Abstract
Early detection and diagnosis of breast cancer are essential for successful treatment. Currently mammography and ultrasound are the basic imaging techniques for the detection and localization of breast tumors. The low sensitivity and specificity of these imaging tools resulted in a demand for new imaging modalities and breast magnetic resonance imaging (MRI) has become increasingly important in the detection and delineation of breast cancer in daily practice. However, the clinical benefits of the use of pre-operative MRI in women with newly diagnosed breast cancer is still a matter of debate. The main additional diagnostic value of MRI relies on specific situations such as detecting multifocal, multicentric or contralateral disease unrecognized on conventional assessment (particularly in patients diagnosed with invasive lobular carcinoma), assessing the response to neoadjuvant chemotherapy, detection of cancer in dense breast tissue, recognition of an occult primary breast cancer in patients presenting with cancer metastasis in axillary lymph nodes, among others. Nevertheless, the development of new MRI technologies such as diffusion-weighted imaging, proton spectroscopy and higher field strength 7.0 T imaging offer a new perspective in providing additional information in breast abnormalities. We conducted an expert literature review on the value of breast MRI in diagnosing and staging breast cancer, as well as the future potentials of new MRI technologies.
Keywords: Breast magnetic resonance imaging, Cancer, Diffusion-weighted imaging, Spectroscopy, 7.0 Tesla
Core tip: Early detection and diagnosis of breast cancer are essential for successful treatment. Magnetic resonance imaging (MRI) has become increasingly important in the detection and delineation of breast cancer in daily practice. However, the clinical benefits of the use of pre-operative MRI in women with newly diagnosed breast cancer is still a matter of debate. We conducted a literature review on the value of breast MRI in diagnosing and staging breast cancer, as well as the future potentials of new MRI technologies, such as MR spectroscopy, diffusion-weighted imaging and higher field strength 7.0 Tesla imaging.
INTRODUCTION
Breast cancer is the most common malignant disease occurring in women worldwide with a lifetime risk of 12.4%[1,2]. Early detection and diagnosis of breast cancer are prerequisites for successful treatment selection. Although mammography and ultrasound are the most commonly imaging tools used for the detection and characterization of breast abnormalities, the relatively low sensitivity and specificity of these techniques (especially in patients with dense breast tissue, with breast implants or postsurgical scar distortions)[3-6] resulted in a demand for new imaging modalities. Contrast enhanced magnetic resonance imaging (CE-MRI) with its high soft tissue contrast, multiplanar sectioning and three dimensional representation of the breast provides high sensitivity (over 90%) in the detection of breast cancer. However, the specificity for lesion characterization is still low to moderate (72%)[7-19], turning the discrimination between cancer and benign lesions into a challenge. The main additional diagnostic value of MRI relies on (1) detecting foci of multifocal, multicentric or contralateral disease unrecognized on conventional assessment (physical examination, mammography and ultrasound); (2) recognition of invasive components in ductal carcinoma in situ (DCIS); (3) assessing the response to neoadjuvant chemotherapy (NAC); (4) detecting an occult primary breast cancer in patients presenting with metastatic cancer in axillary nodes; and (5) detection of cancer in dense breast tissue[14,20-26]. The development of new technologies has also resulted in information gain concerning breast lesions. We reviewed the recent literature to clarify the role of MRI in diagnosing and staging breast cancer with focus on the implementation of new techniques, such as MR spectroscopy, diffusion-weighted imaging (DWI) and higher field strength 7.0 T imaging.
SEARCH
In this expert literature review, we conducted a literature search on Pubmed in papers published between 1990 and 2013 using the keywords “breast”, “MRI”, “staging”, “spectroscopy”, “diffusion-weighted imaging” and “high field breast MRI”. Articles published in English pertaining to adult humans with available abstracts were included. References of articles were also included. First we present main guidelines on the use of MRI in diagnosing and staging of breast cancer and, subsequently, we will discuss the new technologies currently available for research.
