Abstract
PC12 cells were manipulated in such a way as to permit the study of differentiation-specific responses independently from proliferative responses. Cells were starved for serum then exposed to nerve growth factor (NGF) or serum. Following addition of serum, cells incorporated thymidine in a synchronous manner. Subsequent to the wave of DNA synthesis, the cell number increased approximately two-fold. Addition of NGF to serum-starved cultures had no measurable effect on either parameter. Neurite outgrowth was more rapid and extensive and appearance of Na+ channels, measured as saxitoxin binding sites, more rapid than when NGF was added to exponentially-growing cells. Epidermal growth factor receptors were heterologously down-regulated by NGF with similar kinetics under both conditions. Induction of the proto-oncogene c-fos by NGF was also greater in the serum-starved cells than in exponentially-growing cultures. These results indicated that serum starvation resulted in synchronisation of the cultures and that NGF action may be cell cycle-specific. Analysis of the cellular response to NGF at different times during the cell cycle showed that c-fos was induced in the G1 phase but not in S or G2. Fluorescence-activated cell sorter analysis demonstrated that addition of NGF to exponentially-growing cells, resulted in their accumulation in a G1-like state. With regard to the study of the mechanism of NGF action, these results illustrate that measurements of NGF effects on specific components in the signal transduction pathway may be confounded by the use of exponentially-growing cultures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein D. E., Greene L. A. Nerve growth factor has both mitogenic and antimitogenic activity. Dev Biol. 1982 Dec;94(2):477–482. doi: 10.1016/0012-1606(82)90364-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Dahmer M. K., Perlman R. L. Insulin and insulin-like growth factors stimulate deoxyribonucleic acid synthesis in PC12 pheochromocytoma cells. Endocrinology. 1988 May;122(5):2109–2113. doi: 10.1210/endo-122-5-2109. [DOI] [PubMed] [Google Scholar]
- Doherty P., Mann D. A., Walsh F. S. Cholera toxin and dibutyryl cyclic AMP inhibit the expression of neurofilament protein induced by nerve growth factor in cultures of naive and primed PC12 cells. J Neurochem. 1987 Dec;49(6):1676–1687. doi: 10.1111/j.1471-4159.1987.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Fujita K., Lazarovici P., Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989 Mar;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greene L. A. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978 Sep;78(3):747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P. W., Landreth G. E., Layer P., Ignatius M., Shooter E. M. Nerve growth factor-induced differentiation of PC12 cells: evaluation of changes in RNA and DNA metabolism. J Neurosci. 1981 Apr;1(4):368–379. doi: 10.1523/JNEUROSCI.01-04-00368.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G., Dickens G., End D. The induction of ornithine decarboxylase by nerve growth factor and epidermal growth factor in PC12 cells. J Neurochem. 1981 Aug;37(2):342–349. doi: 10.1111/j.1471-4159.1981.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff K., End D., Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J Cell Biol. 1981 Jan;88(1):189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
