Abstract
AIM: To investigate the role of exercise training the past 25 years on major physiological-psychological outcomes studied thus far in this patient population.
METHODS: PubMed, MedlinePlus, the Cochrane Library, Web of Science, SportDiscus, Embase, Scorpus, and Google Scholar were searched from September to November 2013 to identify exercise training studies that used objective measurements of fitness and/or patient reported outcomes assessed pre and post-exercise training with statistical analyses performed in at least one of the following outcome measurements: Cardiorespiratory function, body composition, muscular strength, fatigue, depression, and overall quality of life. Five reviewers independently identified the studies that met the criteria for the review and discrepancies were resolved by consensus among all authors.
RESULTS: Fifty-one studies were included in this review with 5 from the period between 1989-1999, 11 from 2000-2006, and 35 from 2007-2013. The evolution of study designs changed from aerobic only exercise training interventions (1989-1999), to a combination of aerobic and resistance training (2000-2006), to studies including an arm of resistance training or examining the effects of resistance training as the main mode of exercise (2007-2013). Overall, the benefits of exercise showed improvements in cardiorespiratory function, body composition, strength, and patient reported outcomes including fatigue, depression, and quality of life.
CONCLUSION: Exercise training appears to be safe for most breast cancer patients and improvements in physiological, psychological, and functional parameters can be attained with regular participation in moderate intensity exercise.
Keywords: Breast cancer, Exercise training, Complementary Alternative Medicine, Oncology, VO2peak, Patients reported outcomes
Core tip: The purpose of this systematic literature review was to investigate the role of exercise training the past 25 years on major physiological-psychological outcomes studied thus far in this patient population. Exercise training appears to be safe for most breast cancer patients and improvements in physiological, psychological, and functional parameters can be attained with regular participation in moderate intensity exercise.
INTRODUCTION
Despite being the most commonly diagnosed cancer among women and the second most diagnosed type of cancer overall in the world (approximately 1.7 million diagnosed cases in 2012, 11.9% of all cancer cases) the incidence of breast cancer continues to rise with a concerning increase of 20% of newly diagnosed cases since 2008[1]. This worldwide increase in new breast cancer cases has been been attributed, in part, to changes in lifestyle in developing countries due to economic growth and societal changes similar in nature to those of developed industrialized nations[1]. These lifestyle changes are often associated with poor dietary choices and lack of physical activity; both considered major risk factors for the development of the disease[2]. However, in developed countries, new diagnostic tools that allows for faster detection and technological advances in anti-cancer treatments, have been credited as the main factors for the increased longevity observed in oncology patients, especially in the breast cancer population[2,3].
Living longer with a history of the disease often comes with the deleterious effects of treatments, which regrettably are associated with reduced quality of life in the patients. Marked reductions in physical capacities (i.e., reduced cardiac function and muscular strength), negative body composition alterations (i.e., increase in body mass, reduction in muscle mass, and increases in fat mass), and detrimental patients reported outcomes (PROs) (i.e., increased levels of fatigue, depression, and anxiety) are among the common side-effects of cancer treatments that negatively impact the overall quality of life of these patients and also put them at a greater risk for the development of other co-morbidities[4]. If not addressed, these side effects can increase the likelihood for the development of secondary cancers as well as diminish life expectancy[2].
During the past 3 decades, complementary therapies known as Complementary Alternative Medicine, have received more attention by the medical community due to its ability to mitigate some of the side-effects commonly experienced by cancer patients during and post-completion of cancer treatments. One intervention that has gained increasingly more attention due its efficacious ability to positively impact many physiological systems that are altered during cancer treatment, its non-invasive nature, and relatively low cost, is exercise.
The effects of exercise training in breast cancer patients began to be examined in the late 1980’s. The first study examining the effects of exercise in breast cancer patients was published in 1989[5] and demonstrated that the patients tolerated exercise training and significant improvement in cardiorespiratory functional capacity [represented by the variable VO2max (L/min)] was observed in the exercise-training group. Since then, an ever-growing number of studies examining the effects of exercise in breast cancer patients has continued to be one of the most studied populations in this relatively new and exciting area of exercise oncology research. The results of the evaluation of these studies are promising and affirming, but clearly warrant further and more extensive investigations.
The purpose of this systematic review of the literature on the effects of exercise training in breast cancer patients was to present and evaluate the results of studies conducted in this area of research during the past 25 years, divided into the following time periods: (1989-1999, 2000-2006, 2007-2013). Most specifically, this review focuses on presenting and discussing the results of the effects of exercise training on the most commonly evaluated outcomes in the area including physiological parameters of cardiorespiratory function, body composition, and muscular strength, and PROs that evaluated fatigue, depression, anxiety, and overall quality of life. For organizational purposes a chronological description of studies results, studies common characteristics, and commentaries on changes observed in this area of research over the past 25 years are discussed.
MATERIALS AND METHODS
A systematic review of the literature using PubMed, MedlinePlus, the Cochrane Library, Web of Science, SportDiscus, Scorpus, Embase, and Google Scholar was conducted from September to November 2013 to identify exercise-training studies in breast cancer patients. The MeSH terms and text word used for the search of studies included: exercise and cancer, exercise oncology, breast cancer, breast cancer and exercise, oncology, exercise training, exercise therapy, neoplasms, and malignancies. All references relevant to the topic were hand-searched.
Studies selection criteria
Included in the review were all exercise training studies that used aerobic training or aerobic in combination with resistance exercise training, or had a study arm evaluating the effects of aerobic training or aerobic in combination with resistance exercise training. These studies objectively assessed the effects that these exercise training modes had in breast cancer patients on at least one of the following fitness and/or patient rated outcomes variables including: cardiorespiratory function, body composition, muscular strength, fatigue, depression, and overall quality of life. These outcome variables were chosen because they have been the most commonly evaluated variables in the area of exercise oncology during the past 25 years. Studies excluded from the review involved those conducted in pediatric patients (age below 18 years); that used non-conventional exercise prescriptions (i.e., Yoga, aqua aerobics, Tai Chi, etc.); not published in English; used traditional exercise training programs in combination with other interventions (i.e., dietary manipulations, psychosocial therapies, counseling, etc.); secondary articles from the same research team with data previously published on initial original article of outcome variables of interest of this review; studies that evaluated the results of the exercise intervention in breast cancer patients mixed with other types of cancers; studies with non-retrievable incomplete data sets, review articles, guidelines articles, letters to editor, and animal based studies. Since very few studies have used resistance training as the main mode of exercise, a separate evaluation summarizing the results of studies that objectively measured muscular strength using the 1 maximum repetition (1-RM) and presented data on at least one of the major outcomes mentioned above are also presented. Five reviewers (Mills RC, Phillips BL, Lee JT, Story CE, Nascimento MGB) independently identified the studies that met the criteria for the review mentioned above and discrepancies were resolved by consensus among all authors.
Statistical analyses
Descriptive statistics were used in the form of mean and standard deviations for the presentation of main outcome variables data at baseline and post-exercise intervention. Comparisons between baseline and post-exercise training data were conducted using dependent samples t tests for both the exercise and control (non exercise training) groups. For studies where there was more than one exercise arm (i.e., self-directed and supervised exercise), when no differences were observed for changes between groups, both groups were included in the analyses. An alpha of 0.05 set a priori was used for all tests. No alpha level adjustment was performed for the multiple comparisons. Due to differences of instruments used to evaluate the main outcome variables included in this review, only variables evaluated by at least two independent studies were included in the analyses. For variables evaluated by only one study, means, standard deviations, and change from baseline to post-intervention values were provided when data was retrievable.
RESULTS
Between September and November of 2013, 1903 potential citations for inclusion in the review were identified. After an initial screening of all of 1903 potential citations, 210 citations were included in a second screening for further evaluation of inclusion eligibility criteria. After the second screening, 51 citations were deemed eligible. The literature review citation selection process flow chart is presented in Figure 1.
Figure 1.