RESULTS
Detection of additional disease
The main evidence in favor of MRI is based on the superior capability of this technique in detecting ipsilateral and contralateral disease, when compared to mammography and ultrasound (Figure 1)[21,26,27]. In a prospective trial, Schelfout et al[26,27] found that MRI detected 96% of multifocal/multicentric disease, while mammography and ultrasound depicted only 28.6% and 26.5%, respectively. Taking the histological types of breast cancer into account, invasive lobular carcinoma (responsible for 5% to 15% of all cases of invasive breast cancers)[28-30] is well known to have a higher incidence of multifocal, multicentric and contralateral disease when compared to invasive ductal carcinoma. MRI is, therefore, particularly important in the preoperative work-up and staging of these patients[7,11,12,30-32]. In a recent retrospective study, Menezes et al[33] also found a high incidence of multifocal, multicentric and contralateral disease in patients with mixed tumors containing different percentages of lobular component. This might corroborate the hypothesis that MRI would also be valuable in the work-up of patients with mixed breast tumors (Figure 2). MRI also has shown higher accuracy in determining tumor size (correlated to histopathology) than ultrasound or mammography[26]. However, some studies emphasize that MRI tends to overestimate lesion size, particularly in patients diagnosed with invasive lobular carcinoma and DCIS[34-38].
Figure 1.
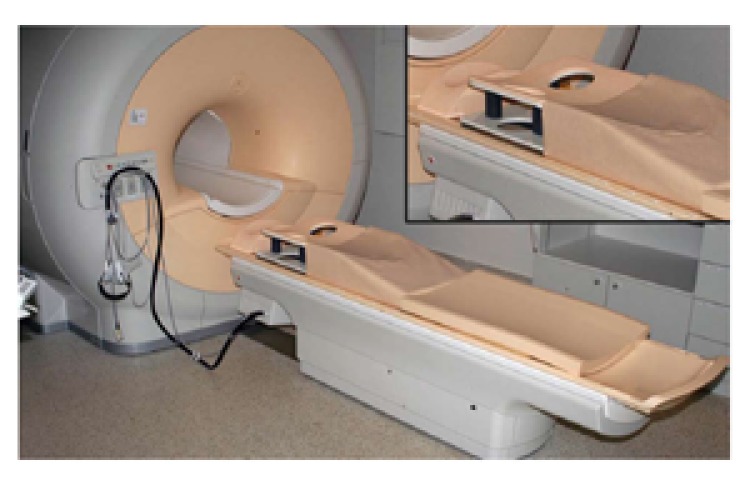
Magnetic resonance imaging scanner with closed bore magnet and a dedicated 8 channel phased-array breast coil (top right). Technically any magnetic resonance imaging scanner could be used in breast image acquisition. However, in daily practice, field strengths of 1.5 T and 3 T are often used due to higher spatial resolution at similar temporal resolution, providing better diagnostic efficacy.
Figure 2.
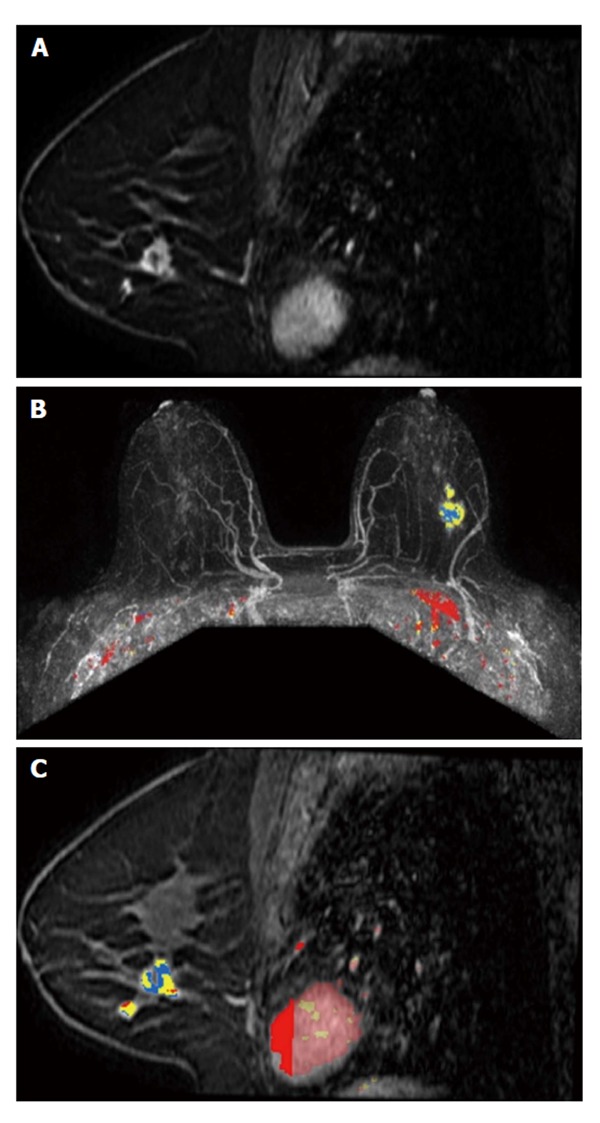
A 48-year-old woman, with positive family history for breast cancer, presented with a palpable lump on the left breast, finally diagnosed as invasive ductolobular carcinoma. A: Sagittal contrast-enhanced fat-suppressed T1-weighted gradient echo images obtained at 3 T shows a spiculated mass, with rim enhancement and small satellite lesion (multifocal disease); B and C: Color parametric enhancement map in axial postcontrast maximum intensity projection and sagittal projection indicates predominantly a plateau enhancement behavior, with some areas of washout.
