Abstract
Bacillus thuringiensis subsp. israelensis (Bti) is the first Bacillus thuringiensis to be found and used as an effective biological control agent against larvae of many mosquito and black fly species around the world. Its larvicidal activity resides in four major (of 134, 128, 72 and 27 kDa) and at least two minor (of 78 and 29 kDa) polypeptides encoded respectively by cry4Aa, cry4Ba, cry11Aa, cyt1Aa, cry10Aa and cyt2Ba, all mapped on the 128 kb plasmid known as pBtoxis. These six δ-endotoxins form a complex parasporal crystalline body with remarkably high, specific and different toxicities to Aedes, Culex and Anopheles larvae. Cry toxins are composed of three domains (perforating domain I and receptor binding II and III) and create cation-selective channels, whereas Cyts are composed of one domain that acts as well as a detergent-like membrane perforator. Despite the low toxicities of Cyt1Aa and Cyt2Ba alone against exposed larvae, they are highly synergistic with the Cry toxins and hence their combinations prevent emergence of resistance in the targets. The lack of significant levels of resistance in field mosquito populations treated for decades with Bti-bioinsecticide suggests that this bacterium will be an effective biocontrol agent for years to come.
Keywords: biological control, mosquito-borne diseases, larvicidal crystal proteins
1. Introduction
Mosquitoes are an enormous public health menace in transmitting various tropical diseases and generally as a nuisance [1]. Many species of the genera Anopheles, Aedes and Culex are vectors of, e.g., malaria, yellow fever, dengue fever, hemorrhagic fever and lymphatic filariasis [2,3,4]. Despite the use of synthetic pesticides over the past 70 years, mosquito-borne diseases are still threatening half of the world's population. Malaria remains one of the leading causes of morbidity and mortality and kills about 660,000 people a year, mainy young children in Africa [5]. Chemical insecticides used in vector control programs harm the environment with adverse impacts on man and nature. Resistance to such insecticides among mosquito species that are vectors of malaria (Anopheles gambiae) and West Nile virus (Culex pipiens) emerged over 25 years ago in Africa, America and Europe and it is frequently due to loss of sensitivity of the insect's acetylcholinesterase to organophosphates and carbamates [6]. Alternative technologies such as biological control offer alternatives to deal with these problems and limitations [7].
2. The Bacterium: Bacillus thuringiensis subsp. israelensis
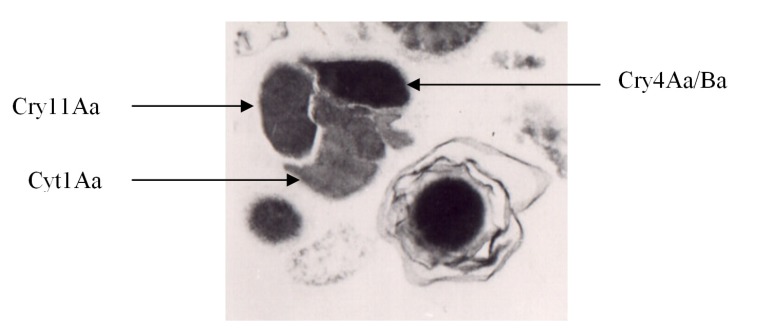
Bacillus thuringiensis subsp. israelensis (Bti) is the first subspecies of B. thuringiensis (Bt) found to be toxic to dipteran larvae. This gram-positive spore-forming subspecies is the most powerful and environmental-friendly biological alternative component in integrated programs to control disease vectors [8,9]. Bti forms a crystalline parasporal body composed of protein protoxins (δ-endotoxins) (Figure 1) that are also used as a commercial bio-pesticide against larvae of noxious arthropod species of the suborder Nematocera, including mosquitoes, black flies and chironomid midges [7,9]. Bti is much more effective against many species of mosquito and black fly larvae than any previously known bio-control agent. Resistance to Bti extensively searched for in field populations of mosquitoes, has not been detected despite nearly 35 years of extensive field usage [10,11,12,13,14]. Several recent studies reported decreased susceptibilities in some field populations [15,16,17,18], but natural variation in such populations and different laboratory strains as well as technical variations inherent in bioassay tests need to be considered in interpreting bioassay results [19]. Thus, lethal concentration values that differ by 5-fold or less are not likely to reliably indicate resistance, and as a general guideline, differences of at least 10-fold are necessary for proof of resistance [15]. The lack of resistance to Bti is mainly attributed to different modes of action and synergistic interactions between the four major toxins, Cry4Aa, Cry4Ba and Cry11Aa and Cyt1Aa [20,21,22].
Figure 1.
B. thuringiensis subsp. israelensis: crystal (left) and spore (right). Modified from Manasherob et al. [49].
In addition to mosquitoes, black flies [23] and chironomid midges [24,25] the expanded host range of Bti includes the following species: Tabanus triceps (Diptera: Tabanidae) [26], Mexican fruit fly, Anastrepha ludens and Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae) [27,28], Tipula paludosa (Diptera: Nematocera) [29], fungus gnats, Bradysia coprophila and Bradysia impatiens (Diptera: Sciaridae) [30,31], nodule-damaging fly Rivellia angulata (Diptera: Platystomatidae) [32], pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea) [33], potato aphid, Macrosiphum euphorbiae (Homoptera: Aphididae) [34], cotton boll weevil Anthonomus grandis (Coleoptera: Tenebrionidae) [35], leaf beetle, Chrysomela scripta (Coleoptera: Chrysomelidae) [36], fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) [37], diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) [38], root-knot nematode, Meloidogyne incognita on barley [39] and trematode species, Schistosoma mansoni and Trichobilharzia szidati (Trematoda: Schistosomatidae) [40].
Originally isolated from a temporary pond with dying Cx. pipiens larvae [41], Bti seems able to reproduce and persist under natural conditions [42,43,44]. Spayed suspension of Bti (spores and crystals) settles within 24–48 h at the bottom of mosquito breeding sites. Ingested spores germinate and recycle in carcasses of Bti-killed mosquito larvae [45,46,47] and pupae [48], and the carcasses are toxic to mosquito larvae.
Other organisms coexisting in mosquito breeding sites may support Bti multiplication in nature, e.g., the ciliate protozoan Tetrahymena pyriformis [49]. Spores and δ-endotoxins are not destroyed in T. pyriformis during the digestion process; the spores germinate in excreted food vacuoles and complete a full growth and sporulation cycle in them [49,50]. In the absence of mosquito larvae, some recycling was observed in laboratory experiments with sediments and vegetation [42], in which case the persistence pattern of the δ-endotoxin components (Cry4 > Cry11 > Cyt) differs from that of the Bti parasporal body crystals [44].
3. δ-Endotoxins of Bti
The original isolate of Bti harbors eight circular plasmids ranging in size between 5 and 210 kb and a linear replicon of approximately 16 kb [51,52]. The larvicidal activity of Bti resides in at least four major crystal protoxins, of 134, 128, 72 and 27 kDa, encoded by cry4Aa, cry4Ba, cry11Aa and cyt1Aa respectively, all mapped on the 128 kb plasmid known as pBtoxis [53,54,55]. In addition, pBtoxis contains cry10Aa, cyt2Ba and cyt1Ca: Cry10Aa and Cyt2Ba accumulate in small amounts in the parasporal body and seem to contribute to the overall toxicity of Bti [56,57,58]. The large protoxins (Cry4Aa and Cry4Ba) have conserved C-terminal halves participating in spontaneous crystal formation via inter- and intra-molecular disulphide bonds [59,60], whereas the smaller (Cry11Aa and Cyt1Aa) do not possess this domain and hence require assistance in crystallization [61,62,63]. The cry11Aa is organized in an operon together with p19 and p20 [64,65]. The P20 accessory protein stabilizes both Cyt1Aa and Cry11Aa in recombinant Escherichia coli [61,62,63,66,67], Pseudomonas putida [63] and Bt [68,69] by interactions with the nascent polypeptides thus protecting these protoxins from proteolysis [61,62,63].
The Cry and Cyt toxins are membrane-perforating proteins although unrelated structurally and differ in their requirement of essential membrane components; the Cry’s bind to membrane receptors [70,71,72,73,74,75] whereas Cyt1Aa binds with high affinities to unsaturated phospholipids [76,77].
3.1. Major Toxins
Cry4Aa, encoded by a sequence of 3543 bp (1180 amino acids), is highly toxic to larvae of Culex and less to Anopheles and Aedes [19,21,70,78,79], and Cry4Ba, encoded by a sequence of 3408 bp (1136 amino acids), has high larvicidal activities against Anopheles and Aedes but very low against Culex [19,21,70,78,79]. Consistent with the differential specificities, the identity between the amino acid sequences of their N-termini toxic portions is only about 30% (55% similarity) [80,81]. Cry11Aa is encoded by a sequence of 1929 bp (643 amino acids) and displays high larvicidities against the larvae of Aedes and Culex but lower against Anopheles [19,21,82].
The larvicidal activity of Cyt1Aa, encoded by a sequence of 744 bp (248 amino acids), is low against all three mosquito genera [19,83,84]. It is cytolytic in vitro to cells of certain vertebrates and invertebrates [85] and highly mosquito species-specific in vivo, implying a specific mode of action [83,86]. The cytotoxicity seems to derive from an interaction between its hydrophobic segment and membrane phospholipids. The sequence of Cyt1Aa has no homology to Cry polypeptides [87] but the toxins plays a critical role in delaying selection for resistance to Bti’s Cry proteins [22,88,89,90].
3.2. Minor Toxins
Cry10Aa, encoded by a sequence of 2025 bp (675 amino acids), accumulates to minor amounts in Bti crystals [57,91] and differs markedly from Cry4Aa and Cry4Ba. The cry10Aa is arranged in an operon 48 bp upstream orf2 [55]. Orf2 is highly homologous (over 65%) to sequences at the carboxylic end of Cry4Aa and Cry4Ba. It can be speculated that together, cry10Aa and orf2 is a variant of the cry4-type genes. Toxicity of Cry10Aa is comparable to those of the other Cry4 toxins and is synergistic with Cyt1Aa against Aedes aegypti larvae [92] and with Cry4Ba against C. pipiens larvae [93].
Cyt2Ba, encoded by a sequence of 789 bp (263 amino acids), is found at very low concentrations in Bti crystals [56]. Proteolytically activated Cyt2Ba is hemolytic in vitro [94,95] and exhibits lower toxicities against larvae of Culex, Aedes and Anopheles than Cyt1Aa [94] but higher than Cyt1Ab from Bt subsp. medellin [96]. Cyt2Ba is synergistic with Cry4Aa, Cry4Ba or Cry11Aa [97,98] and with the B sphaericus binary toxin [96]; it may thus contribute to the overall toxicity of Bti.