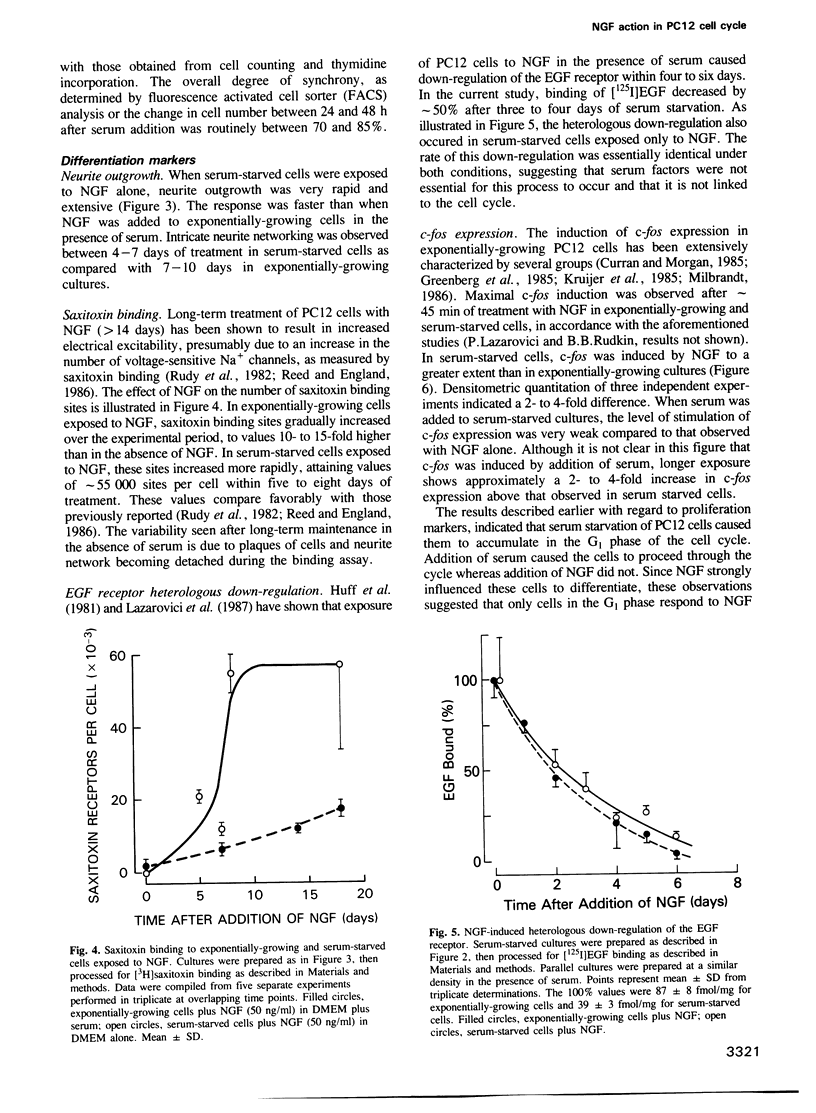
- Lazarovici P., Dickens G., Kuzuya H., Guroff G. Long-term, heterologous down-regulation of the epidermal growth factor receptor in PC12 cells by nerve growth factor. J Cell Biol. 1987 Jun;104(6):1611–1621. doi: 10.1083/jcb.104.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovici P., Levi B. Z., Lelkes P. I., Koizumi S., Fujita K., Matsuda Y., Ozato K., Guroff G. K-252a inhibits the increase in c-fos transcription and the increase in intracellular calcium produced by nerve growth factor in PC12 cells. J Neurosci Res. 1989 May;23(1):1–8. doi: 10.1002/jnr.490230102. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Claude P. Nerve growth factor is a mitogen for cultured chromaffin cells. Nature. 1985 Oct 17;317(6038):632–634. doi: 10.1038/317632a0. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monard D. Role of protease inhibition in cellular migration and neuritic growth. Biochem Pharmacol. 1987 May 1;36(9):1389–1392. doi: 10.1016/0006-2952(87)90103-1. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
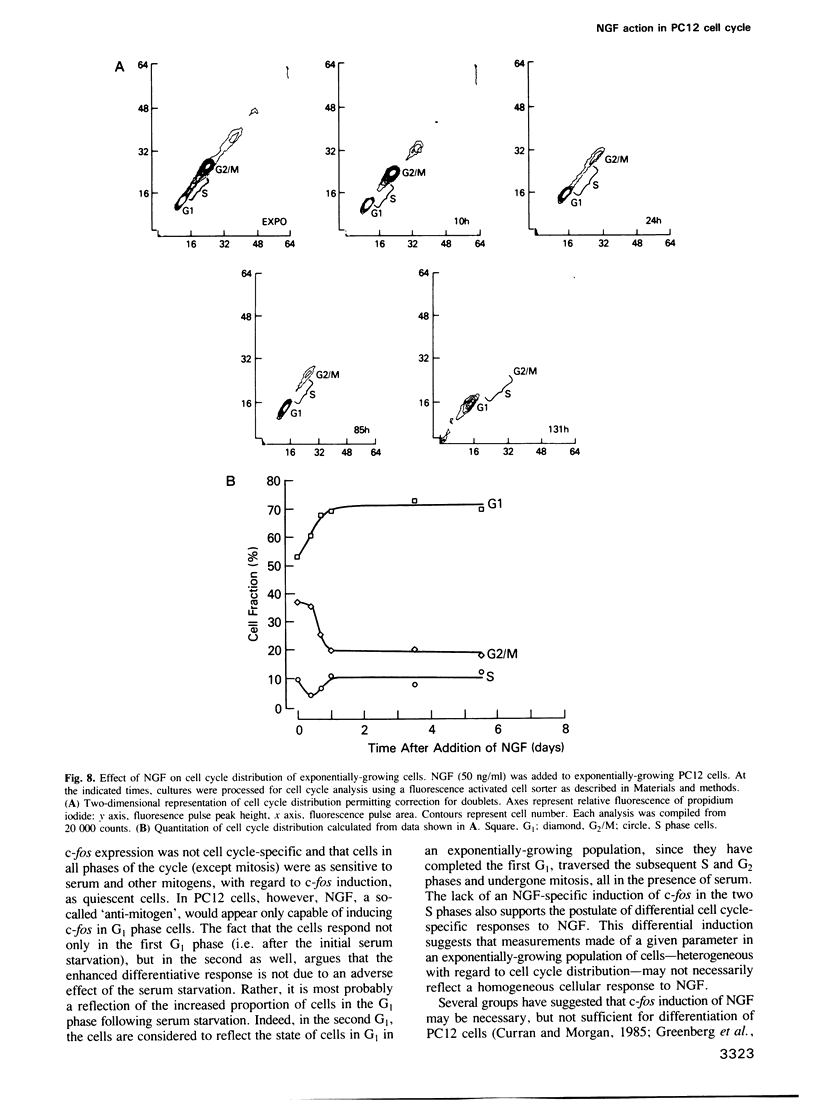
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Reed J. K., England D. The effect of nerve growth factor on the development of sodium channels in PC12 cells. Biochem Cell Biol. 1986 Nov;64(11):1153–1159. doi: 10.1139/o86-152. [DOI] [PubMed] [Google Scholar]
- Rudy B., Kirschenbaum B., Greene L. A. Nerve growth factor-induced increase in saxitoxin binding to rat PC12 pheochromocytoma cells. J Neurosci. 1982 Oct;2(10):1405–1411. doi: 10.1523/JNEUROSCI.02-10-01405.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Schmidt-Glenewinkel T. Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J Neurochem. 1975 Apr;24(4):791–795. [PubMed] [Google Scholar]
- Sierra F., Lichtler A., Marashi F., Rickles R., Van Dyke T., Clark S., Wells J., Stein G., Stein J. Organization of human histone genes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1795–1799. doi: 10.1073/pnas.79.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper S. D., Selak I., Varon S. Serum vulnerability and time-dependent stabilization of neurites induced by nerve growth factor in PC12 pheochromocytoma cells. J Neurosci Res. 1983;10(3):303–315. doi: 10.1002/jnr.490100309. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Pledger W. J., Tucker R. W., Martin R. G., Scher C. D. Regulation of the Balb/c-3T3 cell cycle-effects of growth factors. J Supramol Struct. 1980;13(4):489–499. doi: 10.1002/jss.400130408. [DOI] [PubMed] [Google Scholar]