An additional value of MRI is the detection of invasive component in DCIS lesions. In a retrospective study, Kim et al[38] concluded that MRI was more accurate for the detection and assessment of the size of DCIS than mammography. In a prospective cohort, Hwang et al[22] demonstrated MRI to be superior to mammography in detecting invasive components in patients diagnosed with DCIS. MRI also showed a higher sensitivity and superior negative predictive value for detection of residual DCIS. However, the small number of patients in this study (51) might not be representative of a large population with DCIS. Further research might be necessary in order to confirm the use of MRI in detecting invasive component in DCIS.
Patients with an increased risk
MRI has an important role in screening high-risk patients. The American Cancer Society Guidelines for the Early Detection of Cancer advices annual breast MRI beginning at the age of 25-30 years in patients carrying a BRCA gene mutation, in women who are untested but have a first-degree relative with a BRCA mutation and women with an approximately 20% to 25% or greater lifetime risk of breast cancer[39-41]. In a cohort of 496 women, Passaperuma et al[42] concluded that MRI surveillance of women with BRCA mutations detects most breast cancers at an early stage. Although the results of this study are promising, data on longer-term follow up is needed in order to encourage MRI surveillance as a safe alternative to prophylactic mastectomy. Likewise, patients diagnosed with breast cancer under the age of 50 have a 20% lifetime risk of recurrence (even after radiotherapy to the tumor bed following surgical approach)[43,44]. In these particular cases, the European Society of Breast Imaging also recommends annual MRI screening[45].
Patients with dense breast parenchyma
Additional MRI can be beneficial in patients with dense breast parenchyma. Mammography has a high false negative rate in patients with dense breast tissue[46-49], and the sensitivity remains low in dense breasts even when computer-aided detection is applied to digital mammography[50]. In a large multicenter study, Schnall et al[51] proved that MRI has superior capability to detect additional occult cancer foci when compared to mammography, particularly in women with radiographically dense breasts and larger index cancers (18% vs 7.2%). Many other studies confirm that MRI has the highest diagnostic value when used in patients with heterogeneous or extremely dense breast parenchyma[21,26,27]. The European Breast Imaging Society also advices the use of pre-operative MRI in staging malignant lesions in patients with dense breast tissue[45]. MRI also has a substantial advantage in detecting breast lesions in scattered fibroglandular breast parenchyma[20,52,53].
Impact on treatment
Despite all advantages of MRI, there is no consistent evidence supporting the clinical benefit of pre-operative MRI for all patients with breast cancer. In the MONET trial, 418 patients with non-palpable BI-RADS 3-5 lesions were randomized to undergo routine clinical care (211 patients) or standard clinical care associated to contrast enhanced MRI (207 patients) prior to large core needle biopsy[54,55]. In total 74 patients had 83 malignant lesions in the MRI group and 75 patients had 80 malignant lesions in the control group. The choice of prioritizing non-palpable breast tumors was based on the difficultness in determining the margins of a lesion that cannot be seen or palpable during surgery. Thus, additional surgical intervention is often required in those cases. The authors hypothesized that the use of CE-MRI of the breast would reduce the need of additional surgical procedure, once MRI would add important three-dimensional information about the lesion, would be an important tool in defining tumor margins and in detecting multifocal and multicentric disease.
