury in nonalcoholic steatohepatitis (Nocito et al., 2007a). Inhibition of serotonin transport by OCT1 could potentiate the pathogenesis of nonalcoholic steatohepatitis. In contrast, increased serotonin levels in resection patients could be beneficial for liver regeneration. In any case, drug-mediated inhibition of serotonin uptake by OCT1 may well have important physiologic consequences.
In conclusion, we have established that serotonin is a viable substrate for human OCT1 and, more importantly, that commonly prescribed drugs inhibit its uptake. Diphenhydramine, fluoxetine, imatinib, and verapamil significantly inhibited serotonin transport in both HEK293 cells and in primary human hepatocytes. Moreover, these compounds appear to be fairly potent inhibitors of serotonin uptake, as IC50 values were determined to be in the low micromolar range for all four drugs. The implications of serotonin uptake inhibition in the liver may be several, and the results of this study bring new insights to the potential for drugs and other xenobiotics to interfere with endogenous substrate transport and elimination.
Acknowledgments
The authors thank Dr. Kathy Giacomini (University of California, San Francisco, San Francisco, CA) for the kind gift of the hOCT1 expression vector and Dr. Sylvie Kandel (Xenotech, LLC, Lenexa, KS) for helpful scientific discussions. The hepatocytes used in this study were derived from samples collected and provided by the KUMC Department of Pharmacology, Toxicology and Therapeutics Hepatocyte Core laboratory and the Kansas University Liver Center.
Abbreviations
- MPP+
1-methyl-4-phenylpyridinium
- OCT
organic cation transporter
- PMAT
plasma membrane monoamine transporter
- rOCT
rat organic cation transporter
- SERT
serotonin reuptake transporter
Authorship Contributions
Participated in research design: Boxberger, Hagenbuch, Lampe.
Conducted experiments: Boxberger.
Performed data analysis: Boxberger, Hagenbuch, Lampe.
Wrote or contributed to the writing of the manuscript: Boxberger, Hagenbuch, Lampe.
Footnotes
This work was supported by the National Institutes of Health Institute of General Medical Sciences [Grant R01-GM077335] and [Grant P20 GM103549-07], National Center for Research Resources [Grant P20-RR021940], and National Institute of Environmental Health and Sciences [Grant T32 ES007079-26A2]; and the Kansas IDeA Network of Biomedical Research Excellence [Grant QH846868-K-INBRE].
The study is sponsored by the Kansas University Medical Center, Department of Pharmacology, Toxicology and Therapeutics Biospecimen Core laboratory and the Liver Center at Kansas University Medical Center.
References
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. (2006) Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 50:941–952 [DOI] [PubMed] [Google Scholar]
- Belzer M, Morales M, Jagadish B, Mash EA, Wright SH. (2013) Substrate-dependent ligand inhibition of the human organic cation transporter OCT2. J Pharmacol Exp Ther 346:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear JL, Orlandi C, Hollenberg NK. (1986) Serotonin and the renal blood supply: role of prostaglandins and the 5HT-2 receptor. Kidney Int 30:304–310 [DOI] [PubMed] [Google Scholar]
- Blyden GT, Greenblatt DJ, Scavone JM, Shader RI. (1986) Pharmacokinetics of diphenhydramine and a demethylated metabolite following intravenous and oral administration. J Clin Pharmacol 26:529–533 [DOI] [PubMed] [Google Scholar]
- Bottlender R, Dobmeier P, Möller HJ. (1998) [The effect of selective serotonin-reuptake inhibitors in blood coagulation]. Fortschr Neurol Psychiatr 66:32–35 [DOI] [PubMed] [Google Scholar]
- Breidert T, Spitzenberger F, Gründemann D, Schömig E. (1998) Catecholamine transport by the organic cation transporter type 1 (OCT1). Br J Pharmacol 125:218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. (1996) Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett 395:153–156 [DOI] [PubMed] [Google Scholar]
- Chen L, Pawlikowski B, Schlessinger A, More SS, Stryke D, Johns SJ, Portman MA, Chen E, Ferrin TE, Sali A, et al. (2010) Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics 20:687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CA, Sindelar DK, Neal DW, Cherrington AD. (1999) Hepatic and gut clearance of catecholamines in the conscious dog. Metabolism 48:259–263 [DOI] [PubMed] [Google Scholar]
- Dresser MJ, Leabman MK, Giacomini KM. (2001) Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J Pharm Sci 90:397–421 [DOI] [PubMed] [Google Scholar]
- Duan H, Wang J. (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 335:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G. (2001) The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther 91:35–62 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349 [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. (2004) Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279:50042–50049 [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132:397–414 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Hahne C, Berkels R, Schömig E. (2003) Agmatine is efficiently transported by non-neuronal monoamine transporters extraneuronal monoamine transporter (EMT) and organic cation transporter 2 (OCT2). J Pharmacol Exp Ther 304:810–817 [DOI] [PubMed] [Google Scholar]