Cyt1Ca is encoded by sequence of 1575 bp (525 amino acids) [98,99]. Its N-terminal half is 52% identical to Cyt1Aa, and at the C terminus it contains an extra domain, which appears to be a β-trefoil carbohydrate-binding motif, similar to the receptor binding domain of ricin-B lectin type found in several ricin-like toxins [99]. Transcripts of cyt1Ca were detected, but Cyt1Ca has not been found [100]; the reason may include instability of the transcript or the protein and failure in message translation. Neither mosquito larvicidal activity nor other biological function has been reported for Cyt1Ca [98,99]. The lack of activity of Cyt1Ca may be related to its inability to undergo a certain conformational change due to its lack of flexibility [101].
3.3. Activation, Three-Dimensional Structure and Mode of Action of Major Cry Toxins
Basic studies of the structures and modes of action of δ-endotoxins and their receptors are important for future development of biopesticides that will not be prone to insect resistance [74]. The level of toxicity depends on the capacity of the target species to activate the protoxin by cleaving it to the active toxic component(s) using specific proteases under the alkaline conditions prevailing in the larval midgut. The activated Cry4Aa and Cry4Ba are ~65 kDa toxins with three distinct-domains. The N-terminal domain I is a seven helix bundle responsible for pore formation, and the following two resemble carbohydrate binding proteins: a β-prism (domain II) and a plant lectin-like β-sandwich (domain III) [81,102,103,104,105]. Their three-dimensional structures are similar to the tertiary structures of other previously-solved Cry’s [106,107,108]. In vitro and in vivo processing yields two fragments, of 45 and 20 kDa for Cry4Aa and 45 and 18 kDa for Cry4Ba [79,109,110]. Processing of Cry11Aa yields similarly two fragments, of 38 and 30 kDa [79,82,111,112,113]. This mode of processing differs from those of the lepidopteran-specific toxins [114].
Subsequent steps involve toxin binding to receptors [72,73,74,75,104,105,114,115,116], oligomerization and membrane insertion leading to formation of gated, cation selective channels [117,118]. Lethality is due to collapse of the trans-membrane potential, with subsequent osmotic lysis of cells lining the midgut [119]. These three toxins bind in vitro to the apical brush border of midgut cells in the gastric caecae and posterior stomach of An. gambiae larvae [120] and to the midgut microvilli of Ae. aegypti [79]. The same cells in Cx. pipiens bind Cry4Aa specifically (both in vitro and in vivo) [75]. Each of the two Cry4Aa fragments of 20 (domain I) and 45 kDa (the α6 and α7 helices of domain I and domains II and III), produced by the intramolecular cleavage of the 65-kDa intermediate, are separately not toxic against larvae of Cx. pipiens, but together they display significant toxicity through association with each other to form an active complex of apparently 60 kDa [109,110].
Different mosquito larvicidal activity spectra of both activated Cry4Aa and Cry4Ba likely stem from the structural differences found within domain II and distinct sites binding to the host receptors [70,81,105]. Domain II consists of three anti-parallel β-sheets packed by a central hydrophobic core and three surface-exposed loops at the apex of the domain which are thought to be involved in receptor binding. Loops 2 and 3 of Cry4Aa are an important determinant of the specific toxicity against larvae of Aedes, Anopheles and Culex [105,121], whereas in Cry4Ba, loops 1 and 2 specify the toxicity for Anopheles and Aedes [70,105,122]. Aminopeptidase N and alkaline phosphatase, anchored to glycosyl-phosphatidyl-inositol (GPI) in the epithelial membrane of the Ae. aegypti larval midgut were identified as the receptors of Cry4Ba [123,124], and α-amylase was identified as such in the midgut brush border membrane vesicles of Anopheles albimanus [125]. Cry4Aa may contains multiple sub-sites spread out in domains II and III that cooperate for receptor binding and thus differ from other well-characterized Cry toxins of Bt in their receptor binding mechanism(s) [126].
Pre-pore trimeric structures of either Cry4Aa or Cry4Ba seem to form in aqueous solution and in lipid monolayer, which may facilitate insertion of their three α4-α5 hairpins into the membrane [104,127,128]. Proteolytically activated Cry4Ba in vitro can also form pre-pore oligomers that are proficient in perforation and formation of stable ion channels even without support of the receptors [129].
The three-dimensional structure of Cry11Aa has still not been solved but an in silico model was obtained based on that of Cry2Aa [115]. The pattern of the protoxin activation involves specific proteolytic removal of 27 N-terminal residues and intra-molecular cleavage into two fragments of about 30–33 and 34–36 kDa. Coexistence of the two fragments is essential for toxicity against larvae of Cx. pipiens and Culex quinquefasciatus [111,112,113]. Cry11Aa binds specifically to 148 kDa and 78 kDa proteins of brush border membrane vesicles of An. stephensi and Tipula oleracea respectively [72]. Its putative receptors were identified as GPI-aminopeptidase N, GPI-alkaline phosphatase, cadherin and α-amylase [71,73,125,130,131]. Cry11Aa-receptor interaction seems to involve at least three exposed regions of domain II (loop α-8, β-4 and loop3) [115]. Loop α-8 plays a significant role in the interaction of the toxin with its receptor and subsequent toxicity [115].
3.4. Activation, Three-Dimensional Structure and Mode of Action of Cyt1Aa and Cyt2Ba Toxins
The crystal structure of the proteolytically activated, monomeric forms of Cyt2Ba and Cyt1Aa were solved to 1.8 Å and 2.2 Å resolutions, respectively [101,132]. The toxins are composed of a single pore-forming domain of α/β architecture with a β-sheet surrounded by two α-helical layers representing a cytolysin fold. This structure is strikingly similar to those of the protoxin form of Cyt2Aa from Bt kyushuensis [133] and the fungal volvatoxin A2 [134], suggesting that the toxic monomer of these proteins has a similar mode of activity against cell membrane.
Based on its structure, toxicity of Cyt1Aa is correlated with ability to undergo conformational changes that must occur prior to membrane insertion and perforation [101,132]. The cytolysin fold allows the α-helical layers to swing away, exposing the β-sheet to insert into the membrane. The putative lipid binding pocket between the β-sheet and the helical layer of Cyt1Aa and the hemolytic activity of Cyt1Aa, which resembles that of the pore-forming agents α-toxin and saponin, support this mechanism [101].
Cyt1Aa do not bind specific receptors but have strong binding affinity to the unsaturated fatty acids that compose the membrane of midgut epithelial cells of dipterans [77,135]. In vitro processing of Cyt1Aa protoxin yields single active 22–25 kDa fragment [136,137] that is about three times more effective than the protoxin [138,139].
Cyt1Aa binds to the apical brush border of midgut cells, to the gastric caecae and to stomach cells of An. gambiae larvae; this may be related to the ability of the toxin to perforate cell membranes without participation of any specific receptor [120] by a mechanism that is still a subject of debate. A higher proportion of unsaturated phospholipids in diptera than in other insects may be the reason for a greater affinity of Cyt δ-endotoxins to dipteran cell membranes and activity in vivo. This implies a specific mode of action that is different to those of Bti Cry’s, but an insect-specific receptor may still be essential for the specificity of the Cyt toxins [133,140].
Two different models were proposed for the mode of action of Cyt toxins: pore-forming [141,142] and detergent-like [143]. According to the former, Cyt binds as a monomer which then undergoes conformational changes, its C-terminal half composed mainly of β-strands is inserted into the membrane and the N-terminal half comprising mainly α-helices is exposed on the outside of the membrane [101,144]. Oligomerization on the cell membrane forms β-barrel pores [133,144,145] that induce equilibration of ions and net influx of water, cell swelling, and eventual colloid-osmotic lysis [117,119,146]. Consistent with a detergent-like mechanism, Cyt1Aa is rather adsorbed onto the surface as aggregates thereby causing nonspecific defects in membrane lipid packing, through which intracellular molecules can leak by all-or-nothing mechanism [138,139,143]. Both models may coexist if one considers a differential activity under different doses (concentration × time) [147]: specific perforation occurs at low toxin concentration or short exposure, whereas membrane disruption occurs at high levels or long times.
4. Synergy between the Toxins and Resistance of Targets
4.1. Synergistic Interactions between Bti δ-Endotoxins
The high, specific mosquito larvicidal properties of Bti δ-endotoxins are attributed to complex interactions between six proteins, Cry4Aa, Cry4Ba, Cry10Aa, Cry11Aa, Cyt1Aa and Cyt2Ba, differing in toxicity levels and against different species of mosquitoes (Table 1) [19,20,21,22,92,93,96,148]. Toxicity of each of the four Cry’s is higher than of the Cyt’s, but the high activity of the whole crystal results in synergies among them [19,20,21,22,92,98]. The combinations of Cry4Aa and Cry4Ba, of Cry4Aa and Cry11Aa, or of the three Cry’s, are synergistic against larvae of Culex, Aedes and Anopheles [19,20,21,66,78,149], whereas Cry4Ba and Cry11Aa are synergistic against Ae. aegypti [20]. Two minor crystal toxins, Cry10Aa and Cyt2Ba contribute to the insecticidal activity of Bti by synergistic interactions: Cry10Aa with Cyt1Aa against Ae. aegypti [92] and with Cry4Ba against Cx. pipiens [93] and Cyt2Ba with Cry4Aa against Ae. aegypti [98]. Despite the low toxicities of Cyt1Aa and of Cyt2Aa of Bt kyushuensis against exposed larvae, they are highly synergistic with the Bti Cry toxins and their combinations [20,22,88,89,90,150,151,152,153,154,155,156,157,158,159]. Each functions as a receptor for Cry4Ba, which binds through its domain II loops, explaining the synergy mechanism [155,159].
Table 1.