Surprisingly the re-excision rate due to positive resection margins after breast conserving therapy was even higher in the MRI group (34%) than in the control group (12%). Also the rate of additional surgical interventions after initial breast conserving therapy was higher in the MRI group than in the control group (45% vs 28%), although significance was not reached (P = 0.069). The COMICE trial attempted to determine whether the addition of breast MRI in 1623 breast cancer patients proven by triple assessment (clinical, radiological and pathological) would aid tumor localization and reduce re-operation rates. Patients were randomized to undergo or not MRI. The results showed no difference in the re-operation rates between the study arms (18.8% in patients who underwent MRI vs 19.3% in patients who did not undergo MRI). The economic analysis of this trial showed no significant difference in cost-effectiveness between the research arms. The addition of MRI to triple assessment did not reduce the re-operation rates, and the use of MRI consumed extra resource with few benefits[56]. Differently from the MONET study, the COMICE trial included patients with breast cancer proved by biopsy and most patients presented with palpable tumors. Both trials have a high level of evidence and, in both studies, the authors admonished the use of pre-operative breast MRI as a routine clinical care in patients with non-palpable breast cancer[55]. Up to now, the results of these randomized controlled trials discourage the standard use of pre-operative MRI in all patients with breast cancer.
TECHNOLOGICAL DEVELOPMENTS AND ONGOING RESEARCH
Morphologic assessment can be a subjective task. It is strongly related to the experience of the radiologist and it is vulnerable to interobserver variations (especially in small lesions and nonmass-like lesions). An adjunct method which could provide a higher specificity would be of value. The use of new available technologies, such as breast MR spectroscopy and DWI is being verified in order to improve the accuracy and specificity of CE-MRI[57-60].
Diffusion weighted imaging
DWI is a non-invasive MRI technique that measures the mobility of water molecules in tissue, providing information such as cellular density, viscosity, membrane integrity, and tissue microstructure, without the need of contrast injection[59,61]. DWI is able to differentiate between tissue types based on the use of the apparent diffusion coefficient (ADC). Malignant breast tumors usually have a higher cellularity and generally present with restricted water diffusion and lower ADC values when compared to benign lesions[62,63]. CE-MRI enables the assessment of morphological and kinetic patterns of benign and malignant breast tumors, but has a low specificity and high false-negative rates[17,64,65]. The use of DWI is being considered as a new approach in order to improve the sensitivity and specificity of breast lesion characterization and may be incorporated into routine breast MRI assessment of breast lesions. In a retrospective study, El Khouli et al[66] selected 93 women with 101 lesions (68 malignant tumors and 33 benign tumors) who underwent MRI using a 3.0 T magnet and both CE-MRI and DWI were performed. The association of DWI with ADC significantly improved the diagnostic performance and lesion characterization when compared to conventional 3D T1-weighted and CE-MRI at 3.0 T. In a study with 70 patients, Partridge et al[67] showed that CE-MRI added to ADC criteria providing a superior positive predictive value than contrast enhanced MRI alone (47% vs 37%). Tan et al[68] analyzed 44 breast lesions (31 malignant and 13 benignant) on 3.0 T MRI. The cut-off ADC values for benign and malignant lesions were 1.21 × 10-3 mm2/s for b = 500 s/mm2 and 1.22 × 10-3 mm2/s for b = 1000 s/mm2, respectively. Although the authors had a small sample size, the values obtained between ADC values of benign and malignant lesions were significant (P < 0.001). The sensitivity of CE-MRI was 100% with a specificity of 66.7%. CE-MRI combined with b = 1000 s/mm2, showed a specificity of 100% and a sensitivity of 90.6%. There was no significant between ADC values and prognostic factors[68]. Marini et al[69] investigated 81 breast lesions. Considering a mean diffusivity threshold value of 1.1 × 10-3 mm2/s, malignant lesions were differentiated from benign lesions with a sensitivity of 80% and specificity of 81%. A meta-analysis from Chen et al[70] described 964 lesions (maximum b = 1000 s/mm2, 95%CI, area under curve of summary receiver operating characteristic 0.9085). ACD measurement of DWI showed a sensitivity and specificity of both 84% to differentiate between benign and malignant lesions. DWI also has the advantage of not requiring the use of intravenous contrast and the use of this technique could be an alternative to CE-MRI. For example, in a retrospective study with 118 breast lesions (12 DCIS, 15 invasive carcinomas end 91 benign lesions), 89% of malignant breast tumors were found to be clearly hyperintense on DWI. ADC values helped in differentiating malignant from benign lesions. In a study of Yabuuchi et al[71], the authors compared the detection of non-palpable breast cancers in mammography, DWI and CE-MRI. DWI was significantly more accurate than mammography, although it has shown to be not as accurate as CE-MRI.
