Abstract
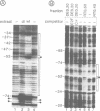
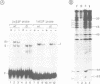
The binding of the cellular E2F transcription factor to the central EIa-responsive element of the adenovirus EIIa early promoter (EIIaE) was compared in extracts of HeLa cells which had been infected with either wild-type adenovirus or the EIa-deficient mutant dl312. No quantitative differences in the E2F-binding activity were detected as a function of EIa gene expression. However, complexes formed by the E2F factor in the presence of EIa were qualitatively different from those formed on the same sequence element in the absence of EIa. Specifically, the formation of complexes containing two E2F molecules is favoured by EIa, probably through the induction of protein-protein interactions. Protein binding to EIIaE promoter in extracts from non-infected F9 embryonal carcinoma cells, prepared before and after in vitro differentiation of these cells was also analysed. The higher expression of EIIaE in undifferentiated cells, which was originally attributed to a cellular EIa-like function, may be correlated with the increased binding activity of a murine E2F-like protein which does not, however, result in the simultaneous occupation of both E2F sites on the EIIaE promoter, suggesting that the viral EIa and the presumptive cellular EIa-like functions trans-activate the EIIaE promoter through different pathways.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Boeuf H., Zajchowski D. A., Tamura T., Hauss C., Kédinger C. Specific cellular proteins bind to critical promoter sequences of the adenovirus early EIIa promoter. Nucleic Acids Res. 1987 Jan 26;15(2):509–527. doi: 10.1093/nar/15.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Regulatory proteins in yeast. Annu Rev Genet. 1987;21:425–452. doi: 10.1146/annurev.ge.21.120187.002233. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Devaux B., Kédinger C. The abundance and in vitro DNA binding of three cellular proteins interacting with the adenovirus EIIa early promoter are not modified by the EIa gene products. Mol Cell Biol. 1987 Oct;7(10):3806–3817. doi: 10.1128/mcb.7.10.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Wintzerith M., Gaire M., Hauss C., Egly J. M., Kédinger C. Purification and functional characterization of a cellular transcription factor that binds to an enhancer element within the adenovirus early EIIa promoter. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2484–2488. doi: 10.1073/pnas.85.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen-Durr P., Boeuf H., Kédinger C. Replication-induced stimulation of the major late promoter of adenovirus is correlated to the binding of a factor to sequences in the first intron. Nucleic Acids Res. 1988 May 11;16(9):3771–3786. doi: 10.1093/nar/16.9.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Ko J. L., Dalie B. L., Goldman E., Harter M. L. Adenovirus-2 early region IA protein synthesized in Escherichia coli extracts indirectly associates with DNA. EMBO J. 1986 Jul;5(7):1645–1651. doi: 10.1002/j.1460-2075.1986.tb04407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2180–2184. doi: 10.1073/pnas.84.8.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987 May 22;49(4):507–513. doi: 10.1016/0092-8674(87)90453-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moncollin V., Miyamoto N. G., Zheng X. M., Egly J. M. Purification of a factor specific for the upstream element of the adenovirus-2 major late promoter. EMBO J. 1986 Oct;5(10):2577–2584. doi: 10.1002/j.1460-2075.1986.tb04537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Activation of a preexisting cellular factor as a basis for adenovirus E1A-mediated transcription control. Proc Natl Acad Sci U S A. 1988 Jan;85(2):387–390. doi: 10.1073/pnas.85.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Developmental control of a promoter-specific factor that is also regulated by the E1A gene product. Cell. 1987 Feb 13;48(3):501–506. doi: 10.1016/0092-8674(87)90200-5. [DOI] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Shaw A. R., Ziff E. B. Selective inhibition of adenovirus type 2 early region II and III transcription by an anisomycin block of protein synthesis. Mol Cell Biol. 1982 Jul;2(7):789–799. doi: 10.1128/mcb.2.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yee A. S., Raychaudhuri P., Jakoi L., Nevins J. R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989 Feb;9(2):578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. S., Reichel R., Kovesdi I., Nevins J. R. Promoter interaction of the E1A-inducible factor E2F and its potential role in the formation of a multi-component complex. EMBO J. 1987 Jul;6(7):2061–2068. doi: 10.1002/j.1460-2075.1987.tb02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajchowski D. A., Boeuf H., Kédinger C. E1a inducibility of the adenoviral early E2a promoter is determined by specific combinations of sequence elements. Gene. 1987;58(2-3):243–256. doi: 10.1016/0378-1119(87)90379-9. [DOI] [PubMed] [Google Scholar]
- Zheng X. M., Moncollin V., Egly J. M., Chambon P. A general transcription factor forms a stable complex with RNA polymerase B (II). Cell. 1987 Jul 31;50(3):361–368. doi: 10.1016/0092-8674(87)90490-9. [DOI] [PubMed] [Google Scholar]