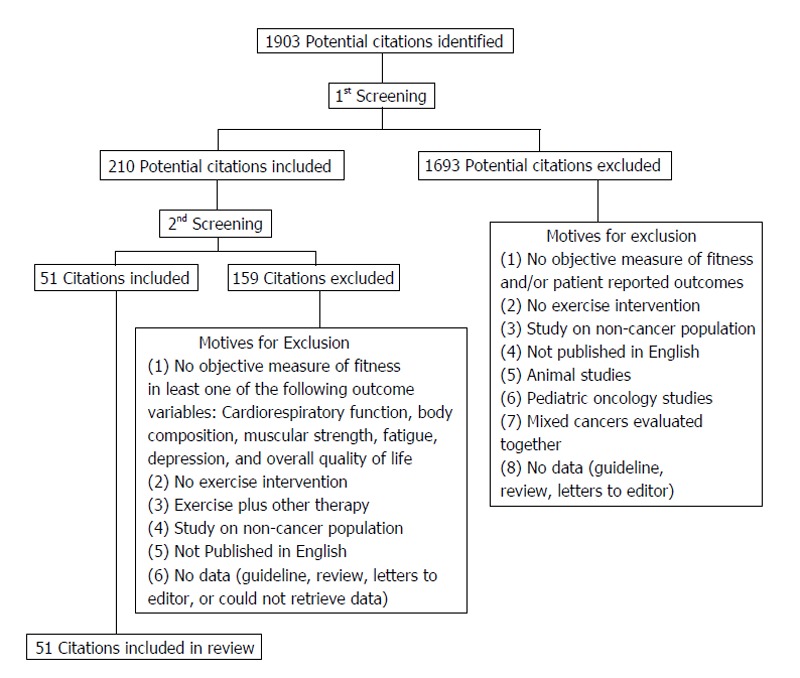
Literature review citation selection flow chart.
For organizational purposes a chronological description of studies results conducted in this area of research during the past 25 years was arbitrarily divided into the following time periods: (1989-1999, 2000-2006, 2007-2013).
1989-1999
During the decade between 1989 and 1999, only 5 studies met the inclusion criteria for this review[5-9]. All 5 studies were conducted while patients were receiving active treatment including chemotherapy and/or radiation therapy and the average age was 46.4 ± 1.5 for the patients who participated in the exercise interventions and 46.6 ± 3.4 for patients in the control groups. Four of the studies were randomized controlled trials and more than half of the studies were home-based interventions (n = 3)[7-9]. The summary of the results of the studies is presented in Table 1.
Table 1.
1989-1999 study results summary
| Measure | No. of studies | N |
Baseline |
Post-intervention |
Change |
P | |||
| Mean | SD | Mean | SD | Mean | 95%CI | ||||
| Cardiorespiratory function | |||||||||
| Bike VO2peak (L/min) | |||||||||
| Exercise | 1 | 45 | 1.02 | - | 1.45 | - | 0.43 | - | - |
| Control | 0 | ||||||||
| 12-min walk test (m) | |||||||||
| Exercise | 2 | 25 | 1123.7 | 7.1 | 1216.7 | 42.3 | 93.1 | -632 | 0.166 |
| Control | 2 | 16 | 1052.6 | 76.2 | 965.5 | 30.1 | -87.1 | -2476 | 0.536 |
| Body composition | |||||||||
| Body mass (kg) | |||||||||
| Exercise | 1 | 22 | 66.57 | - | - | ||||
| Control | 1 | 24 | 62.16 | - | - | ||||
| Body fat (%) | |||||||||
| Exercise | 1 | 12 | - | - | -0.51 | ||||
| Control | 1 | 12 | - | - | 2.19 | ||||
| Lean body mass (kg) | |||||||||
| Exercise | 1 | 12 | - | - | 2.04 | ||||
| Control | 1 | 12 | - | - | -1.26 | ||||
| Quality of life | |||||||||
| QOL Index, 0–100 | |||||||||
| Exercise | 1 | 27 | 61.4 | - | 56.8 | - | -4.60 | - | - |
| Control | 0 | - | - | - | - | - | - | - | - |
| Fatigue | |||||||||
| SympASF, 0-100 | |||||||||
| Exercise | 2 | 31 | 12.5 | 2.1 | 22.0 | 5.7 | 9.50 | -63.5 | 0.164 |
| Control | 2 | 29 | 19.5 | 7.8 | 41.5 | 5.0 | 22 | -50.8 | 0.058 |
| Depression | |||||||||
| SymptASD, 0-100 | |||||||||
| Exercise | 2 | 31 | 11.5 | 6.4 | 11.5 | 2.1 | 0.00 | -76.2 | 1.000 |
| Control | 2 | 29 | 9.5 | 2.1 | 14.5 | 9.1 | 5.00 | -127 | 0.500 |
Lower scores reflect lower fatigue and lower depression. VO2peak: Peak oxygen uptake; QOL: Quality of life; SympASF: Symptom Assessment Scale-Fatigue; SymptASD: Symptom assessment Scale-Depression.
Only 1 study[6] assessed the effects of exercise training on cardiorespiratory function using a direct measurement through a cardiopulmonary exercise test (CPET) on a cycle ergometer with gas exchange; 2 evaluated overall physical function using the 12-min walk test; 1 focused on the effects of exercise training on body weight and body composition[5]; the other 3 studies focused on patient reported outcomes[7-9]. The overall exercise prescription used in these 5 initial studies included 100% aerobic exercise training prescriptions, with two studies using an interval-training methodology[5,6]. The frequency of training ranged from 3-5 sessions per week (average of 3.7 sessions per week), with training sessions of 15-45 min (average of 27 min). The only modes of exercise used on these studies included either walking or cycle ergometry with an exercise training program length varying from 6-24 wk of training (average of 11 wk). For the studies that used heart rate for the prescription of exercise intensity, 60%-85% of heart rate reserve was used. The majority of studies that used walking as the mode of exercise, the description of exercise intensity was subjective and included terms such as brisk walk, self-paced, low to moderate, or incremental exercise.
No statistical significance was observed for changes in any outcome variable analyzed. However, within the results the most noticeable effect of the exercise training was observed for the variable fatigue. A trend towards significance was observed for the variable fatigue in the control group with increased values from baseline to post-exercise training of 22 points in a scale from 0-100 representing a 22% increase in fatigue levels. This increase was of clinical relevance since the exercise group showed a much lower fatigue response. No data on body composition was analyzed, however, a trend towards a reduction in body fat % and gain in lean body mass was observed in the exercise group while the control group experienced the opposite effect. Even though no statistical analysis was performed on the variable cardiorespiratory function (only one study), an increase of VO2max of 0.43 L/min was observed, representing a clinically relevant improvement on this variable with exercise training. No adverse events resulting from the exercise training was reported in any study.