The use of DWI in patients with DCIS has also been described. Partridge et al[72] reported that ADC values of DCIS were lower when compared to benign lesions and invasive carcinoma. Rahbar et al[73] found 96% of pure DCIS lesions to be hyperintense in DWI.
Considering pre-treatment prediction of response to NAC in breast cancer patients, results suggest DWI associated to ADC to be useful for predicting tumor response. Park et al[74] performed DW-MRI (1.5 T, b values 0 and 750 s/mm2) and CE-MRI of 53 invasive breast cancers before and after chemotherapy prior to surgery. The percentage of ADC increase in responders was bigger than in non-responders (P < 0.001), the best cutoff to differentiate responders from non-responders was 1.17 × 10-3 mm2/s (sensitivity of 94% and a specificity of 71%).
Sharma et al[75] assessed the response of 56 patients with breast malignant lesions at four different times, before and after three cycles of NAC. ADC has shown a statistically significant change in volume and diameter in responders (sensitivity 68% and specificity 100%) and the authors suggested ADC would be useful in predicting early tumor response. According to Pickles et al[76], significant alterations on ADC could be observed even before changes in tumor size in patients undergoing chemotherapy. Therefore the authors suggested that DWI might have the ability to provide indication of response to treatment, preceding changes in tumor size.
Proton spectroscopy
Spectroscopy is an additional non-invasive method that can provide chemical information from a selected region in the body. In clinical practice, spectroscopy is used mainly for brain applications and prostate cancer[77,78]. Breast cancer spectroscopy is slightly behind that of prostate in development and in determining the suitability of this technique for clinical practice.
In mammary gland area, total choline (tCho), or just Cho, is considered the most important metabolite in proton MR spectroscopy. Many different metabolites overlap and contribute to the Cho peak, such as choline, phosphocholine, glycerophsphocoline, taurine and myo-inositol, among others[79-81]. The Cho peak is centered at 3.2 ppm.
Cholines are precursors of phospholipids which are components of cell membranes and increased Cho signals are associated with increased cellular turnover[82-84].
The use of breast MR spectroscopy to distinguish between benign and malignant lesions (using elevated tCho level as an indicator of malignancy) can potentially improve the accuracy of an MRI scan by offering increased specificity. In a recent systematic review and meta - analysis, Baltzer et al[85] included 19 studies with 1183 patients in order to evaluate the diagnostic performance of spectroscopy in differentiating breast lesions in field strengths of 1.5 and 3.0 T. They found a high pooled specificity (88%) and sensitivity (73%). Higher field strength, post contrast acquisition or qualitative vs quantitative MR spectroscopy had no significant influence on the results. Katz-Brull et al[86] performed a similar meta-analysis and found a combined sensitivity and specificity of 83% and 85%, respectively.
In a study with 184 patients with breast cancer, Shin et al[87] have shown that the use of absolute tCho-containing compound peak integral, normalized tCho-containing compound integral, and signal-to-noise-ratio determined by spectroscopy could be valuable in differentiating between IDC and DCIS. These same parameters could also be useful in determining tumor aggressiveness.
Many researchers suggest measurements if tCho with breast spectroscopy to be useful to monitor the response to NAC.
In a recent study, Tozaki et al[81] concluded that changes in Cho after NAC determined by 1H MR spectroscopy are more sensitive to predict the pathological response than changes in the tumor size. Meisamy et al[88] used a 4 T strength field to evaluate the concentration of tCho in patients diagnosed with breast cancer before NAC, within 24 h after the first dose and after the fourth dose. Twenty-four hours after the first dose there was a significant variation in concentration of tCho (compared to baseline) and this change had a significant positive correlation with the change in lesion size (P = 0.001). The change observed in tCho concentration after first dose of NAC was significantly different between responders and non-responders (P = 0.007).