B. thuringiensis subsp. israelensis crystal δ-endotoxins.
| Toxin | Activated form (kDa) | Toxicity | Synergistic with |
|---|---|---|---|
| Cry4Aa (134 kDa) | 20 and 45 | Cx > An ≥ Ae | Cry4Ba, Cry11Aa, Cyt1Aa, Cyt2Ba |
| Cry11Aa (72 kDa) | 18 and 45 | An ≥ Ae > Cx | Cry4Aa, Cry11Aa, Cry10Aa, Cyt1Aa, Cyt2Aa |
| Cry11Aa (72 kDa) | 30–33 and 34–38 | Ae ≥ Cx > An | Cry4Aa, Cry4Ba, Cyt1Aa, Cyt2Ba |
| Cyt1Aa (27 kDa) | 22–25 | Cx ≥ Ae > An | Cry4Aa, Cry4Ba, Cry11Aa, Cry10Aa |
| Cry10Aa (78 kDa) | 58–68 | Ae > Cx | Cyt1Aa, Cry4Ba |
| Cyt2Ba (29 kDa) | 22.5 | Cx ≥ Ae > An | Cry4Aa, Cry4Ba, Cry11Aa |
| Cyt1Ca (57 kDa) | ND | ND | ND |
The suggestion that Cyt1Aa synergizes Cry11Aa by facilitating the latter’s interaction with the target cell or translocation of the corresponding toxic fragment [157] was later confirmed [154,158]: the interaction is based on their binding as follows. Cyt1Aa, which functions as a membrane-bound receptor, inserts its β-sheet into the membrane after conformational changes, two of its components (loop β6-αE and part of β7) bind with high affinity to Cry11Aa, which subsequently is inserted into the larval epithelial membranes [114,154,158]; residues K198 on β7, and E204 on α6 and K225 on β8 are involved in this process. Inconsistent with this model, these three residues seem to be inserted into the cell’s membrane [101], and an alternative mechanism suggests that Cry11Aa binds to Cyt1Aa using these exposed, charged residues prior to its membrane insertion. This mechanism was recently confirmed [160]: the synergy is retained in mutants of Cyt1Aa helix α-C that were affected in oligomerization, membrane insertion, hemolytic and insecticidal activities. Binding between Cyt1Aa and Cry11Aa may occur in solution or in the membrane plane, promoting oligomerization of Cry11Aa and thus synergizing its toxicity.
4.2. Resistance of Targets to Bti δ-Endotoxins
Field and laboratory resistance of Cx. quinquefasciatus and Cx. pipiens to Bti have been found, regardless of their origin or the level of selection pressure applied [15], but only insignificant levels of resistance were attained against Ae. aegypti. In both examples, resistance was unstable in the absence of larval selection pressure and declined by 50% over three generations. Under laboratory selection pressure against individual Cry4Aa, Cry4Ba and Cry11Aa or in their combinations, larvae of Cx. quinquefasciatus evolve variable levels of resistance and cross-resistance, but only negligible resistance emerged when selected against all four major toxins, three Cry’s and Cyt1Aa [88,89,90]. Moreover, in the presence of moderate Cyt1Aa concentrations, the strains resistant to these Cry toxins (without Cyt1Aa) retained their original wild type sensitivity levels even to this highly effective combination [15]. Thus, increasing the number of Cry toxins delayed the evolution of resistance, but including Cyt1Aa in the combination used for selection was essential to the process [15,22,88,89]. The synergy between Cyt1Aa and Cry’s is significantly high against resistant larvae [22,90] due to the unique feature of Cyt1Aa that serves as an additional receptor for Bti Cry’s. Genetically, Cx. quinquefasciatus evolve multiple-loci resistance to the Bti Cry toxins, but progeny of reciprocal crosses to a sensitive strain exhibited autosomal inheritance with intermediate levels of resistance [161]. The interactions in the diverse mixture of Bti δ-endotoxins, particularly with Cyt1Aa, allow a long term use of Bti as a biological control means against mosquitoes and black flies.
5. Antibacterial and Anticancer Activities of Bti δ-Endotoxins
Expression of cyt1Aa alone in recombinant acrystalliferous Bt kurstaki and in E. coli causes loss of colony-forming ability [68,162]; the latter cells arrest growth and DNA replication leading to strong nucleoid compaction and partial lysis [163,164]. These findings support the suggestion that, in addition to its membrane perforating activity, Cyt1Aa specifically disrupts nucleoid associations with the cytoplasmic membrane. Simultaneous, high-affinity interactions of Cyt1Aa with zwitterionic phospholipids as well as with DNA may enhance detachment of DNA from the membrane and hence affect nucleoid compaction [163]. Co-expression with p20 (encoding a putative chaperonin) preserves cell viability [68,165]. Antibacterial activity of expressed N terminus-truncated Cyt1Ca in E. coli causes instant arrest in biomass growth and decreased viability [99].
Cyt1Aa, Cry4Ba and Cry11Aa, as well as two proteins (of 36 and 34 kDa) isolated from Bti, are antibacterial also exogenously, against E. coli and Gram-positive species (Micrococcus luteus, Streptomyces chrysomallus and Staphyloccocus aureus) [137,166,167]. Cyt1Aa is bactericidal for E. coli, whereas it is bacteriostatic for S. aureus as reflected in morphological changes and ion balance alteration [137]. Cyt1Aa may bind to the outer membrane of Gram-negative cells and easily penetrate the cytoplasmic membrane, whereas in Gram positive cells, it must cross the massive peptidoglycan layer before reaching the cytoplasmic membrane. Furthermore, Cyt1Aa can contribute to the antibacterial activity of some antibiotics through partial disruption of the outer membrane, enabling better penetration of the antibiotic [137].
Cry toxins from other Bt subspecies (kurstaki, galleriae, tenebrionis) are toxic to the anaerobic Gram-positive bacteria Clostridium butyricum and Clostridium acetobutylicum and the archaea Methanosarcina barkeri [168].
Cyt1Aa and Cyt2Ba display anti-cancer activities as well; conjugation of activated Cyt1Aa to a peptide carrier molecule is toxic against murine hybridoma cells [169] whereas activated Cyt2Ba exhibits some cytotoxicity to human breast cancer cells (MCF-7) [170]. Cyt1Aa may be useful in other medical applications: specific toxicity against cells bearing a high number of insulin receptors is enhanced by linking it to insulin [171].
6. Limitations of Bti and Recombinant Bacteria
Applying Bti for mosquito control is limited by short residual activity of current preparations under field conditions [9] due to: (i) sinking to the bottom of the water body; (ii) adsorption onto silt particles and organic matter; (iii) consumption by other organisms to which it is nontoxic; and (iv) inactivation by sunlight. In order to overcome these shortcomings, the δ-endotoxin genes have already been expressed individually or in combinations in various Gram-positive and -negative species [7,9]. Best results were achieved by expressing the genes encoding binary toxin of B. sphaericus in Bti [172]. The recombinant bacteria were highly potent against fourth instar larvae of Cx. quinquefasciatus and Cx. tarsalis, even to lines selected for resistance to the binary toxin. Higher toxicity against fourth-instar Cx. quinquefasciatus was achieved in recombinant acrystalliferous Bti strain that produces the combination of B. sphaericus binary toxin together with Cyt1Aa of Bti and Cry11Ba from Bt subsp. jegathesan [173].
Several attempts have been made during the last two decades to produce transgenic mosquito larvicidal cyanobacteria [9,174]. Most promising results were obtained when cry4Aa and cry11Aa alone or with cyt1Aa were expressed from the dual constitutive and efficient promoters PpsbA and PA1 in the filamentous, nitrogen-fixing cyanobacterium Anabaena PCC 7120 [174,175,176,177,178,179]. LC50 values of these clones against third and fourth instar Ae. aegypti larvae were 8.3 × 104 and 3.5 × 104 cells mL−1 respectively, the lowest reported values for engineered cyanobacterial cells with Bti toxin genes. Toxicity of the Anabaena clone expressing constitutively cry4Aa and cry11Aa with p20 is retained following irradiation by high doses of UV-B, doses that partially inactivate Bti toxicity [177]. This latter recombinant strain exhibited decent toxicity against larvae of An. merus, An. arabiensis and An. gambiae, but very weak activity against An. funestus [178]. Optimizing growth conditions in a photobioreactor was described for this cyanobacterial clone [179].
7. Concluding Remark
Bti is environmentally friendly and a safe alternative means to control mosquitoes and blackflies. Emergence of resistant variants has not been found despite three decades of extensive use, likely due to the complex and diverse δ-endotoxins composition of its crystal. To overcome or prevent theoretical, future emergence of resistance, recombinant microorganisms can be engineered to co-express toxins with different modes of action or chimeric toxins with improved efficacy [180]. Enhancing Bti’s mosquito larvicidal activity can be achieved by totally different mechanisms that wane larval survival, e.g., chitinase (damaging the peritrophic membrane) [181] and Trypsin Modulating Oostatic Factor (causing larval starvation) [182,183].