2000-2006
From 2000 to 2006, 11 studies[10-20] met the review inclusion criteria with 6 of them being randomized controlled trials. More than half of the studies during this time period were conducted in patients who had completed chemotherapy and/or radiation therapy (n = 6, 54.5%) while all of the other studies (45.5%) were conducted with patients undergoing treatment (n = 4), and 1 study including patients in and off-treatment as part of the study design[12]. The average age for patients that participated in the exercise interventions was 51.09 ± 4.6 and 51.03 ± 4.9 for patients in the control groups. The majority of studies used an exercise prescription that combined aerobic with resistance training (68.8%) with only 31.2% of the studies using aerobic training only. The major modes of aerobic exercise included walking, swimming and cycling. No studies during this time period used resistance training exclusively as the major mode of exercise. On average, aerobic exercise was prescribed 2-4 d/wk for approximately 30 min, with intensities varying from 50%-80% of peak heart rate, 50%-75% of heart rate reserve, rate of perceived exertion between 11-15 with overall training duration between 8-14 wk. The resistance training portion of the exercise prescription lasted approximately 40 min (37 ± 17 min) and consisted of exercises targeting major muscles groups performed for 2-3 sets, with approximately 10-12 repetitions using weight training machines, dumbbells, and rubber bands. The description of exercise intensity used in the 2000-2006 studies that included resistance training as a portion of the exercise intervention varied significantly and clear descriptions of the intensities used were often missing. For example, one study used the patient’s own body weight to induce a training response[14] while one study would increase the resistance between 5%-10% of the previous load as soon as patients were able to achieve the maximum number of repetitions prescribed[19]. All other studies that used resistance training did not describe the intensity used during the resistance portion of the exercise intervention. The duration of training in studies that used aerobic or a combination of aerobic and resistance training was approximately 11 wk (10.9 ± 6.78). As mentioned, six studies were randomized controlled trials[11,13,16,17,19,20]. The primary mean for prescribing and monitoring exercise intensity was heart rates, which varied from 70%-75% of VO2peak, 40%-70% of estimated VO2max, 60%-90% of predicted maximum heart rate, and 60%-70% of heart rate reserve. This heterogeneity in prescribing exercise intensity is troublesome since significant differences in the determination of exercise intensity from using one method versus the other can produce significantly different prescriptions. Eleven studies were performed between 2000-2006[10-20]. Seven were conducted in a supervised setting[11,12,13,15,16,19,20], while 3 were home-based interventions[10,14,17,18]. One study used a supervised exercise session plus 2 self-monitored exercise sessions at home per week[14], while 1 study used a period of two weeks familiarizing subjects to the exercise intervention in a supervised setting followed by a home-based self-monitored exercise training for the remaining of the study[18]. The summary of the results of the studies conducted between 2000-2006 is presented in Table 2.
Table 2.
2000-2006 study results summary
| Measure | No. of studies | N |
Baseline |
Post-intervention |
Change |
P | |||
| Mean | SD | Mean | SD | Mean | 95%CI | ||||
| Cardiorespiratory function | |||||||||
| Bike VO2peak (mL/kg per minute) | |||||||||
| Exercise | 5 | 81 | 23.26 | 2.95 | 25.40 | 2.75 | 2.14 | -4.28 | 0.000 |
| Control | 2 | 36 | 23.10 | 6.08 | 21.95 | 5.30 | -1.15 | -13.98 | 0.284 |
| Bike VO2peak (L/min) | |||||||||
| Exercise | 1 | 22 | 1.67 | 0.35 | 1.81 | 0.37 | 0.14 | - | - |
| Control | 1 | 19 | 1.6 | 0.36 | 1.63 | 0.35 | 0.03 | - | - |
| Est. Treadmill. VO2peak (mL/kg per minute) | |||||||||
| Exercise | 1 | 40 | 30.58 | - | 35.20 | - | 4.62 | - | - |
| Control | 0 | - | - | ||||||
| VO2peak (L/min) | |||||||||
| Exercise | 1 | 22 | 1.67 | - | 1.81 | - | 0.14 | - | - |
| Control | 1 | 19 | 1.6 | - | 1.63 | - | 0.03 | - | - |
| mCAFT VO2peak (mL/kg per minute) | |||||||||
| Exercise | 1 | 82 | 25.7 | 0.28 | 26.45 | 0.49 | -0.75 | - | - |
| Control | 1 | 41 | 25.1 | 6.10 | 25.10 | 6.10 | 0.00 | - | - |
| 12-min walk test (m) | |||||||||
| Exercise | 2 | 56 | 1032.35 | 8.98 | 1264.8 | 126.15 | 232.45 | -2428.16 | 0.248 |
| Control | 2 | 37 | 1045.2 | 59.11 | 944.8 | 194.03 | -100.4 | -2424.34 | 0.484 |
| 6-min walk test (m) | |||||||||
| Exercise | 1 | 9 | 672.9 | - | 776.03 | - | 103.13 | - | - |
| Control | 0 | ||||||||
| 1 mile walk test (min) | |||||||||
| Exercise | 1 | 43 | 17.45 | - | 16.34 | - | -1.11 | - | - |
| Control | 1 | 43 | 17.65 | - | 17.85 | - | 0.20 | - | - |
| Body composition | |||||||||
| Body mass (kg) | |||||||||
| Exercise | 9 | 248 | 69.47 | 10.36 | 68.43 | 9.54 | -1.04 | -2.83 | 0.131 |
| Control | 7 | 183 | 75.80 | 6.46 | 76.64 | 6.31 | 0.84 | -2.62 | 0.166 |
| Fat mass (kg) | |||||||||
| Exercise | 4 | 75 | 25.95 | 9.73 | 23.93 | 8.57 | -2.03 | -7.57 | 0.187 |
| Control | 3 | 73 | 32.90 | 8.18 | 33.20 | 6.52 | -0.30 | -8.44 | 0.789 |
| Body fat (%) | |||||||||
| Exercise | 8 | 218 | 33.82 | 8.25 | 32.31 | 8.03 | 1.51 | 0.37-2.66 | 0.017 |
| Control | 6 | 165 | 36.48 | 9.29 | 36.66 | 8.96 | 0.18 | -2.03 | 0.673 |
| Strength | |||||||||
| 1RM UB composite (kg) | |||||||||
| Exercise | 1 | 21 | 9.1 | 1.93 | 10.16 | 2.08 | 0.96 | - | - |
| Control | 1 | 15 | 8.77 | 1.75 | 8.59 | 1.29 | -0.18 | - | - |
| 1RM UB (kg) | |||||||||
| Exercise | 2 | 67 | 22.63 | 10.15 | 30.23 | 13.39 | 7.61 | -58.33 | 0.187 |
| Control | 0 | ||||||||
| 1RM LB (kg) | |||||||||
| Exercise | 3 | 76 | 99.87 | 31.29 | 133.19 | 57.10 | 33.32 | -141.42 | 0.180 |
| Control | 0 | ||||||||
| Psychosocial Measures | |||||||||
| FACT-G | |||||||||
| Exercise | 2 | 37 | 78.75 | 9.55 | 87.60 | 5.23 | 8.85 | -77.50 | 0.211 |
| Control | 2 | 38 | 83.90 | 6.93 | 82.70 | 9.33 | -1.20 | -43.20 | 0.609 |
| FACT-B | |||||||||
| Exercise | 3 | 47 | 100.3 | 9.30 | 111.03 | 7.42 | 10.73 | -15.37 | 0.027 |
| Control | 2 | 38 | 105.75 | 13.79 | 105.05 | 15.20 | -0.70 | -25.42 | 0.611 |
| SF-36 | |||||||||
| Exercise | 1 | 42 | 76.30 | 0.28 | 94.75 | 8.98 | 18.45 | - | - |
| Control | 1 | 41 | 83.50 | - | 102.4 | - | 18.90 | - | - |
| WHO-QOL BREF | |||||||||
| Exercise | 1 | 27 | 8.2 | 0.92 | 8.8 | 0.78 | 0.60 | - | - |
| Control | 0 | ||||||||
| PFS | |||||||||
| Exercise | 2 | 22 | 5.07 | 0.24 | 3.47 | 0.47 | -1.61 | 0.50-4.81 | 0.194 |
| Control | 1 | 10 | 4.87 | 2.50 | 4.62 | 2.50 | -0.25 | - | - |
| SCFS | |||||||||
| Exercise | 1 | 44 | 10.70 | 3.75 | 10.20 | 6.00 | -0.50 | - | - |
| Control | 1 | 27 | 10.80 | 0.00 | 13.40 | 5.60 | 2.60 | - | - |
| LAS | |||||||||
| Exercise | 1 | 43 | 42.47 | 23.54 | 27.08 | 21.41 | -15.39 | - | - |
| Control | 1 | 43 | 41.66 | 25.04 | 42.28 | 26.20 | 0.62 | - | - |
| BDI | |||||||||
| Exercise | 1 | 40 | 5.84 | 4.90 | 3.92 | 4.00 | -1.92 | - | - |
| Control | 0 | ||||||||
| HRSD | |||||||||
| Exercise | 1 | 40 | 9.46 | 6.00 | 6.10 | 5.00 | -3.36 | - | - |
| Control | 0 | ||||||||
VO2peak: Peak oxygen uptake; mCAFT: Modified Canadian Fitness Test; UB composite: Upper body combined strength measures; UB: Upper body strength test; LB: Lower body strength test; EORTC: European Organization for Research and Treatment of Cancer; FACT-G: Functional Assessment of Cancer Therapy-General; FACT-B: Functional Assessment of Cancer Therapy-Breast; SF-36: Short-Form-36; WHO-QOL BREF: The World Health Organization-BREF Quality of Life Instrument; PFS: Piper Fatigue Scale; SCFS: Schwartz Cancer Fatigue Scale; LAS: Linear Analog Scale; BDI: Beck Depression Inventory; HRSD: The Hamilton Rating Scale for Depression; Est. Treadmill VO2peak: VO2peak estimated via submaximal CPET; CPET: Cardiopulmonary exercise test.