Jacobs et al[89] evaluated NAC response using magnetic resonance spectroscopy, and 23Na magnetic resonance. According to the authors, multiparametric and multinuclear imaging parameters were reduced after the first cycle of NAC in responders, specifically, Cho signal-to-noise ratio and sodium (P ≤ 0.01).
To evaluate if applying DWI and spectroscopy together would help to improve the differentiation of breast lesions at 3.0 T, Tsougos et al[90] selected 51 women with known breast abnormalities (18 benign lesions and 33 malignant lesions). DWI and spectroscopy together provided higher accuracy and higher specificity for the differentiation between malignant and benign lesions when compared to these techniques used separately.
High field breast MRI at 7.0 Tesla
Recently, high-field MRI (7.0 T) has become available for research. 7.0 T MRI has an inherent advantage over lower field’s strengths and is, therefore, able to provide better signal to noise ratio, improve morphology assessment of breast lesions and increase the modality’s sensitivity and specificity (Figure 3)[57,58,60]. However, there are limitations in the use of 7.0 T. Higher magnetic field results in longer T1 relaxation time, shorter T2* decay time, greater radiofrequency, specific absorption rate, and increased B1 + field inhomogeneity. Nevertheless, some studies indicate that these disadvantages can be overcome.
Figure 3.
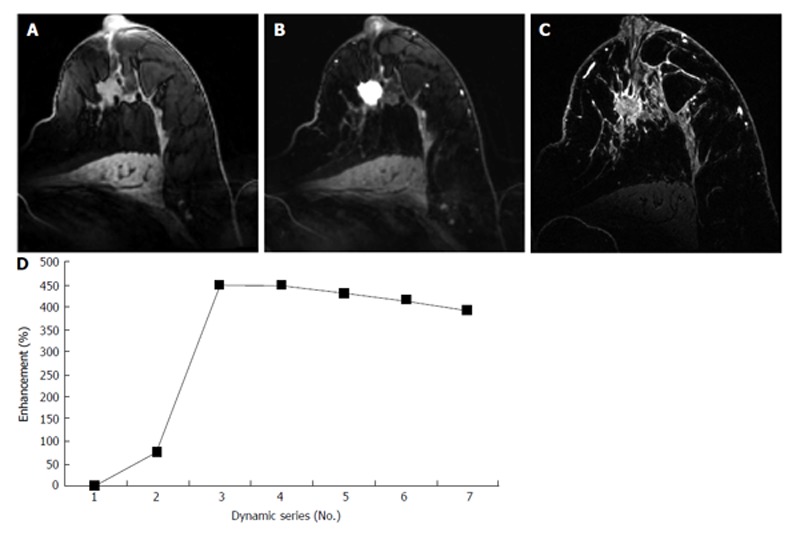
A 62-year-old patient with nipple withdrawal, finally diagnosed as ductolobular carcinoma. A, B and C: Axial T1-weighted gradient-echo images obtained at 7 T before and after contrast injection. An irregular mass with spiculated margins can be observed on pré-contrast imaging (A). An intense homogeneous enhancement (B) and a rapid wash-out kinetic curve (D) can be observed following contrast administration. In Figure 3C, an ultra-high-resolution T1-weighted gradient-echo sequence with fat suppression was performed, and the morphological aspects of the lesion can be more clearly seen.
Stehouwer et al[57] observed 7.0 T images in 20 patients with 23 suspicious breast lesions. The radiologist correctly identified all malignant tumors (BI-RADS 4 or 5) and in most cases image quality was considered good or excellent by both radiologists.