Acknowledgments
Arieh Zaritsky is gratefully acknowledged for the thorough scrutiny of the manuscript and for his critical and helpful comments. I thank Robert Manasherob, Kamal Khawaled, Vadim Khasdan and Shmulik Cohen for cooperation in research that we have conducted over many years and Monica Einav for many years of great help in all aspects of our laboratory life.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Service M.W. Medical Entomology for Students. 3rd ed. Cambridge University Press; Cambridge, UK: 2004. [Google Scholar]
- 2.Sinka M.E., Bangs M.J., Manguin S., Rubio-Palis Y., Chareonviriyaphap T., Coetzee M., Mbogo C.M., Hemingway J., Patil A., Temperley W.H. A global map of dominant malaria vectors. Parasit Vectors. 2012;5 doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyapong J.O., Twum-Danso N.A.Y. Editorial: Global elimination of lymphatic filariasis: Fact or fantasy? Trop. Med. Int. Health. 2006;11:125–128. doi: 10.1111/j.1365-3156.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 4.Reisen W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010;55:461–483. doi: 10.1146/annurev-ento-112408-085419. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO; Geneva, Switzland: 2012. World Malaria Report 2012. [Google Scholar]
- 6.Weill M., Lutfalla G., Mogensen K., Chandre F., Berthomieu A., Berticat C., Pasteur N., Philips A., Fort P., Raymond M. Insecticide resistance in mosquito vectors. Nature. 2003;423:136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 7.Federici B.A., Park H.W., Bideshi D.K. Overview of the basic biology of Bacillus thuringiensis with emphasis on genetic engineering of bacterial larvicides for mosquito control. OpenToxinol. J. 2010;3:83–100. [Google Scholar]
- 8.Fillinger U., Lindsay S.W. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop. Med. Int. Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- 9.Margalith Y., Ben-Dov E. Biological control by Bacillus thuringiensis subsp. israelensis. In: Rechcigl J.E., Rechcigl N.A., editors. Insect Pest Management:Techniques for Environmental Protection. CRC Press; Boca Raton, FL, USA: 2000. pp. 243–301. [Google Scholar]
- 10.Becker N., Ludwig M. Investigations on possible resistance in Aedes vexans field populations after a 10-years application of Bacillus thuringiensis israelensis. J. Am. Mosq. Control Assoc. 1993;9:221–224. [PubMed] [Google Scholar]
- 11.Kamgang B., Marcombe S., Chandre F., Nchoutpouen E., Nwane P., Etang J., Corbel V., Paupy C. Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Parasit Vectors. 2011;4 doi: 10.1186/1756-3305-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loke S.R., Andy-Tan W.A., Benjamin S., Lee H.L., Sofian-Azirun M. Susceptibility of field-collected Aedes aegypti (L.) (Diptera: Culicidae) to Bacillus thuringiensis israelensis and temephos. Trop. Biomed. 2010;27:493–503. [PubMed] [Google Scholar]
- 13.Tetraeu G., Stalinski R., David J.P., Despres L. Monitoring resistance to Bacillus thuringiensis israelensis (Bti) in the field by performing bioassays with each Cry toxin superlatively. Mem. Inst. Oswaldo Cruz. 2013;108:894–900. doi: 10.1590/0074-0276130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasquez M.I., Violaris M., Hadjivassilis A., Wirth M.C. Susceptibility of Culex pipiens (Diptera: Culicidae) field populations in Cyprus to conventional organic insecticides, Bacillus thuringiensis subsp. israelensis and methoprene. J. Med. Entomol. 2009;46:881–887. doi: 10.1603/033.046.0421. [DOI] [PubMed] [Google Scholar]
- 15.Wirth M.C. Mosquito resistance to bacterial larvicidal toxins. OpenToxinol. J. 2010;3:126–140. [Google Scholar]
- 16.Boyer S., Paris M., Jego S., Lemperiere G., Ravanel P. Influence of insecticide Bacillus thuringiensis subsp. israelensis treatments on resistance and enzyme activities in Aedes rusticus larvae (Diptera: Culicidae) Biol. Control. 2012;62:75–81. doi: 10.1016/j.biocontrol.2012.02.001. [DOI] [Google Scholar]
- 17.Paul A., Harrington L.C., Zhang L., Scott J.G. Insecticide resistance in Culex pipiens from New York. J. Am. Mosq. Control Assoc. 2005;21:305–309. doi: 10.2987/8756-971X(2005)21[305:IRICPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H.Y., Yang C.J., Huang J.Y., Lu L. Susceptibility of field populations of Anopheles sinensis (Diptera: Culicidae) to Bacillus thuringiensis subsp. israelensis. Biocontrol Sci.Technol. 2004;14:321–325. doi: 10.1080/09583150310001639187. [DOI] [Google Scholar]
- 19.Otieno-Ayayo Z.N., Zaritsky A., Wirth M.C., Manasherob R., Khasdan V., Cahan R., Ben-Dov E. Variations in the mosquito larvicidal activities of toxins from Bacillus thuringiensis ssp. israelensis. Environ. Microbial. 2008;10:2191–2199. doi: 10.1111/j.1462-2920.2008.01696.x. [DOI] [PubMed] [Google Scholar]
- 20.Crickmore N., Bone E.J., Williams J.A., Ellar D.J. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol. Lett. 1995;131:249–254. [Google Scholar]
- 21.Poncet S., Delecluse A., Klier A., Rapoport G. Evaluation of synergistic interactions among the CryIVA, CryIVB, and CryIVD toxic components of B. thuringiensis subsp. israelensis crystals. J. Invertebr. Pathol. 1995;66:131–135. doi: 10.1006/jipa.1995.1075. [DOI] [Google Scholar]
- 22.Wirth M.C., Park H.-W., Walton W.E., Federici B.A. Cyt1A of Bacillus thuringiensis delays evolution of resistance to Cry11A in the mosquito Culex quinquefasciatus. Appl. Environ. Microbial. 2005;71:185–189. doi: 10.1128/AEM.71.1.185-189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira E., Teles B., Martins E., Praà L., Santos A., Ramos F., Berry C., Monnerat R. Comparative toxicity of Bacillus thuringiensis berliner strains to larvae of simuliidae (Insecta: Diptera) Bt Res. 2013;4:8–13. [Google Scholar]
- 24.Ali A. A concise review of chironomid midges (Diptera: Chironomidae) as pests and their management. J. Vector Ecol. 1996;21:105–121. [Google Scholar]
- 25.Kondo S., Ohba M., Ishii T. Comparative susceptibility of chironomid larvae (Dipt., Chironomidae) to Bacillus thuringiensis serovar israelensis with special reference to altered susceptibility due to food difference. J. Appl. Entomol. 1995;119:123–125. doi: 10.1111/j.1439-0418.1995.tb01256.x. [DOI] [Google Scholar]
- 26.Saraswathi A., Ranganathan L.S. Larvicidal effect of Bacillus thuringiensis var. israelensis on Tabanus triceps (Thunberg) (Diptera: Tabanidae) Indian J. Exp. Biol. 1996;34:1155–1157. [Google Scholar]
- 27.Robacker D.C., Martinez A.J., Garcia J.A., Diaz M., Romero C. Toxicity of Bacillus thuringiensis to Mexican fruit fly (Diptera: Tephritidae) J. Econ. Entomol. 1996;89:104–110. [Google Scholar]
- 28.Vidal-Quist J.C., Castañera P., González-Cabrera J. Cyt1Aa protein from Bacillus thuringiensis (Berliner) serovar israelensis is active against the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) Pest Manag. Sci. 2010;66:949–955. doi: 10.1002/ps.1965. [DOI] [PubMed] [Google Scholar]
- 29.Oestergaard J., Belau C., Strauch O., Ester A., van Rozen K., Ehlers R.U. Biological control of Tipula paludosa (Diptera: Nematocera) using entomopathogenic nematodes (Steinernema spp.) and Bacillus thuringiensis subsp. israelensis. Biol. Control. 2006;39:525–531. doi: 10.1016/j.biocontrol.2006.07.003. [DOI] [Google Scholar]
- 30.Harris M.A., Oetting R.D., Gardner W.A. Use of entomopathogenic nematodes and a new monitoring technique for control of fungus gnats, Bradysia coprophila (Diptera: Sciaridae), in floriculture. Biol. Control. 1995;5:412–418. doi: 10.1006/bcon.1995.1049. [DOI] [Google Scholar]
- 31.Taylor M.D., Willey R.D., Noblet R. A 24-h potato-based toxicity test for evaluating Bacillus thuringiensis var. israelensis (H-14) against darkwinged fungus gnat Bradysia impatiens Johannsen (Diptera: Sciaridae) larvae. Int. J. Pest Manag. 2007;53:77–81. [Google Scholar]
- 32.Nambiar P.T.C., Ma S.W., Iyer V.N. Limiting an insect infestation of nitrogen-fixing root nodules of the pigeon pea (Cajanus cajan) by engineering the expression of an entomocidal gene in its root nodules. Appl. Environ. Microbiol. 1990;56:2866–2869. doi: 10.1128/aem.56.9.2866-2869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porcar M., Grenier A.M., Federici B., Rahbé Y. Effects of Bacillus thuringiensis δ-endotoxins on the Pea aphid (Acyrthosiphon pisum) Appl. Environ. Microbiol. 2009;75:4897–4900. doi: 10.1128/AEM.00686-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters F.S., English L.H. Toxicity of Bacillus thuringiensis δ-endotoxins toward the potato aphid in an artificial diet bioassay. Entomol. Exp. Appl. 1995;77:211–216. doi: 10.1111/j.1570-7458.1995.tb02003.x. [DOI] [Google Scholar]
- 35.Monnerat R., Martins E., Praça L., Dumas V., Berry C. Activity of a Brazilian strain of Bacillus thuringiensis israelensis against the cotton boll weevil Anthonomus grandis Boheman (Coleoptera: Tenebrionidae) Neotrop. Entomol. 2012;41:62–67. doi: 10.1007/s13744-011-0008-6. [DOI] [PubMed] [Google Scholar]
- 36.Federici B.A., Bauer L.S. Cyt1Aa protein of Bacillus thuringiensis is toxic to the cottonwood leaf beetle, Chrysomela scripta, and suppresses high levels of resistance to Cry3Aa. Appl. Environ. Microbiol. 1998;64:4368–4371. doi: 10.1128/aem.64.11.4368-4371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Souza J.D.A., Jr., Jain S., de Oliveira C.M.F., Ayres C.F., Lucena W.A. Toxicity of a Bacillus thuringiensis israelensis-like strain against Spodoptera frugiperda. BioControl. 2009;54:467–473. doi: 10.1007/s10526-008-9191-8. [DOI] [Google Scholar]
- 38.Sayyed A.H., Crickmore N., Wright D.J. Cyt1Aa from Bacillus thuringiensis subsp. israelensis is toxic to the diamondback moth, Plutella xylostella, and synergizes the activity of Cry1Ac towards a resistant strain. Appl. Environ. Microbiol. 2001;67:5859–5861. doi: 10.1128/AEM.67.12.5859-5861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma R.D. Bacillus thuringiensis: A biocontrol agent of Meloidogyne incognita on barley. Nematol. Brasileira. 1994;18:79–84. [Google Scholar]