Cardiorespiratory function, when assessed directly using maximal CPET with gas exchange via cycle ergometer, showed significant improvements in patients who underwent exercise training (P < 0.0001). An improvement corresponding to an approximately 9% (2.14 mL/kg per minute) from baseline testing was observed for the exercise group while the control group showed no significant changes[11,13,14,18,19]. When cardiorespiratory function was indirectly measured (i.e., using submaximal/estimated VO2max testing protocols), no significant changes in either the exercise or control groups were observed, however, a trend towards improvements in the exercise group was observed. Conversely, patients in the control group did not change or end up decreasing cardiorespiratory function from baseline to completion of study protocols[6]. Due to the small number of studies that have assessed some strength parameter and the lack of a control group in studies conducted during the period of 2000-2006, it is not possible at this time to provide an evaluation on the effects of a combined aerobic and resistance training exercise intervention on measurements of strength in breast cancer patients.
The only body composition parameter that significantly changed from baseline was body fat % in the exercise group (change of 1.51%). No significant changes in any other body composition parameter was observed in either exercise or control groups. However, a trend towards positive changes for the exercise group (i.e., reduction in body mass and fat mass) and some negative changes in the control group such as increases in body mass, fat mass, and body fat %, suggesting that exercise training may be a promising intervention in alleviating negative body composition changes in breast cancer patients warranting further research[5].
Significant improvements in overall quality of life assessed using the Functional Assessment of Cancer Therapy-Breast was observed in the exercise group (P = 0.027) with no changes in the control group. No other PROs were significant in either group; however, trends of reductions in fatigue and depression were observed in the exercise but not in the control group[9]. A few adverse events were reported during 2000-2006 and included lymphedema (3 cases), gynecologic complications (1 case), and influenza (1 case)[13].
2007-2013
From 2007 to November 2013, 35 exercise studies involving breast cancer patients were conducted[21-55]. Twenty-seven (77%) of all studies conducted during this time period were randomized controlled trials. Out of all 35 exercise studies, 22 examined the effects of exercise training in patients undergoing treatment (62.8%), 1 study studied patients in and off treatment[40] and 1 study examined the effects of exercise in patients receiving neo-adjuvant therapy[55]. The average age for patients that participated in the exercise interventions was 58.0 ± 3.9 and 53.7 ± 4.5 for patients in the control groups. Most of the studies were conducted in a supervised setting (n = 27, 77% of all studies conducted between 2007 and November 2013). Sixteen studies used aerobic exercise as the main mode of exercise training, 12 used a combination of aerobic and resistance exercise training, while only 7 used resistance exercise training as the main mode of exercise training or had a study arm that examined the effects of resistance training on outcome measurement/variables of interest to this review. The modes of exercise for the aerobic exercise training interventions included walking, jogging, treadmill, cycle ergometer, elliptical, low-impact aerobics, stepping, arm ergometer, rowing ergometer, mini-trampolines, step-up blocks, and circuit training. For studies that used resistance training as their major mode of exercise or as the mode of a study arm, weight machines, free weights, elastic bands, tubing, therapeutic balls, and resistance-training circuits were used. The average frequency of training was 3 d/wk ranging from 2-5 training sessions per week, with overall training duration average of 23 wk (range of 3-48 wk). Each training session lasted on average 46 min, with training sessions ranging from 15-90 min. The intensity for aerobic training varied from 40%-85% of maximum heart rate, 40%-90% of VO2peak, while the intensity for resistance training ranged from 55%-85% of 1RM. Minimal adverse events due to exercise were reported; when reported, events such as lightheaded, hypotension, nausea, and weakness during exercise testing were basically the extent of the complications with patients recovering quickly afterwards.
Most studies assessed cardiorespiratory function using estimated measurement protocols. When using estimated measurements derived from a submaximal treadmill testing protocol, a significant improvement in cardiorespiratory function was observed for the exercise group (improvement from baseline of 3.54 mL/kg per minute, P < 0.0005). When cardiorespiratory function was measured directly using a cycle ergometer maximal oxygen uptake test protocol with gas exchange, improvement from baseline approached significance in the exercise group (improvement from baseline of 2.38 mL/kg per minute, P = 0.057). Significant decrease in meters walked during a 6 min and 2 km walk tests was observed in the control group (-9.52 m, -0.61 m, P = 0.008 and P = 0.010 respectively) while improvements in a 12 min walk test approached significance in the exercise group (improvement of +162 m, P = 0.062). A summary of the results of the effects of exercise on cardiorespiratory function is presented in Table 3.
Table 3.
2007-2013 cardiorespiratory function study results summary
| Measure | No. of studies | N |
Baseline |
Post-intervention |
Change |
P | |||
| Mean | SD | Mean | SD | Mean | 95%CI | ||||
| Cardiorespiratory function | |||||||||
| Treadmill VO2peak (mL/kg per minute) | |||||||||
| Exercise | 1 | 71 | 25.20 | - | 25.70 | - | 0.5 | - | - |
| Control | 1 | 73 | 24.80 | - | 23.50 | - | -1.3 | - | - |
| Treadmill VO2peak (L/min) | |||||||||
| Exercise | 1 | 71 | 1.72 | - | 1.77 | - | 0.05 | - | - |
| Control | 1 | 73 | 1.76 | - | 1.68 | - | -0.08 | - | - |
| Est. Treadmill VO2peak (mL/kg per minute) | |||||||||
| Exercise | 9 | 265 | 23.51 | 4.56 | 27.05 | 5.12 | 3.54 | -4.84-2.26 | < 0.0005 |
| Control | 5 | 88 | 24.45 | 6.08 | 25.02 | 6.80 | 0.57 | -2.85 | 0.328 |
| Bike VO2peak (mL/kg per minute) | |||||||||
| Exercise | 2 | 37 | 22.11 | 3.68 | 24.49 | 3.38 | 2.38 | -5.47 | 0.057 |
| Control | 2 | 33 | 21.98 | 6.34 | 20.83 | 6.82 | -1.15 | -8.77 | 0.185 |
| Bike VO2peak (L/min) | |||||||||
| Exercise | 1 | 10 | 1.41 | - | 1.59 | - | 0.18 | - | - |
| Control | 1 | 10 | 1.34 | - | 1.20 | - | -0.14 | - | - |
| Est. Bike VO2peak (mL/kg per minute) | |||||||||
| Exercise | 1 | 18 | 21.07 | - | 23.88 | - | 2.81 | - | - |
| Control | 0 | 0 | - | - | - | - | - | - | - |
| 6-min walk test (m) | |||||||||
| Exercise | 2 | 30 | 463.23 | 56.37 | 482.57 | 49.84 | 19.34 | -117.4 | 0.149 |
| Control | 2 | 32 | 450.14 | 33.57 | 440.62 | 33.40 | -9.52 | 8.05-10.98 | 0.008 |
| 12-min walk test (m) | |||||||||
| Exercise | 3 | 129 | 931.00 | 102.93 | 1093.0 | 160.18 | 162 | -364.7 | 0.062 |
| Control | 3 | 124 | 921.03 | 148.50 | 888.13 | 132.92 | -32.9 | -258.0 | 0.387 |
| 2 km walk test (min) | |||||||||
| Exercise | 3 | 313 | 18.05 | 0.44 | 17.38 | 0.42 | -0.67 | -1.60 | 0.070 |
| Control | 3 | 280 | 17.94 | 0.33 | 17.33 | 0.23 | -0.61 | 0.34-0.86 | 0.010 |
VO2peak: Peak oxygen uptake; Treadmill VO2peak: VO2peak obtained directly through CPET with gas exchange; Est. Treadmill VO2peak: VO2peak estimated via submaximal CPET; CPET: Cardiopulmonary exercise test; Est.: Estimated.