- Haenisch B, Drescher E, Thiemer L, Xin H, Giros B, Gautron S, Bönisch H. (2012) Interaction of antidepressant and antipsychotic drugs with the human organic cation transporters hOCT1, hOCT2 and hOCT3. Naunyn Schmiedebergs Arch Pharmacol 385:1017–1023 [DOI] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Bertrand M, Fardel O. (2005) Functional expression of sinusoidal drug transporters in primary human and rat hepatocytes. Drug Metab Dispos 33:1418–1422 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Schinkel AH. (2004) Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3). J Pharmacol Exp Ther 308:2–9 [DOI] [PubMed] [Google Scholar]
- Kerb R, Brinkmann U, Chatskaia N, Gorbunov D, Gorboulev V, Mornhinweg E, Keil A, Eichelbaum M, Koepsell H. (2002) Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics 12:591–595 [DOI] [PubMed] [Google Scholar]
- Koepsell H. (2013) The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med 34:413–435 [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24:1227–1251 [DOI] [PubMed] [Google Scholar]
- Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. (2006) Platelet-derived serotonin mediates liver regeneration. Science 312:104–107 [DOI] [PubMed] [Google Scholar]
- Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, Ermert L, Kummer W, Koepsell H. (2005) Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol 33:79–88 [DOI] [PubMed] [Google Scholar]
- Martínez-Guerrero LJ, Wright SH. (2013) Substrate-dependent inhibition of human MATE1 by cationic ionic liquids. J Pharmacol Exp Ther 346:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minematsu T, Giacomini KM. (2011) Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther 10:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Lips KS, Metzner L, Neubert RHH, Koepsell H, Brandsch M. (2005) Drug specificity and intestinal membrane localization of human organic cation transporters (OCT). Biochem Pharmacol 70:1851–1860 [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Damme K and Schwab M (2011) Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol 201:105–167. [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, Zanger UM, Keppler D, Schwab M, Schaeffeler E. (2009) Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50:1227–1240 [DOI] [PubMed] [Google Scholar]
- Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, Renner EL, Clavien PA. (2007a) Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology 133:608–618 [DOI] [PubMed] [Google Scholar]
- Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. (2007b) Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology 45:369–376 [DOI] [PubMed] [Google Scholar]
- Page IH, McCubbin JW. (1953) The variable arterial pressure response to serotonin in laboratory animals and man. Circ Res 1:354–362 [DOI] [PubMed] [Google Scholar]
- Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, Sawyers CL, Rosamilia M, Ford J, Lloyd P, et al. (2004) Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 22:935–942 [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. (1993) Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA 90:2542–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MM. (1949) Serum vasoconstrictor (serotonin) the presence of creatinine in the complex; a proposed structure of the vasoconstrictor principle. J Biol Chem 180:961–969 [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. (1998) Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem 71:1289–1297 [DOI] [PubMed] [Google Scholar]
- Thomas DP, Vane JR. (1967) 5-hydroxytryptamine in the circulation of the dog. Nature 216:335–338 [DOI] [PubMed] [Google Scholar]
- Toh CC. (1954) Release of 5-hydroxytryptamine (serotonin) from the dog’s gastrointestinal tract. J Physiol 126:248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler M, Dickinson K, Homer-Vanniasinkam S. (2007) Platelet function and antiplatelet therapy. Br J Surg 94:674–682 [DOI] [PubMed] [Google Scholar]
- Tyce GM. (1990) Origin and metabolism of serotonin. J Cardiovasc Pharmacol 16 (Suppl 3):S1–S7 [PubMed] [Google Scholar]
- Umehara KI, Iwatsubo T, Noguchi K, Kamimura H. (2007) Comparison of the kinetic characteristics of inhibitory effects exerted by biguanides and H2-blockers on human and rat organic cation transporter-mediated transport: Insight into the development of drug candidates. Xenobiotica 37:618–634 [DOI] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. (1998) Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem 273:32776–32786 [DOI] [PubMed] [Google Scholar]
- Zhang L, Schaner ME, Giacomini KM. (1998) Functional characterization of an organic cation transporter (hOCT1) in a transiently transfected human cell line (HeLa). J Pharmacol Exp Ther 286:354–361 [PubMed] [Google Scholar]
- Zhou M, Engel K, Wang J. (2007) Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem Pharmacol 73:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]