- 40.Horak P., Weiser J., Mikes L., Kolarova L. The effect of Bacillus thuringiensis M-exotoxin on trematode cercariae. J. Invertebr. Pathol. 1996;68:41–49. doi: 10.1006/jipa.1996.0056. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg L.J., Margalit J. A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univittatus, Aedes aegypti and Culex pipiens. Mosq. News. 1977;37:355–358. [Google Scholar]
- 42.Boisvert M., Boisvert J. Persistence of toxic activity and recycling of Bacillus thuringiensis var. israelensis in cold water: Field experiments using diffusion chambers in a pond. Biocontrol Sci. Technol. 1999;9:507–522. [Google Scholar]
- 43.Tilquin M., Paris M., Reynaud S., Despres L., Ravanel P., Geremia R.A., Gury J. Long lasting persistence of Bacillus thuringiensis subsp. israelensis (Bti) in mosquito natural habitats. PLoS ONE. 2008;3:e3432. doi: 10.1371/journal.pone.0003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tetreau G., Alessi M., Veyrenc S., Périgon S., David J.P., Reynaud S., Després L. Fate of Bacillus thuringiensis subsp. israelensis in the field: Evidence for spore recycling and differential persistence of toxins in leaf litter. Appl. Environ. Microbiol. 2012;78:8362–8367. doi: 10.1128/AEM.02088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aly C., Mulla M.S., Federici B.A. Sporulation and toxin production by Bacillus thuringiensis var. israelensis in cadavers of mosquito larvae (Diptera: Culicidae) J. Invertebr. Pathol. 1985;46:251–258. [Google Scholar]
- 46.Zaritsky A., Khawaled K. Toxicity in carcasses of Bacillus thuringiensis var. israelensis-killed Aedes aegypti larvae against scavenging larvae: Implications to bioassay. J. Am. Mosq. Control Assoc. 1986;2:555–559. [PubMed] [Google Scholar]
- 47.Khawaled K., Barak Z., Zaritsky A. Feeding behavior of Aedes aegypti larvae and toxicity of dispersed and of naturally encapsulated Bacillus thuringiensis var. israelensis. J. Invertebr. Pathol. 1988;52:419–426. doi: 10.1016/0022-2011(88)90054-7. [DOI] [PubMed] [Google Scholar]
- 48.Khawaled K., Ben-Dov E., Zaritsky A., Barak Z. The fate of Bacillus thuringiensis var. israelensis in B. thuringiensis var. israelensis-killed pupae. J. Invertebr. Pathol. 1990;56:312–316. doi: 10.1016/0022-2011(90)90117-O. [DOI] [PubMed] [Google Scholar]
- 49.Manasherob R., Ben-Dov E., Zaritsky A., Barak Z. Germination, growth, and sporulation of Bacillus thuringiensis subs. israelensis in excreted food vacuoles of the protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 1998;64:1750–1758. doi: 10.1128/aem.64.5.1750-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Dov E., Zalkinder V., Shagan T., Barak Z., Zaritsky A. Spores of Bacillus thuringiensis var. israelensis as tracers for ingestion rates by Tetrahymena pyriformis. J. Invertebr. Pathol. 1994;63:220–222. doi: 10.1006/jipa.1994.1042. [DOI] [PubMed] [Google Scholar]
- 51.Carlton B.C., Gonzalez J.M., Jr. Plasmids and delta-endotoxin production in different subspecies of Bacillus thuringiensis. In: Hoch J.A., Setlow P., editors. Molecular Biology of Microbial Differentiation. American Society for Microbiology; Washington, DC, USA: 1985. pp. 246–252. [Google Scholar]
- 52.Sekar V. Genetics of Bacillus thuringiensis israelensis. In: de Barjac H., Sutherland D.J., editors. Bacterial Control of Mosquitoes and Black Flies. Rutgers University Press; New Brunswick, NJ, USA: 1990. pp. 66–77. [Google Scholar]
- 53.Ben-Dov E., Einav M., Peleg N., Boussiba S., Zaritsky A. Restriction map of the 125-kilobase of Bacillus thuringiensis subsp. israelensis carrying the genes that encode delta-endotoxins active against mosquito larvae. Appl. Environ. Microbiol. 1996;62:3140–3145. doi: 10.1128/aem.62.9.3140-3145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Dov E., Nissan G., Peleg N., Manasherob R., Boussiba S., Zaritsky A. Refined, circular restriction map of the Bacillus thuringiensis subsp. israelensis plasmid carrying the mosquito larvicidal genes. Plasmid. 1999;42:186–191. doi: 10.1006/plas.1999.1415. [DOI] [PubMed] [Google Scholar]
- 55.Berry C., O’Neil S., Ben-Dov E., Jones A.F., Murphy L., Quail M.A., Holden M.T.G., Harris D., Zaritsky A., Parkhill J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2002;68:5082–5095. doi: 10.1128/AEM.68.10.5082-5095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerchicoff A., Ugalde R.A., Rubinstein C.P. Identification and characterization of a previously undescribed cyt gene in Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 1997;63:2716–2721. doi: 10.1128/aem.63.7.2716-2721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S.G., Eckblad W., Bulla A., Jr. Diversity of protein inclusion bodies and identification of mosquitocidal protein in Bacillus thuringiensis subsp. israelensis. Biochem. Biophys. Res. Commun. 1985;126:953–960. doi: 10.1016/0006-291X(85)90278-5. [DOI] [PubMed] [Google Scholar]
- 58.Thorne L., Garguno F., Thompson T., Decker D., Zounes M., Wild M., Walfieldand A.M., Pollock T.J. Structural similarity between the Lepidoptera- and Diptera-specific isecticidal endotoxins genes of Bacillus thuringiensis subsp. kurstaki and israelensis. J. Bacteriol. 1986;166:801–811. doi: 10.1128/jb.166.3.801-811.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bietlot H.P.L., Vishnubhatla I., Carey P.R., Pozsgay M., Kaplan H. Characterization of the cysteine residues and disulfide linkages in the protein crystal of Bacillus thuringiensis. Biochem. J. 1990;267:309–315. doi: 10.1042/bj2670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Couche G.A., Pfannenstiel M.A., Nickerson K.W. Structural disulfide bonds in the Bacillus thuringiensis subsp. israelensis protein crystal. J. Bacteriol. 1987;169:3281–3288. doi: 10.1128/jb.169.7.3281-3288.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams L.F., Visick J.E., Whiteley H.R. A 20-kilodalton protein is required for efficient production of the Bacillus thuringiensis subsp. israelensis 27-kilodalton crystal protein in Escherichia coli. J. Bacteriol. 1989;171:521–530. doi: 10.1128/jb.171.1.521-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visick J.E., Whiteley H.R. Effect of a 20-kilodalton protein from Bacillus thuringiensis subsp. israelensis on production of the CytA protein by Escherichia coli. J. Bacteriol. 1991;173:1748–1756. doi: 10.1128/jb.173.5.1748-1756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Y., Nagai M., Bagdasarian M., Smith T.W., Walker E.D. Expression of the p20 gene from Bacillus thuringiensis H-14 increases Cry11A toxin production and enhances mosquito-larvicidal activity in recombinant gram-negative bacteria. Appl. Environ. Microbiol. 2001;67:3010–3015. doi: 10.1128/AEM.67.7.3010-3015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agaisse H., Lereclus D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J. Bacteriol. 1995;177:6027–6032. doi: 10.1128/jb.177.21.6027-6032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dervyn E., Poncet S., Klier A., Rapoport G. Transcriptional regulation of the cryIVD gene operon from Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 1995;177:2283–2291. doi: 10.1128/jb.177.9.2283-2291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben-Dov E., Boussiba S., Zaritsky A. Mosquito larvicidal activity of Escherichia coli with combinations of genes from Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 1995;177:2851–2857. doi: 10.1128/jb.177.10.2851-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLean K.M., Whiteley H.R. Expression in Escherichia coli of a cloned crystal protein gene of Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 1987;169:1017–1023. doi: 10.1128/jb.169.3.1017-1023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu D., Federici B.A. A 20-kilodalton protein preserves cell viability and promotes CytA crystal formation during sporulation in Bacillus thuringiensis. J. Bacteriol. 1993;175:5276–5280. doi: 10.1128/jb.175.16.5276-5280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu D., Federici B.A. Improved production of the insecticidal CryIVD protein in Bacillus thuringiensis using cryIA(c) promoters to express the gene for an associated 20-kDa protein. Appl. Microbiol. Biotechnol. 1995;45:697–702. doi: 10.1007/BF00171947. [DOI] [PubMed] [Google Scholar]
- 70.Abdullah M.A.F., Alzate O., Mohammad M., McNall R.J., Adang M.J., Dean D.H. Introduction of Culex toxicity into Bacillus thuringiensis Cry4Ba by protein engineering. Appl. Environ. Microbiol. 2003;69:5343–5353. doi: 10.1128/AEM.69.9.5343-5353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen J., Likitvivatanavong S., Aimanova K.G., Gill S.S. A 104 kDa Aedes aegypti aminopeptidase N is a putative receptor for the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Insect Biochem. Molec. Biol. 2013;43:1201–1208. doi: 10.1016/j.ibmb.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feldmann F., Dullemans A., Waalwijk C. Binding of the CryIVD toxin of Bacillus thuringiensis subsp. israelensis to larval dipteran midgut proteins. Appl. Environ. Microbiol. 1995;61:2601–2605. doi: 10.1128/aem.61.7.2601-2605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez L.E., Aimanova K.G., Gill S.S., Bravo A., Soberón M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem. J. 2006;394:77–84. doi: 10.1042/BJ20051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pigott C.R., Ellar D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Molec. Biol. Rev. 2007;71:255–281. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamagiwa M., Kamauchi S., Okegawa T., Esaki M., Otake K., Amachi T., Komano T., Sakai H. Binding properties of Bacillus thuringiensis Cry4A toxin to the apical microvilli of larval midgut of Culex pipiens. Biosci. Biotechnol. Biochem. 2001;11:2419–2427. doi: 10.1271/bbb.65.2419. [DOI] [PubMed] [Google Scholar]