Positive body composition changes with exercise training were observed in the exercise group, with the most notorious changes in significant decrease in body fat % (P = 0.037) and increase in lean body mass (P = 0.002). The control group experience significant increases in body fat % (P = 0.009) and a trend toward an increase in overall body mass (P = 0.065). Table 4 provides a summary of studies that evaluated changes in body composition outcomes.
Table 4.
2007-2013 body composition study results summary
| Measure | No. of studies | N |
Baseline |
Post-intervention |
Change |
P | |||
| Mean | SD | Mean | SD | Mean | 95%CI | ||||
| Body composition | |||||||||
| Body mass (kg) | |||||||||
| Exercise | 10 | 550 | 74.66 | 3.94 | 74.25 | 3.84 | -0.41 | -0.38 to 1.20 | 0.268 |
| Control | 9 | 527 | 73.59 | 4.63 | 74.72 | 4.69 | +1.13 | -2.35 to 0.09 | 0.065 |
| Fat mass (kg) | |||||||||
| Exercise | 1 | 262 | 25.73 | - | 26.43 | - | +0.70 | - | - |
| Control | 1 | 236 | 24.45 | - | 25.12 | - | +0.67 | - | - |
| Body fat (%) | |||||||||
| Exercise | 13 | 609 | 38.00 | 5.03 | 37.15 | 5.80 | -0.85 | 0.06 to 1.64 | 0.037 |
| Control | 11 | 536 | 38.50 | 4.41 | 39.00 | 4.12 | +0.50 | -0.84 to -0.16 | 0.009 |
| Lean body mass (kg) | |||||||||
| Exercise | 3 | 330 | 43.91 | 0.32 | 44.29 | 0.31 | +0.38 | -0.45 to -0.31 | 0.002 |
| Control | 3 | 308 | 43.45 | 0.37 | 43.27 | 0.56 | -0.18 | -0.49 to 0.87 | 0.358 |
| Lean body mass (%) | |||||||||
| Exercise | 1 | 10 | 71.00 | - | 74.10 | - | +3.10 | - | - |
| Control | 1 | 10 | 69.10 | - | 68.90 | - | -0.20 | - | - |
Quality of life significantly improved in the exercise group when the Functional Assessment of Cancer Therapy-General was used (improvement of +6.90 points, P = 0.022). Also, when using the Beck Inventory, the effects of exercise training significantly reduced depression levels in the exercise group (reduction of -3.40 points, P = 0.037). Even though many of the analyses performed on other PROs showed no significant changes in either group, there is a clear trend towards greater improvement in quality of life, reduction in fatigue and depression in the exercise group when compared to the control group. The summary of the analyses performed on reported PROs is presented in Table 5.
Table 5.
2007-2013 patient reported outcomes study results summary
| Measure | No. of studies | N |
Baseline |
Post-intervention |
Change |
P | |||
| Mean | SD | Mean | SD | Mean | 95%CI | ||||
| Quality of life | |||||||||
| EORTC, 0-100 | |||||||||
| Exercise | 2 | 296 | 74.05 | 13.36 | 79.95 | 7.71 | +5.90 | -101.64 | 0.379 |
| Control | 1 | 236 | 80.90 | - | 84.30 | - | 3.40 | - | - |
| FACT-G, 0-104 | |||||||||
| Exercise | 4 | 165 | 78.98 | 3.30 | 85.88 | 4.22 | 6.90 | -10.05 | 0.022 |
| Control | 4 | 178 | 79.74 | 6.89 | 79.63 | 10.88 | -0.11 | -21.22 | 0.976 |
| FACT-B, 0-104 | |||||||||
| Exercise | 5 | 99 | 103.92 | 6.75 | 110.32 | 4.25 | 6.40 | -17.44 | 0.111 |
| Control | 5 | 82 | 108.76 | 5.72 | 106.53 | 9.98 | -2.23 | -22.82 | 0.616 |
| SF-36 physical function, 0-100 | |||||||||
| Exercise | 1 | 25 | 47.41 | - | 50.80 | - | 3.39 | - | - |
| Control | 0 | 0 | - | - | - | - | - | - | - |
| Fatigue1 piper, 0-10 | |||||||||
| Exercise | 3 | 140 | 3.88 | 1.05 | 3.08 | 0.41 | -0.80 | -4.34 | 0.250 |
| Control | 2 | 45 | 3.78 | 0.17 | 3.91 | 0.35 | 0.13 | -3.30 | 0.500 |
| FSI, 1-10 | |||||||||
| Exercise | 1 | 11 | 3.40 | - | 3.40 | - | 0.00 | - | - |
| Control | 1 | 9 | 3.25 | - | 3.30 | - | 0.05 | - | - |
| FACIT-F | |||||||||
| Exercise | 4 | 373 | 37.85 | 2.95 | 39.58 | 5.87 | 1.73 | -10.49 | 0.372 |
| Control | 4 | 351 | 40.11 | 4.07 | 40.08 | 3.73 | -0.03 | -6.12 | 0.975 |
| Depression2 BDI, 0-63 | |||||||||
| Exercise | 2 | 93 | 11.75 | 0.07 | 8.35 | 0.35 | -3.40 | 0.86-5.94 | 0.037 |
| Control | 2 | 102 | 10.96 | 2.89 | 11.21 | 0.42 | 0.25 | -44.48 | 0.910 |
| R-Beck Inventory | |||||||||
| Exercise | 2 | 295 | 8.53 | 7.11 | 4.60 | 1.98 | -3.93 | -92.24 | 0.475 |
| Control | 2 | 271 | 7.15 | 5.15 | 6.67 | 5.18 | -0.48 | 0.23-0.73 | 0.027 |
| CES-D, 0-24 | |||||||||
| Exercise | 1 | 71 | 12.80 | - | 9.70 | - | -3.10 | - | - |
| Control | 1 | 73 | 13.90 | - | 10.80 | - | -3.10 | - | - |
| HADS, 0-21 | |||||||||
| Exercise | 1 | 30 | 4.17 | - | 2.70 | - | -1.47 | - | - |
| Control | 1 | 28 | 4.79 | - | 4.64 | - | -0.15 | - | - |
Lower scores reflect lower fatigue;
Lower scores reflect lower depression. BDI: Beck Depression Inventory; EORTC: European Organization for Research and Treatment of Cancer; FACT-G: Functional Assessment of Cancer Therapy-General; FACT-B: Functional Assessment of Cancer Therapy-Breast; SF-36: Short-Form-36; FSI: The Fatigue Symptom Inventory; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; BDI: Beck Depression Inventory; R-Beck Inventory: R-Beck Depression Inventory; CES-D: Center for epidemiologic studies depression scale (short-form); HADS: Hospital anxiety and depression scale.
Resistance training
Due to the limited number of studies that used resistance training as the main mode of exercise training[42,46,52,54], and those that used resistance training as an arm part of the study design[27,29,43], we were unable to conduct analyses to evaluate the effects of resistance training on cardiorespiratory function measured directly through CPET with gas analyses. However, the effects of resistance training on cardiorespiratory function evaluated using a 12-min walk test, showed improvement in the exercise group of 35 m while a decline of 91 m was observed for the control group. Regarding the effects of resistance training on changes in body composition, a significant increase in body mass and lean body mass was observed in the exercise group (increase of 1.20 and 0.65 kg, P = 0.016 and P = 0.049, respectively). Upper body strength significantly increased in the exercise group (increase of +5.31 kg, P = 0.005) while for lower body, strength significantly increased in both, exercise and control groups (P = 0.25, P = 0.008 respectively) with a greater increase observed in the exercise group (increase of +17.82 kg vs +5.42 kg, respectively). No significant changes in quality of life were observed in either the exercise and control groups. The summary of the results of the effects of resistance training on outcomes included in this review is presented in Table 6.
Table 6.
2007-2013 resistance exercise study results summary
| Measure | No. of studies | N |
Baseline |
Post-intervention |
Change |
P | |||
| Mean | SD | Mean | SD | Mean | 95%CI | ||||
| Cardiorespiratory fitness | |||||||||
| Treadmill VO2peak (mL/kg per minutes) | |||||||||
| Exercise | 1 | 77 | 25.5 | - | 24.20 | - | -1.30 | - | - |
| Control | 1 | 73 | 24.8 | - | 23.50 | - | -1.30 | - | - |
| Treadmill VO2peak (L/min) | |||||||||
| Exercise | 1 | 77 | 1.73 | - | 1.67 | - | -0.06 | - | - |
| Control | 1 | 73 | 1.76 | - | 1.68 | - | -0.08 | - | - |
| Est. Treadmill VO2peak (mL/kg per minutes) | |||||||||
| Exercise | 1 | 9 | 23.46 | - | 24.22 | - | 0.76 | - | - |
| Control | 0 | 0 | - | - | - | - | - | - | - |
| 12-min walk test (m) | |||||||||
| Exercise | 1 | 21 | 1020.00 | - | 1055.00 | - | 35.00 | - | - |
| Control | 1 | 23 | 1035.00 | - | 944.00 | - | -91.00 | - | - |
| Body composition | |||||||||
| Body mass (kg) | |||||||||
| Exercise | 3 | 136 | 72.53 | 2.96 | 73.73 | 2.70 | 1.20 | -1.32 | 0.016 |
| Control | 3 | 129 | 72.33 | 1.81 | 73.30 | 0.95 | 0.97 | -4.29 | 0.192 |
| Fat mass (kg) | |||||||||
| Exercise | 2 | 59 | 28.10 | 3.25 | 28.70 | 3.11 | 0.60 | -2.54 | 0.105 |
| Control | 2 | 56 | 26.70 | 3.25 | 27.50 | 2.12 | 0.80 | -20.32 | 0.500 |
| Body fat (%) | |||||||||
| Exercise | 4 | 145 | 36.75 | 2.82 | 36.60 | 3.05 | -0.15 | -1.18 | 0.476 |
| Control | 3 | 129 | 37.27 | 2.57 | 37.97 | 2.15 | 0.70 | -3.94 | 0.266 |
| Lean body mass (kg) | |||||||||
| Exercise | 2 | 59 | 44.35 | 1.34 | 45.00 | 1.41 | 0.65 | -1.29 to -0.01 | 0.049 |
| Control | 2 | 56 | 44.70 | 0.00 | 45.15 | 0.07 | +0.45 | -1.28 | 0.070 |
| Muscular strength | |||||||||
| 1-RM upper body composite (kg) | |||||||||
| Exercise | 1 | 43 | 32.93 | - | 39.38 | - | 6.45 | - | - |
| Control | 1 | 19 | 31.45 | - | 32.30 | - | 0.85 | - | - |
| 1-RM upper body single test (kg) | |||||||||
| Exercise | 5 | 166 | 26.37 | 5.03 | 31.68 | 4.78 | 5.31 | -7.94 to -2.69 | 0.005 |
| Control | 4 | 152 | 27.60 | 3.79 | 28.65 | 3.15 | +1.05 | -2.27 | 0.059 |
| 1-RM lower body single test (kg) | |||||||||
| Exercise | 5 | 200 | 70.58 | 29.80 | 88.40 | 38.01 | 17.82 | -32.06 to -3.58 | 0.025 |
| Control | 5 | 171 | 68.19 | 25.36 | 73.61 | 27.74 | +5.42 | -8.51 to -2.33 | 0.008 |
| Quality of Life | |||||||||
| SF-36 physical function, 0-100 | |||||||||
| Exercise | 2 | 79 | 47.80 | 3.54 | 50.28 | 2.02 | +2.48 | -27.31 | 0.261 |
| Control | 2 | 50 | 49.00 | 3.96 | 48.85 | 4.88 | -0.15 | -16.52 | 0.856 |
| Fatigue1 SCFS, 6-36 | |||||||||
| Exercise | 1 | 36 | 9.90 | - | 10.10 | - | 0.20 | - | - |
| Control | 1 | 31 | 9.30 | - | 9.00 | - | -0.30 | - | - |
| FACIT-F | |||||||||
| Exercise | 1 | 77 | 34.30 | - | 36.30 | - | 2.00 | - | - |
| Control | 1 | 73 | 34.60 | - | 34.90 | - | 0.30 | - | - |
| Depression2 CES-D, 0-24 | |||||||||
| Exercise | 1 | 77 | 13.80 | - | 10.60 | - | -3.20 | - | - |
| Control | 1 | 73 | 13.90 | - | 10.80 | - | -3.10 | - | - |
Lower scores reflect lower fatigue;
Lower scores reflect lower depression. VO2peak: Peak oxygen uptake; Est. Treadmill VO2peak: VO2peak estimated via submaximal CPET; CPET: Cardiopulmonary exercise test; Est.: Estimated; RM: Repetition Maximum; SF-36: Short-Form-36; SCFS: Schwartz cancer fatigue scale; FACIT-F: Functional assessment of chronic illness therapy-fatigue; CES-D: Center for Epidemiologic Studies Depression Scale (short-form).
DISCUSSION
During the past 25 years, studies examining the effects of exercise training in breast cancer survivors have steadily increased. Based of the inclusion criteria adopted by this systematic review, from 5 initial studies published between 1989-1999, 35 studies were published during the last 7 years. The increase in published studies in this area of research is a testament of the growing interest by health care professionals and the medical community on exploring complementary therapies that have the potential to improve the overall care and health of breast cancer patients while promoting alleviation of cancer treatment-related side-effects. The evolution of the science examining the effects of exercise in breast cancer patients has progressed from studies using simpler exercise prescriptions (i.e., aerobic exercise only prescriptions) to more complex designs incorporating prescriptions that combined aerobic and resistance training exercises. Interestingly, however, of the studies conducted during first decade[5-9], all examined the effects of exercise while patients were undergoing major cancer treatments (i.e., surgery, chemotherapy and/or radiation therapy) and the majorities were home-based interventions[7-9]. Besides some limitations presented by these initial studies[5-9] (including relatively small sample sizes and lack of more rigorous exercise prescriptions) the overall results were promising. Breast cancer patients, who exercised while undergoing cancer treatments, were not only able to tolerate the exercise prescriptions but also presented a more favorable trend towards alleviation of decrements in functional capacity, fatigue levels, and depression when compared to patients who did not exercise. Also, and very importantly, no adverse events were reported and patients seemed to have no problems engaging in regular exercise training. Although limited data were available for a more precise evaluation of these initial studies, the results were promising and served as the basis for subsequent trials conducted with breast cancer patients.
The most noticeable differences between the initial studies conducted between 1989-1999 and those between 2000-2006 were the use of aerobic exercise combined with resistance training in the majority of the studies (68.8%) and the fact that over 50% of these studies were conducted in patients who had completed their major cancer treatments. Furthermore, the heterogeneity of exercise prescriptions and a variety of different measurements used to assess the major outcome variables included in this review makes it impossible for direct comparison to be made between studies. Importantly during 2000-2006, a significant increase in number of breast cancer patients studied and overall improvements in the quality of study methodologies, marked a new era in this area of research with significant improvements observed in physiological (i.e., improved VO2max, decreases in body fat %) and overall quality of life in the exercise group, while the control patients continued to present not so favorable outcomes during and post completion of cancer treatments. Out of studies that utilized exercise prescriptions following a training progression (i.e., considered by the exercise physiology scientific community as efficacious training methodologies in the promotion of more pronounced training responses) a study conducted by Courneya et al[14], showed a remarkable improvement in cardiorespiratory function using a prescription derived from the results of a gold standard evaluation of cardiopulmonary function (CPET with gas analyses). Not only did Courneya’s study show an improvement of 17.4% in cardiorespiratory function in patients randomized into the exercise group, the patients in the control group decreased their cardiopulmonary function by 3.4%. Furthermore, these authors provided a better description of the sample, exercise testing procedures, and exercise prescription, along with the reporting of adverse events that occurred during the study improved the scientific rigor in this area, which was later followed by many groups around the world.