- 76.Thomas W.E., Ellar D.J. Mechanism of action of Bacillus thuringiensis var. israelensis insecticidal δ-endotoxin. FEBS Lett. 1983;154:362–368. doi: 10.1016/0014-5793(83)80183-5. [DOI] [PubMed] [Google Scholar]
- 77.Cantón P.E., López-Díaz J.A., Gill S.S., Bravo A., Soberón M. Membrane binding and oligomer membrane insertion are necessary but insufficient for Bacillus thuringiensis Cyt1Aa toxicity. Peptides. 2013 doi: 10.1016/j.peptides.2013.10.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Angsuthanasombat C., Crickmore N., Ellar D.J. Comparison of Bacillus thuringiensis subsp. israelensis CryIVA and CryIVB cloned toxins reveals synergism in vivo. FEMS Microbiol. Lett. 1992;94:63–68. doi: 10.1111/j.1574-6968.1992.tb05290.x. [DOI] [PubMed] [Google Scholar]
- 79.Beltrão H.B.M., Silva-Filha M.H.N.L. Interaction of Bacillus thuringiensis svar. israelensis Cry toxins with binding sites from Aedes aegypti (Diptera: Culicidae) larvae midgut. FEMS Microbiol. Lett. 2007;266:163–169. doi: 10.1111/j.1574-6968.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 80.Sen K., Honda G., Koyama N., Nishida M., Neki A., Sakai H., Himeno M., Komano T. Cloning and nucleotide sequences of the two 130 kDa insecticidal protein genes of Bacillus thuringiensis var. israelensis. Agric. Biol. Chem. 1988;52:873–878. doi: 10.1271/bbb1961.52.873. [DOI] [Google Scholar]
- 81.Angsuthanasombat C., Uawithya P., Leetachewa S., Pornwiroon W., Ounjai P., Kerdcharoen T., Katzenmeier G., Panyim S. Bacillus thuringiensis Cry4A and Cry4B mosquito-larvicidal proteins: Homology-based 3D model and implications for toxin activity. J. Biochem. Molec. Biol. 2004;37:304–313. doi: 10.5483/BMBRep.2004.37.3.304. [DOI] [PubMed] [Google Scholar]
- 82.Revina L.P., Kostina L.I., Ganushkina L.A., Mikhailova A.L., Zalunin I.A., Chestukhina G.G. Reconstruction of Bacillus thuringiensis ssp. israelensis Cry11A endotoxin from fragments corresponding to its N- and C moieties restores its original biological activity. Biochemistry. 2004;69:181–187. doi: 10.1023/b:biry.0000018949.70836.dc. [DOI] [PubMed] [Google Scholar]
- 83.Thiery I., Delecluse A., Tamayo M.C., Orduz S. Identification of a gene for Cyt1A-like hemolysin from Bacillus thuringiensis subsp. medellin and expression in a crystal-negative B. thuringiensis strain. Appl. Environ. Microbiol. 1997;63:468–473. doi: 10.1128/aem.63.2.468-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bourgouin C., Klier A., Rapoport G. Characterization of the genes encoding the haemolytic toxin and mosquitocidal δ-endotoxin of Bacillus thuringiensis var. israelensis. Mol. Gen. Genet. 1986;205:390–397. doi: 10.1007/BF00338072. [DOI] [PubMed] [Google Scholar]
- 85.Thomas W.E., Ellar D.J. Bacillus thuringiensis var. israelensis crystal δ-endotoxin: Effects on insect and mammalian cells in vitro and in vivo. J. Cell Sci. 1983;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- 86.Cheong H., Gill S.S. Cloning and characterization of a cytolytic and mosquitocidal δ-endotoxin from Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 1997;63:3254–3260. doi: 10.1128/aem.63.8.3254-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crickmore N., Zeigler D.R., Feitelson J., Schnepf E., Van Rie J., Lereclus D., Baum J., Dean D.H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998;62:807–813. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Georghiou G.P., Wirth M.C. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae) Appl. Environ. Microbiol. 1997;63:1095–1101. doi: 10.1128/aem.63.3.1095-1101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wirth M.C., Georghiou G.P. Cross-resistance among CryIV toxins of Bacillus thuringiensis subsp. israelensis in Culex quinquefasciatus (Diptera: Culicidae) J. Econ. Entomol. 1997;90:1471–1477. [Google Scholar]
- 90.Wirth M.C., Georghiou G.P., Federici B.A. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefasciatus. Proc. Natl. Acad. Sci. USA. 1997;94:10536–10540. doi: 10.1073/pnas.94.20.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garguno F., Thorne L., Walfield A.M., Pollock T.J. Structural relatedness between mosquitocidal endotoxins of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 1988;54:277–279. doi: 10.1128/aem.54.1.277-279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hernández-Soto A., Del Rincón-Castro M.C., Espinoza A.M., Ibarra J.E. Parasporal body formation via overexpression of the Cry10Aa toxin of Bacillus thuringiensis subsp. israelensis, and Cry10Aa-Cyt1Aa synergism. Appl. Environ. Microbiol. 2009;75:4661–4667. doi: 10.1128/AEM.00409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delecluse A., Bourgouin C., Klier A., Rapoport G. Specificity of action on mosquito larvae of Bacillus thuringiensis var. israelensis toxins encoded by two different genes. Mol. Gen. Genet. 1988;214:42–47. doi: 10.1007/BF00340177. [DOI] [PubMed] [Google Scholar]
- 94.Juárez-Pérez V., Guerchicoff A., Rubinstein C., Delécluse A. Characterization of Cyt2Bc toxin from Bacillus thuringiensis subsp. medellin. Appl. Environ. Microbiol. 2002;68:1228–1231. doi: 10.1128/AEM.68.3.1228-1231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nisnevitch M., Cohen S., Ben-Dov E., Zaritsky A., Sofer Y., Cahan R. Cyt2Ba of Bacillus thuringiensis israelensis: Activation by putative endogenous protease Biochem. Biophys. Res. Commun. 2006;344:99–105. doi: 10.1016/j.bbrc.2006.03.134. [DOI] [PubMed] [Google Scholar]
- 96.Wirth M.C., Delécluse A., Walton W.E. Cyt1Ab1 and Cyt2Ba1 from Bacillus thuringiensis subsp. medellin and B. thuringiensis subsp. israelensis synergize Bacillus sphaericus against Aedes aegypti and resistant Culex quinquefasciatus (Diptera: Culicidae) Appl. Environ. Microbiol. 2001;67:3280–3284. doi: 10.1128/AEM.67.7.3280-3284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Purcell M., Ellar D.J. The identification and characterization of novel proteinacious components of the Bacillus thuringiensis subsp. israelensis parasporal inclusion; Proceedings of the 30th Annual Meeting of the Society for Invertebrate Pathology; Banff, Canada. 24–29 August 1997; p. 53. [Google Scholar]
- 98.Manasherob R., Itsko M., Sela-Baranes N., Ben-Dov E., Berry C., Cohen S., Zaritsky A. Cyt1Ca from Bacillus thuringiensis subsp. israelensis: production in Escherichia coli and comparison of its biological activities with those of other Cyt-like proteins. Microbiology. 2006;152:2651–2659. doi: 10.1099/mic.0.28981-0. [DOI] [PubMed] [Google Scholar]
- 99.Itsko M., Zaritsky A. Exposing cryptic antibacterial activity in Cyt1Ca from Bacillus thuringiensis israelensis by genetic manipulations. FEBS Lett. 2007;581:1775–1782. doi: 10.1016/j.febslet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 100.Stein C., Jones G.W., Chalmers T., Berry C. Transcriptional analysis of the toxin-coding plasmid pBtoxis from Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2006;72:1771–1776. doi: 10.1128/AEM.72.3.1771-1776.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohen S., Albeck S., Ben-Dov E., Cahan R., Firer M., Zaritsky A., Dym O. Cyt1Aa toxin: Crystal structure reveals implications for its membrane-perforating function. J. Mol. Biol. 2011;413:804–814. doi: 10.1016/j.jmb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 102.Angsuthanasombat C. Structural basis of pore formation by mosquito-larvicidal proteins from Bacillus thuringiensis. Open Toxinol. J. 2010;3:119–125. [Google Scholar]
- 103.Boonserm P., Angsuthanasombat C., Lescar J. Crystallization and preliminary crystallographic study of the functional form of the Bacillus thuringiensis mosquito-larvicidal Cry4Aa mutant toxin. Acta Crystallogr. Sect. D. 2004;60:1315–1318. doi: 10.1107/S0907444904011205. [DOI] [PubMed] [Google Scholar]
- 104.Boonserm P., Davis P., Ellar D.J., Li J. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 2005;348:363–382. doi: 10.1016/j.jmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 105.Boonserm P., Mo M., Angsuthanasombat C., Lescar J. Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-angstrom resolution. J. Bacteriol. 2006;188:3391–3401. doi: 10.1128/JB.188.9.3391-3401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grochulski P., Masson L., Borisova S., Puztai-Carey M., Schwartz J.-L., Brousseau R., Cygler M. Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation. J. Mol. Biol. 1995;254:447–464. doi: 10.1006/jmbi.1995.0630. [DOI] [PubMed] [Google Scholar]
- 107.Li J., Carroll J., Ellar D.J. Crystal structure of insecticidal δ-endotoxin from Bacillus thuringiensis at 2.5 Å resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- 108.Morse R.J., Yamamoto T., Stroud R.M. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure. 2001;9:409–417. doi: 10.1016/S0969-2126(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 109.Komano T., Yamigawa M., Nishimoto T., Yoshisue H., Tanabe K., Sen K., Sakai H. Activation process of the insecticidal proteins CryIVA and CryIVB produced by Bacillus thuringiensis subsp. israelensis. Isr. J. Entomol. 1998;32:185–198. [Google Scholar]
- 110.Yamagiwa M., Esaki M., Otake K., Inagaki M., Komano T., Amachi T., Sakai H. Activation process of dipteran-specific insecticidal protein produced by Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 1999;65:3464–3469. doi: 10.1128/aem.65.8.3464-3469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dai S.-M., Gill S.S. In vitro and in vivo proteolysis of the Bacillus thuringiensis subsp. israelensis CryIVD protein by Culex quinquefasciatus larval midgut proteases. Insect Biochem. Molec. Biol. 1993;23:273–283. doi: 10.1016/0965-1748(93)90008-G. [DOI] [PubMed] [Google Scholar]
- 112.Yamagiwa M., Ogawa R., Yasuda K., Natsuyama H., Sen K., Sakai H. Active form of dipteran-specific insecticidal protein Cry11A produced by Bacillus thuringiensis subsp. israelensis. Biosci. Biotech. Biochem. 2002;66:516–522. doi: 10.1271/bbb.66.516. [DOI] [PubMed] [Google Scholar]
- 113.Yamagiwa M., Sakagawa K., Sakai H. Functional analysis of two processed fragments of Bacillus thuringiensis Cry11A toxin. Biosci. Biotech. Biochem. 2004;68:523–528. doi: 10.1271/bbb.68.523. [DOI] [PubMed] [Google Scholar]
- 114.Bravo A., Gill S.S., Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandez L.E., Perez C., Segovia L., Rodriguez M.H., Gill S.S., Bravo A., Soberon M. Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop α-8 of domain II. FEBS Lett. 2005;579:3508–3514. doi: 10.1016/j.febslet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 116.Chayaratanasin P., Moonsom S., Sakdee S., Chaisri U., Katzenmeier G., Angsuthanasombat C. High level of soluble expression in Escherichia coli and characterisation of the cloned Bacillus thuringiensis Cry4Ba Domain III fragment. J. Biochem. Mol. Biol. 2007;40:58–64. doi: 10.5483/bmbrep.2007.40.1.058. [DOI] [PubMed] [Google Scholar]
- 117.Knowles B.H. Mechanism of action of Bacillus thuringiensis insecticidal δ-endotoxins. Adv. Insect Physiol. 1994;24:275–308. [Google Scholar]
- 118.Aronson A.I., Shai Y. Why Bacillus thuringiensis insecticidal toxins are so effective: Unique features of their mode of action. FEMS Microbiol. Lett. 2001;195:1–8. doi: 10.1111/j.1574-6968.2001.tb10489.x. [DOI] [PubMed] [Google Scholar]
- 119.Knowles B.H., Ellar D.J. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificities. Biochim. Biophys. Acta. 1987;924:509–518. doi: 10.1016/0304-4165(87)90167-X. [DOI] [Google Scholar]
- 120.Ravoahangimalala O., Charles J.-F. In vitro binding of Bacillus thuringiensis var. israelensis individual toxins to midgut cells of Anopheles gambiae (Diptera: Culicidae) FEBS Lett. 1995;362:111–115. doi: 10.1016/0014-5793(95)00220-4. [DOI] [PubMed] [Google Scholar]
- 121.Howlader M.T.H., Kagawa Y., Sakai H., Hayakawa T. Biological properties of loop-replaced mutants of Bacillus thuringiensis mosquitocidal Cry4Aa. J. Biosci. Bioeng. 2009;108:179–183. doi: 10.1016/j.jbiosc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 122.Buzdin A.A., Revina L.P., Kostina L.I., Zalunin I.A., Chestukhina G.G. Interaction of 65- and 62-kD proteins from the apical membranes of the Aedes aegypti larvae midgut epithelium with Cry4B and Cry11A endotoxins of Bacillus thuringiensis. Biochemistry. 2002;67:540–546. doi: 10.1023/a:1015594127636. [DOI] [PubMed] [Google Scholar]
- 123.Saengwiman S., Aroonkesorn A., Dedvisitsakul P., Sakdee S., Leetachewa S., Angsuthanasombat C., Pootanakit K. In vivo identification of Bacillus thuringiensis Cry4Ba toxin receptors by RNA interference knockdown of glycosylphosphatidylinositol-linked aminopeptidase N transcripts in Aedes aegypti larvae. Biochem. Biophys. Res. Commun. 2011;407:708–713. doi: 10.1016/j.bbrc.2011.03.085. [DOI] [PubMed] [Google Scholar]
- 124.Thammasittirong A., Dechklar M., Leetachewa S., Pootanakit K., Angsuthanasombat C. Aedes aegypti membrane-bound alkaline phosphatase expressed in Escherichia coli retains high-affinity binding for Bacillus thuringiensis Cry4Ba toxin. Appl. Environ. Microbiol. 2011;77:6836–6840. doi: 10.1128/AEM.05775-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fernandez-Luna M.T., Lanz-Mendoza H., Gill S.S., Bravo A., Soberon M., Miranda-Rios J. An α-amylase is a novel receptor for Bacillus thuringiensis ssp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae) Environ. Microbiol. 2010;12:746–757. doi: 10.1111/j.1462-2920.2009.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Howlader M.T.H., Kagawa Y., Miyakawa A., Yamamoto A., Taniguchi T., Hayakawa T., Sakai H. Alanine scanning analyses of the three major loops in domain II of Bacillus thuringiensis mosquitocidal toxin Cry4Aa. Appl. Environ. Microbiol. 2010;76:860–865. doi: 10.1128/AEM.02175-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Likitvivatanavong S., Katzenmeier G., Angsuthanasombat C. Asn183 in α5 is essential for oligomerisation and toxicity of the Bacillus thuringiensis Cry4Ba toxin. Arch. Biochem. Biophys. 2006;445:46–55. doi: 10.1016/j.abb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 128.Taveecharoenkool T., Angsuthanasombat C., Kantchanawarin C. Combined molecular dynamics and continuum solvent studies of the pre-pore Cry4Aa trimer suggest its stability in solution and how it may form a pore. PMC Biophys. 2010;3:1–16. doi: 10.1186/1757-5036-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodríguez-Almazán C., Reyes E.Z., Zúñiga-Navarrete F., Muñoz-Garay C., Gómez I., Evans A.M., Likitvivatanavong S., Bravo A., Gill S.S., Soberón M. Cadherin binding is not a limiting step for Bacillus thuringiensis subsp. israelensis Cry4Ba toxicity to >italic>Aedes aegypti larvae. Biochem. J. 2012;443:711–717. doi: 10.1042/BJ20111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen J., Aimanova K.G., Fernandez L.E., Bravo A., Soberon M., Gill S.S. Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Biochem. J. 2009;424:191–200. doi: 10.1042/BJ20090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J., Aimanova K.G., Pan S., Gill S.S. Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem. Mol. Biol. 2009;39:688–699. doi: 10.1016/j.ibmb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen S., Dym O., Albeck S., Ben-Dov E., Cahan R., Firer M., Zaritsky A. High-resolution crystal structure of activated Cyt2Ba monomer from Bacillus thuringiensis subsp. israelensis. J. Mol. Biol. 2008;380:820–827. doi: 10.1016/j.jmb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 133.Li J., Koni P.A., Ellar D.J. Structure of the mosquitocidal δ-endotoxin CytB from Bacillus thuringiensis sp. kyushuensis and implications for membrane pore formation. J. Mol. Biol. 1996;257:129–152. doi: 10.1006/jmbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- 134.Lin S.C., Lo Y.C., Lin J.Y., Liaw Y.C. Crystal structures and electron micrographs of fungal volvatoxin A2. J. Mol. Biol. 2004;343:477–491. doi: 10.1016/j.jmb.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 135.Gill S.S., Singh G.J.P., Hornung J.M. Cell membrane interaction Bacillus thuringiensis subsp. israelensis cytolytic toxins. Infec. Immunol. 1987;55:1300–1308. doi: 10.1128/iai.55.5.1300-1308.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-yahyaee S.A.S., Ellar D.J. Maximal toxicity of cloned CytA δ-endotoxin from Bacillus thuringiensis subsp. israelensis requires proteolytic processing from both the N- and C-termini. Microbiology. 1995;141:3141–3148. doi: 10.1099/13500872-141-12-3141. [DOI] [Google Scholar]
- 137.Cahan R., Friman H., Nitzan Y. Antibacterial activity of Cyt1Aa from Bacillus thuringiensis subsp. israelensis. Microbiology. 2008;154:3529–3536. doi: 10.1099/mic.0.2008/020784-0. [DOI] [PubMed] [Google Scholar]
- 138.Butko P., Huang F., Pusztai-Carey M., Surewicz W.K. Membrane permeabilization induced by cytolyticδ-endotoxin CytA from Bacillus thuringiensis var. israelensis. Biochemistry. 1996;35:11355–11360. doi: 10.1021/bi960970s. [DOI] [PubMed] [Google Scholar]
- 139.Butko P., Huang F., Pusztai-Carey M., Surewicz W.K. Interaction of the δ-endotoxin CytA from Bacillus thuringiensis var. israelensis with lipid membranes. Biochemistry. 1997;36:12862–12868. doi: 10.1021/bi9702389. [DOI] [PubMed] [Google Scholar]
- 140.Koni P.A., Ellar D.J. Cloning and characterization of novel Bacillus thuringiensis cytolitic delta-endotoxin. J. Mol. Biol. 1993;229:319–327. doi: 10.1006/jmbi.1993.1037. [DOI] [PubMed] [Google Scholar]
- 141.Promdonkoy B., Ellar D.J. Membrane pore architecture of a cytolytic toxin from Bacillus thuringiensis. Biochem. J. 2000;350:275–282. doi: 10.1042/0264-6021:3500275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Promdonkoy B., Ellar D.J. Investigation of the poreforming mechanism of a cytolytic δ-endotoxin from Bacillus thuringiensis. Biochem. J. 2003;374:255–259. doi: 10.1042/BJ20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manceva S.D., Pusztai-Carey M., Russo P.S., Butko P. A detergent-like mechanism of action of the cytolytic toxin Cyt1A from Bacillus thuringiensis var. israelensis. Biochemistry. 2005;44:589–597. doi: 10.1021/bi048493y. [DOI] [PubMed] [Google Scholar]
- 144.Rodriguez-Almazan C., Ruiz de Escudero I., Canton P.E., Munoz-Garay C., Perez C., Gill S.S., Soberón M., Bravo A. The amino- and carboxyl-terminal fragments of the Bacillus thuringensis Cyt1Aa toxin have differential roles in toxin oligomerization and pore formation. Biochemistry. 2010;50:388–396. doi: 10.1021/bi101239r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li J., Derbyshire D.J., Promdonkoy B., Ellar D.J. Structural implications for the transformation of the Bacillus thuringiensis delta-endotoxins from water-soluble to membrane-inserted forms. Biochem. Soc. Trans. 2001;29:571–577. doi: 10.1042/BST0290571. [DOI] [PubMed] [Google Scholar]
- 146.Knowles B.H., Blatt M.R., Tester M., Horsnell J.M., Carroll J., Menestrina G., Ellar D.J. A cytolytic δ-endotoxin from Bacillus thuringiensis var. israelensis forms cation-selective channels in planar lipid bilayers. FEBS Lett. 1989;244:259–262. doi: 10.1016/0014-5793(89)80540-X. [DOI] [PubMed] [Google Scholar]
- 147.Butko P. Cytolytic toxin Cyt1A and its mechanism of membrane damage: Data and hypotheses. Appl. Environ. Microbiol. 2003;69:2415–2422. doi: 10.1128/AEM.69.5.2415-2422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Van Frankenhuyzen K. Cross-order and cross-phylum activity of Bacillus thuringiensis pesticidal proteins. J. Invertebr. Pathol. 2013;114:76–85. doi: 10.1016/j.jip.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 149.Delecluse A., Poncet S., Klier A., Rapoport G. Expression of cryIVA and cryIVB genes, independently or in combination, in a crystal-negative strain of Bacillus thuringiensis subs. israelensis. Appl. Environ. Microbiol. 1993;59:3922–3927. doi: 10.1128/aem.59.11.3922-3927.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khasdan V., Ben-Dov E., Manasherob R., Boussiba S., Zaritsky A. Toxicity and synergism in transgenic Escherichia coli expressing four genes from Bacillus thuringiensis subsp. israelensis. Environ. Microbiol. 2001;3:798–806. doi: 10.1046/j.1462-2920.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- 151.Khasdan V., Ben-Dov E., Manasherob R., Boussiba S., Zaritsky A. Mosquito larvicidal activity of transgenic Anabaena PCC 7120 expressing toxin genes from Bacillus thuringiensis ssp. israelensis. FEMS Microbiol. Lett. 2003;227:189–195. doi: 10.1016/S0378-1097(03)00679-7. [DOI] [PubMed] [Google Scholar]