Overall, the results of all studies conducted during 2000-2006 continued to show promising results, mainly on cardiorespiratory function, body composition, and overall quality of life of patients, increasing even more the interest of the medical community for the use of exercise as an intervention to alleviate treatment-related side effects. With improved science approaches, the challenges faced by investigators around the globe began to surface even more. The heterogeneity in exercise prescriptions characteristics, different instruments that were used to evaluate major outcome variables, different treatment regiments, and testing of different exercise interventions designed to provide the most benefit to patients were among major issues that preclude ones ability to evaluate the real benefit of exercise in breast cancer patients.
During the last 7 years of research evaluating the effects of exercise in breast cancer patients, more pronounced improvements were observed in all major outcome variables included in this review when compared to previous years. Significant improvements in cardiorespiratory function of 2.38 mL/kg per minute, when measured directly using cycle ergometry and slightly higher values (3.54 mL/kg per minute) observed when cardiorespiratory function was estimated used submaximal testing protocols were reported. The larger improvement in cardiorespiratory function can be explained, in part, by the more rigorous exercise prescriptions as well as due to the inclusion of the resistance training as a component of training. Furthermore, more pronounced improvements were also observed in various body composition parameters. Significant decreases in body fat % and increases in lean body mass were observed in the exercise group while significant increase and no change in body fat % and lean body respective were observed for the control group. Furthermore, a trend of overall weight gain was more noticeable in the control group than the exercise group. Again, the inclusion of resistance training on study design of a large portion of the studies conducted between 2007-2013 may help explain the more pronounced positive changes observed in the exercise groups. Lastly, significant decrease in depression along with improvements in quality of life observed in the exercise group (PROs), while no change in quality of life and slight increases in depression in patients on the control group, once again support the idea that exercise during breast cancer help patients to live a higher overall quality of life than patients that do not engage in regular exercise training.
The inclusion of resistance training on the study designs during 2007-2013 as a study arm or even as the main exercise training mode, has been utilized with the objective of producing more effective changes in overall functionality and to alleviate the negative alterations in body composition commonly observed in breast cancer patients during the entire cancer continuum (i.e., increases in body mass, with concomitant increase in fat mass and loss of muscle mass; condition known as sarcopenic obesity). The inclusion of this type of training has also provided for variety in the training prescription of cancer patients, which results in increased adherence; a key factor to promote more robust physiological adaptations. A few studies did not meet the inclusion criteria for this review, however, those studies examined the effects of exercise on biological markers associated with the negative changes in body composition. This area of study is highly significant due to the influence negative changes in body composition may have on the development of secondary cancers, mortality and co-morbidities (e.g., sarcopenic obesity development consequences)[2]. Recently, an exercise-related model proposed by our group on the alleviation of negative changes in body composition and its potential associations with biomarkers of inflammation and androgenic hormones has been developed and initial investigations are promising[56].
Very little data available today allow for a good understanding on the effects of resistance training on the outcomes included on this review. However, as expected, due to the known effects of resistance training on muscle mass gain and muscular strength, significant improvements were observed on lean body mass and upper and lower body strength on patients who participated in exercise training and were objectively measured on these outcomes.
Unfortunately, due to the small number of studies that met the rigorous criteria for inclusion in this review, the data on effects of strength training on PROs are very limited and no definite conclusion can be drawn at this time regarding the effects of resistance training on fatigue, depression, and overall quality of life in breast cancer patients. More research is desperately needed in this area of study.
There are limitations within this systematic review on the effects of exercise in breast cancer survivors that must be considered when evaluating the overall results; including a small number of studies per outcome variable included in this review, the heterogeneity of tools used to evaluate the outcome variables of interest, significant differences in the characteristics of the exercise prescription utilized, and to a much lesser extent, the evaluation of patients in and off treatment included together in the analyses of the data. The later could impose difficulty in interpreting the results, however, a priori evaluation of the overall changes in the parameters included in this review indicated very little clinical difference between results of studies that evaluated patients undergoing treatment and those off treatment. Therefore, we decided that for a chronological overview of the current literature in the topic, the clinical difference observed between patients in and off treatment would not drastically under or overestimate the evaluation of the outcome variables studied. Since this is a relatively new area of research and until larger randomized trials are completed (controlling for many variables that can confound study results such as type, frequency, intensity of exercise, different types of cancer treatments, age of patients, previous fitness levels, and other co-morbidities that can further diminish the tolerability for exercise participation) definite conclusions and more precise exercise summary guidelines can be presented.
Despite these methodological limitations and our inability to currently provide specific exercise guidelines for breast cancer patients, general-generic exercise guidelines[2] are available and can be used to guide safer participation in exercise programs It is recommended by the exercise guidelines set forth by the American College of Sports Medicine[2], that patients should engage, whenever possible, in 150 min/wk of moderate-intensity exercise spread throughout the week, that the exercise prescription should include aerobic and resistance modes of exercise, and that the prescription should be individualized taking into consideration the limitations of each patients.
In conclusion, order to improve the knowledge in the area of research examining the effects of exercise in breast cancer patients more studies are necessary. These studies should include larger samples sizes, involve randomized clinical controlled trials, provide more detailed descriptions of the sample studied (i.e., presence of other co-morbidities, previous physical activity levels, amount of therapies received, etc.) and all testing and exercise protocols (extremely important consideration for improvement in the quality of the science) in their published reports, and provide better reporting on adverse events due to exercise testing and training. Furthermore, studies examining the mechanisms involved in the plasticity of different physiological systems due to different exercise training protocols in breast cancer patients are critical for continued progress of this area of research. Nevertheless, based on the current data available in this area of research, exercise training appears to be safe for most patients and improvements in physiological, psychological, and functional parameters can be attained with regular participation in moderate intensity exercise.
COMMENTS
Background
An ever-growing number of studies in the area of exercise oncology, especially those examining the effects of exercise training in breast cancer survivors, speak loudly to the increased interest by the medical community in exploring complementary interventions that can alleviate treatment-related side effects and improve quality of life of cancer patients.
Research frontiers
Most specifically, this review focuses on presenting and discussing the results of the effects of exercise training on the most commonly evaluated outcomes in the area including physiological parameters of cardiorespiratory function, body composition, and muscular strength, and patients reported outcomes that evaluated fatigue, depression, anxiety, and overall quality of life.
Innovations and breakthroughs
For organizational purposes a chronological description of studies results, studies common characteristics, and commentaries on changes observed in this area of research over the past 25 years are discussed.
Peer review
The arm of this study was to review the effect of exercises for patients with breast cancer in the past 25-years. It is fairly well designed and the statistical analyses appear reasonable. It is worthy of recommending for publication.