- 152.Wirth M.C., Zaritsky A., Ben-Dov E., Manasherob R., Khasdan V., Boussiba S., Walton W.E. Cross-resistance spectra of Culex quinquefasciatus resistant to mosquitocidal toxins of Bacillus thuringiensis towards recombinant Escherichia coli expressing genes from B. thuringiensis ssp. israelensis. Environ. Microbial. 2007;9:1393–1401. doi: 10.1111/j.1462-2920.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 153.Wu D., Johnson J.J., Federici B.A. Synergism of mosquitocidal toxicity between CytA and CryIVD proteins using inclusions produced from cloned genes of Bacillus thuringiensis. Mol. Microbiol. 1994;13:965–972. doi: 10.1111/j.1365-2958.1994.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 154.Pérez C., Fernandez L.E., Sun J., Folch J.L., Gill S.S., Soberón M., Bravo A. Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc. Natl. Acad. Sci. USA. 2005;102:18303–18308. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lailak C., Khaokhiew T., Promptas C., Promdonkoy B., Pootanakit K., Angsuthanasombat C. Bacillus thuringiensis Cry4Ba toxin employs two receptor-binding loops for synergistic interactions with Cyt2Aa2. Biochem. Biophys. Res. Commun. 2013;435:216–221. doi: 10.1016/j.bbrc.2013.04.078. [DOI] [PubMed] [Google Scholar]
- 156.Promdonkoy B., Promdonkoy P., Panyim S. Co-expression of Bacillus thuringiensis Cry4Ba and Cyt2Aa2 in Escherichia coli revealed high synergism against Aedes aegypti and Culex quinquefasciatus larvae. FEMS Microbiol. Lett. 2005;252:121–126. doi: 10.1016/j.femsle.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 157.Chang C., Yu Y.-M., Dai S.-M., Law S.K., Gill S.S. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein, and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl. Environ. Microbiol. 1993;59:815–821. doi: 10.1128/aem.59.3.815-821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pérez C., Muñoz-Garay C., Portugal L.C., Sánchez J., Gill S.S., Soberón M., Bravo A. Bacillus thuringiensis ssp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell. Microbiol. 2007;9:2931–2937. doi: 10.1111/j.1462-5822.2007.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cantón P.E., Reyes E.Z., De Escudero I.R., Bravo A., Soberón M. Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides. 2011;32:595–600. doi: 10.1016/j.peptides.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.López-Diaz J.A., Cantón P.E., Gill S.S., Soberón M., Bravo A. Oligomerization is a key step in Cyt1Aa membrane insertion and toxicity but not necessary to synergize Cry11Aa toxicity in Aedes aegypti larvae. Environ. Microbiol. 2013;15:3030–3039. doi: 10.1111/1462-2920.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wirth M.C., Walton W.E., Federici B.A. Inheritance, stability, and dominance of Cry resistance in Culex quinquefasciatus (Diptera: Culicidae) selected with the three Cry toxins of Bacillus thuringiensis subsp. israelensis. J. Med. Entomol. 2012;49:886–894. doi: 10.1603/me11192. [DOI] [PubMed] [Google Scholar]
- 162.Douek J., Einav M., Zaritsky A. Sensitivity to plating of Escherichia coli cells expressing the cytA gene from Bacillus thuringiensis var. israelensis. Mol. Gen. Genet. 1992;232:162–165. doi: 10.1007/BF00299149. [DOI] [PubMed] [Google Scholar]
- 163.Manasherob R., Zaritsky A., Metzler Y., Ben-Dov E., Itsko M., Fishov I. Compaction of the Escherichia coli nucleoid caused by Cyt1Aa. Microbiology. 2003;149:3553–3564. doi: 10.1099/mic.0.26271-0. [DOI] [PubMed] [Google Scholar]
- 164.Sazhenskiy V., Zaritsky A., Itsko M. Expression in Escherichia coli of the native cyt1Aa from Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2010;76:3409–3411. doi: 10.1128/AEM.03068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Manasherob R., Zaritsky A., Ben-Dov E., Saxena D., Barak Z., Einav M. Effect of accessory proteins P19 and P20 on cytolytic activity of Cyt1Aa from Bacillus thuringiensis subsp. israelensis in Escherichia coli. Curr. Microbiol. 2001;43:355–364. doi: 10.1007/s002840010316. [DOI] [PubMed] [Google Scholar]
- 166.Revina L.P., Kostina L.I., Dronina M.A., Zalunin I.A., Chestukhina G.G., Yudina T.G., Konukhova A.V., Izumrudova A.V. Novel antibacterial proteins from entomocidal crystals of Bacillus thuringiensis ssp. israelensis. Can. J. Microbiol. 2005;51:141–148. doi: 10.1139/w04-121. [DOI] [PubMed] [Google Scholar]
- 167.Yudina T.G., Konukhova A.V., Revina L.P., Kostina L.I., Zalunin I.A., Chestukhina G.G. Antibacterial activity of Cry- and Cyt-proteins from Bacillus thuringiensis ssp. israelensis. Can. J. Microbiol. 2003;49:37–44. doi: 10.1139/w03-007. [DOI] [PubMed] [Google Scholar]
- 168.Yudina T.G., Brioukhanov A.L., Zalunin I.A., Revina L.P., Shestakov A.I., Voyushina N.E., Chestukhina G.G., Netrusov A.I. Antimicrobial activity of different proteins and their fragments from Bacillus thuringiensis parasporal crystals against clostridia and archaea. Anaerobe. 2007;13:6–13. doi: 10.1016/j.anaerobe.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 169.Cohen S., Cahan R., Ben-Dov E., Nisnevitch M., Zaritsky A., Firer M.A. Specific targeting to murine myeloma cells of Cyt1Aa toxin from Bacillus thuringiensis subspecies israelensis. J. Biol. Chem. 2007;282:28301–28308. doi: 10.1074/jbc.M703567200. [DOI] [PubMed] [Google Scholar]
- 170.Corrêa R.F.T., Ardisson-Araújo D.M.P., Monnerat R.G., Ribeiro B.M. Cytotoxicity analysis of three Bacillus thuringiensis subsp. israelensis δ-endotoxins towards insect and mammalian cells. PLoS ONE. 2012;7:e46121. doi: 10.1371/journal.pone.0046121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Al-yahyaee S.A.S., Ellar D.J. Cell targeting of a pore-forming toxin, CytA δ-endotoxin from Bacillus thuringiensis subspecies israelensis, by conjugating CytA with anti-Thy 1 monoclonal antibodies and insulin. Bioconjug. Chem. 1996;7:451–460. doi: 10.1021/bc960030k. [DOI] [PubMed] [Google Scholar]
- 172.Park H.-W., Bideshi D.K., Wirth M.C., Johnson J.J., Walton W.E., Federici B.A. Recombinant larvicidal bacteria with markedly improved efficacy against Culex vectors of West Nile virus. Am. J. Trop. Med. Hyg. 2005;72:732–738. [PubMed] [Google Scholar]
- 173.Park H.-W., Bideshi D.K., Federici B.A. Recombinant strain of Bacillus thuringiensis producing Cyt1A, Cry11B, and the Bacillus sphaericus binary toxin. Appl. Environ. Microbiol. 2003;69:1331–1334. doi: 10.1128/AEM.69.2.1331-1334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boussiba S., Wu X.-Q., Ben-Dov E., Zarka A., Zaritsky A. Nitrogen-fixing cyanobacteria as gene delivery system for expressing mosquitocidal toxins of Bacillus thuringiensis subsp. israelensis. J. Appl. Phycol. 2000;12:461–467. doi: 10.1023/A:1008114929490. [DOI] [Google Scholar]
- 175.Lluisma A.O., Karmacharya N., Zarka A., Ben-Dov E., Zaritsky A., Boussiba S. Suitability of Anabaena PCC 7120 expressing mosquitocidal toxin genes from Bacillus thuringiensis subsp. israelensis for biotechnological application. Appl. Microbiol. Biotechnol. 2001;57:161–166. doi: 10.1007/s002530100776. [DOI] [PubMed] [Google Scholar]
- 176.Wu X., Vennison S.J., Liu H., Ben-Dov E., Zaritsky A., Boussiba S. Mosquito larvicidal activity of transgenic Anabaena strain PCC 7120 expressing combinations of genes from Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 1997;63:4971–4974. doi: 10.1128/aem.63.12.4971-4974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Manasherob R., Ben-Dov E., Wu X., Boussiba S., Zaritsky A. Protection from UV-B damage of mosquito larvicidal toxins from Bacillus thuringiensis subsp. israelensis expressed in Anabaena PCC 7120. Curr. Microbiol. 2002;45:217–220. doi: 10.1007/s00284-001-0106-5. [DOI] [PubMed] [Google Scholar]
- 178.Ketseoglou I., Bouwer G. The susceptibility of five African Anopheles species to Anabaena PCC 7120 expressing Bacillus thuringiensis subsp. israelensis mosquitocidal cry genes. Parasites Vectors. 2012;5 doi: 10.1186/1756-3305-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ketseoglou I., Bouwer G. Optimization of photobioreactor growth conditions for a cyanobacterium expressing mosquitocidal Bacillus thuringiensis Cry proteins. J. Biotechnol. 2013;167:64–71. doi: 10.1016/j.jbiotec.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 180.Sun Y., Zhao Q., Zheng D., Ding X., Wang J., Hu Q., Yuan Z., Park H.-W., Xia L. Construction and characterization of the interdomain chimeras using Cry11Aa and Cry11Ba from Bacillus thuringiensis and identification of a possible novel toxic chimera. Biotechnol. Lett. 2014;36:105–111. doi: 10.1007/s10529-013-1330-3. [DOI] [PubMed] [Google Scholar]
- 181.Sirichotpakorn N., Rongnoparut P., Choosang K., Panbangred W. Coexpression of chitinase and the cry11Aa1 toxin genes in Bacillus thuringiensis serovar israelensis. J. Invertebr. Pathol. 2001;78:160–169. doi: 10.1006/jipa.2001.5058. [DOI] [PubMed] [Google Scholar]
- 182.Borovsky D., Khasdan V., Nauwelaers S., Theunis C., Bertier L., Ben-Dov E., Zaritsky A. Synergy between Aedes aegypti trypsin modulating oostatic factor and δ-endotoxins. Open Toxinol. J. 2010;3:116–125. [Google Scholar]
- 183.Borovsky D., Nauwelaers S., Van Mileghem A., Meyvis Y., Laeremans A., Theunis C., Bertier L., Boons E. Control of mosquito larvae with TMOF and 60 kDa Cry4Aa expressed in Pichia pastoris. Pestycydy. 2011;1:5–15. [Google Scholar]