Footnotes
P- Reviewers: Iams W, Wu YT S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.World Health Organization. International agency for research on cancer. 2013. pp. 1–3. Available from: http: //www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf. [Google Scholar]
- 2.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 5.Winningham ML, MacVicar MG, Bondoc M, Anderson JI, Minton JP. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. 1989;16:683–689. [PubMed] [Google Scholar]
- 6.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients‘ functional capacity. Nurs Res. 1989;38:348–351. [PubMed] [Google Scholar]
- 7.Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney-Tedder S, Schwager LP, Liebman M. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 1994;21:899–907; discussion 908. [PubMed] [Google Scholar]
- 8.Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, Quitasol W, Mitchell S, Chakravarthy A, Gage I. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24:991–1000. [PubMed] [Google Scholar]
- 9.Schwartz AL. Fatigue mediates the effects of exercise on quality of life. Qual Life Res. 1999;8:529–538. doi: 10.1023/a:1008978611274. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz AL. Exercise and weight gain in breast cancer patients receiving chemotherapy. Cancer Pract. 2002;8:231–237. doi: 10.1046/j.1523-5394.2000.85007.x. [DOI] [PubMed] [Google Scholar]
- 11.Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 12.Kolden GG, Strauman TJ, Ward A, Kuta J, Woods TE, Schneider KL, Heerey E, Sanborn L, Burt C, Millbrandt L, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology. 2002;11:447–456. doi: 10.1002/pon.591. [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 14.Turner J, Hayes S, Reul-Hirche H. Improving the physical status and quality of life of women treated for breast cancer: a pilot study of a structured exercise intervention. J Surg Oncol. 2004;86:141–146. doi: 10.1002/jso.20065. [DOI] [PubMed] [Google Scholar]
- 15.Hutnick NA, Williams NI, Kraemer WJ, Orsega-Smith E, Dixon RH, Bleznak AD, Mastro AM. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37:1827–1835. doi: 10.1249/01.mss.0000175857.84936.1a. [DOI] [PubMed] [Google Scholar]
- 16.Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 18.Cheema BS, Gaul CA. Full-body exercise training improves fitness and quality of life in survivors of breast cancer. J Strength Cond Res. 2006;20:14–21. doi: 10.1519/R-17335.1. [DOI] [PubMed] [Google Scholar]
- 19.Herrero F, San Juan AF, Fleck SJ, Balmer J, Pérez M, Cañete S, Earnest CP, Foster C, Lucía A. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27:573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 20.Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29:156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Nikander R, Sievänen H, Ojala K, Oivanen T, Kellokumpu-Lehtinen PL, Saarto T. Effect of a vigorous aerobic regimen on physical performance in breast cancer patients - a randomized controlled pilot trial. Acta Oncol. 2007;46:181–186. doi: 10.1080/02841860600833145. [DOI] [PubMed] [Google Scholar]
- 22.Herrero F, San Juan AF, Fleck SJ, Foster C, Lucia A. Effects of detraining on the functional capacity of previously trained breast cancer survivors. Int J Sports Med. 2007;28:257–264. doi: 10.1055/s-2006-924348. [DOI] [PubMed] [Google Scholar]
- 23.Battaglini C, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D, Hackney AC. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007;125:22–28. doi: 10.1590/S1516-31802007000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews CE, Wilcox S, Hanby CL, Der Ananian C, Heiney SP, Gebretsadik T, Shintani A. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 25.Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, Kearney N, Walker A, Ritchie D. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334:517. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25:1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34:627–633. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 28.Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat. 2007;104:145–152. doi: 10.1007/s10549-006-9410-x. [DOI] [PubMed] [Google Scholar]
- 29.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 30.Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, Adloff K, Keshaviah A, Winer EP. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 31.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 32.Portela AL, Santaella CL, Gómez CC, Burch A. Feasibility of an Exercise Program for Puerto Rican Women who are Breast Cancer Survivors. Rehabil Oncol. 2008;26:20–31. doi: 10.1901/jaba.2008.26-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes DC, Leung P, Naus MJ. Using single-system analyses to assess the effectiveness of an exercise intervention on quality of life for Hispanic breast cancer survivors: a pilot study. Soc Work Health Care. 2008;47:73–91. doi: 10.1080/00981380801970871. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CC, Sprod LK, Hydock DS, Carter SD, Hayward R, Schneider CM. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008;35:909–915. doi: 10.1188/08.ONF.909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 36.Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, Chung GG, Jones B, Knobf MT, DiPietro L. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009;17:1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 38.Rahnama N, Nouri R, Rahmaninia F, Damirchi A, Emami H. The effects of exercise training on maximum aerobic capacity, resting heart rate, blood pressure and anthropometric variables of postmenopausal women with breast cancer. J Res Med Sci. 2010;15:78–83. [PMC free article] [PubMed] [Google Scholar]
- 39.Ligibel JA, Partridge A, Giobbie-Hurder A, Campbell N, Shockro L, Salinardi T, Winer EP. Physical and psychological outcomes among women in a telephone-based exercise intervention during adjuvant therapy for early stage breast cancer. J Womens Health (Larchmt) 2010;19:1553–1559. doi: 10.1089/jwh.2009.1760. [DOI] [PubMed] [Google Scholar]
- 40.DeNysschen CA, Brown JK, Cho MH, Dodd MJ. Nutritional symptom and body composition outcomes of aerobic exercise in women with breast cancer. Clin Nurs Res. 2011;20:29–46. doi: 10.1177/1054773810379402. [DOI] [PubMed] [Google Scholar]
- 41.Mehnert A, Veers S, Howaldt D, Braumann KM, Koch U, Schulz KH. Effects of a physical exercise rehabilitation group program on anxiety, depression, body image, and health-related quality of life among breast cancer patients. Onkologie. 2011;34:248–253. doi: 10.1159/000327813. [DOI] [PubMed] [Google Scholar]
- 42.Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127:447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musanti R. A study of exercise modality and physical self-esteem in breast cancer survivors. Med Sci Sports Exerc. 2012;44:352–361. doi: 10.1249/MSS.0b013e31822cb5f2. [DOI] [PubMed] [Google Scholar]
- 44.Brdareski Z, Djurović A, Susnjar S, Zivotić-Vanović M, Ristić A, Konstantinović L, Vucković-Dekić L, Tankosić M. Effects of a short-term differently dosed aerobic exercise on maximum aerobic capacity in breast cancer survivors: a pilot study. Vojnosanit Pregl. 2012;69:237–242. [PubMed] [Google Scholar]
- 45.Saarto T, Sievänen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, Kautiainen H, Järvenpää S, Penttinen HM, Utriainen M, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012;23:1601–1612. doi: 10.1007/s00198-011-1761-4. [DOI] [PubMed] [Google Scholar]
- 46.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6:189–199. doi: 10.1007/s11764-011-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikander R, Sievänen H, Ojala K, Kellokumpu-Lehtinen PL, Palva T, Blomqvist C, Luoto R, Saarto T. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients--a 12-month RCT. J Musculoskelet Neuronal Interact. 2012;12:127–135. [PubMed] [Google Scholar]
- 48.Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10:188–194. doi: 10.1016/j.suponc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Saarto T, Penttinen HM, Sievänen H, Kellokumpu-Lehtinen PL, Hakamies-Blomqvist L, Nikander R, Huovinen R, Luoto R, Kautiainen H, Järvenpää S, et al. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32:3875–3884. [PubMed] [Google Scholar]
- 50.Fernández-Lao C, Cantarero-Villanueva I, Ariza-Garcia A, Courtney C, Fernández-de-las-Peñas C, Arroyo-Morales M. Water versus land-based multimodal exercise program effects on body composition in breast cancer survivors: a controlled clinical trial. Support Care Cancer. 2013;21:521–530. doi: 10.1007/s00520-012-1549-x. [DOI] [PubMed] [Google Scholar]
- 51.Milecki P, Hojan K, Ozga-Majchrzak O, Molińska-Glura M. Exercise tolerance in breast cancer patients during radiotherapy after aerobic training. Contemp Oncol (Pozn) 2013;17:205–209. doi: 10.5114/wo.2013.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, Luoh SW, Schwartz A. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24:1637–1646. doi: 10.1007/s00198-012-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Vicari S, Rao K, Edson B, Verhulst S, Courneya KS, Hoelzer K. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12:323–335. doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cormie P, Pumpa K, Galvão DA, Turner E, Spry N, Saunders C, Zissiadis Y, Newton RU. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7:413–424. doi: 10.1007/s11764-013-0284-8. [DOI] [PubMed] [Google Scholar]
- 55.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE, Coan A, Gutierrez A, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 56.Battaglini CL, Hackney AC, Goodwin ML. Cancer cachexia: muscle physiology and exercise training. Cancers (Basel) 2012;4:1247–1251. doi: 10.3390/cancers4041247. [DOI] [PMC free article] [PubMed] [Google Scholar]