Field strength is considered an important factor affecting sensibility of spectroscopy. Particularly, 7.0 T is expected to provide increased signal to noise ratio and achieve more accurate information between closely overlapping resonances in the spectral domain[57,58,60,91,92]. In a recent study, Korteweg et al[92] selected 3 patients who received NAC and tried to establish if DWI and spectroscopy could provide diagnostic information in breast cancer patients. One of the patients had nonspecific reaction to NAC and, during the whole NAC course, an increment of values of ADC was observed, suggesting either tumor responsiveness or cystic development/tumoral necrosis. In addition a decrease in Cho concentration during NAC cycles was reported, which could also mean responsiveness of the tumor. After the last NAC course, Cho concentrations and tumor size increased, suggesting acquired resistance to treatment. Pathology assessment confirmed this hypothesis. A second patient had no visible index lesion on 3.0 T or at 7.0 T and Cho was undetectable on both examinations. No tumor was observed in pathologic analysis, which is suggestive that NAC was effective. Even low Cho levels (0.77 mmol/kgwater) were detected, suggesting high sensitivity of 7.0 T in detecting alterations in Cho Metabolism.
7.0 T is still in its early stages and studies with larger number of patients are required on order to confirm these results and check clinical applications.
CONCLUSION
To date, pre-operative MRI is indicated in defined groups of patients in which a potential benefit of local staging is expected, i.e., women with mammographically heterogeneous or extremely dense breasts, at high risk for breast cancer, diagnosed with invasive lobular carcinoma and/or with multifocal, multicentric or contralateral disease[93-95]. These recommendations are based on high-quality randomized controlled trials with narrow confidence intervals and on The American Cancer Society Guidelines for the Early Detection of Cancer and on the Guidelines from the European Breast Imaging Society[45,55,56,95]. MR spectroscopy, DWI and 7.0 T MRI of the breast are promising, but the clinical value of these techniques still remains unclear mostly due to the fact that the number of studies investigating these techniques is small and they are still in early stage Larger studies with more statistical power are necessary to confirm the clinical value and the cost-benefit of these new modalities.
Footnotes
P- Reviewers: Behzatoglu K, Hernanz F S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2013;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. Previous Version: SEER Cancer Statistics Review, 1975-2010. 2013. Available from: http: //seer.cancer. gov/csr/1975_2010/ [Google Scholar]
- 3.Samuels JR, Haffty BG, Lee CH, Fischer DB. Breast conservation therapy in patients with mammographically undetected breast cancer. Radiology. 1992;185:425–427. doi: 10.1148/radiology.185.2.1329142. [DOI] [PubMed] [Google Scholar]
- 4.Leibman AJ, Kruse B. Breast cancer: mammographic and sonographic findings after augmentation mammoplasty. Radiology. 1990;174:195–198. doi: 10.1148/radiology.174.1.2152981. [DOI] [PubMed] [Google Scholar]
- 5.Jackson VP, Hendrick RE, Feig SA, Kopans DB. Imaging of the radiographically dense breast. Radiology. 1993;188:297–301. doi: 10.1148/radiology.188.2.8327668. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am. 1992;30:107–138. [PubMed] [Google Scholar]
- 7.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, Ioffe OB. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 8.Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T, Hendriks JH, Ruys SH. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995;197:743–747. doi: 10.1148/radiology.197.3.7480749. [DOI] [PubMed] [Google Scholar]
- 9.Boetes C, Strijk SP, Holland R, Barentsz JO, Van Der Sluis RF, Ruijs JH. False-negative MR imaging of malignant breast tumors. Eur Radiol. 1997;7:1231–1234. doi: 10.1007/s003300050281. [DOI] [PubMed] [Google Scholar]
- 10.Boetes C, Veltman J, van Die L, Bult P, Wobbes T, Barentsz JO. The role of MRI in invasive lobular carcinoma. Breast Cancer Res Treat. 2004;86:31–37. doi: 10.1023/B:BREA.0000032921.10481.dc. [DOI] [PubMed] [Google Scholar]
- 11.Butler RS, Venta LA, Wiley EL, Ellis RL, Dempsey PJ, Rubin E. Sonographic evaluation of infiltrating lobular carcinoma. AJR Am J Roentgenol. 1999;172:325–330. doi: 10.2214/ajr.172.2.9930776. [DOI] [PubMed] [Google Scholar]
- 12.Chapellier C, Balu-Maestro C, Bleuse A, Ettore F, Bruneton JN. Ultrasonography of invasive lobular carcinoma of the breast: sonographic patterns and diagnostic value: report of 102 cases. Clin Imaging. 2004;24:333–336. doi: 10.1016/s0899-7071(00)00234-5. [DOI] [PubMed] [Google Scholar]
- 13.Harms SE, Flamig DP, Hesley KL, Meiches MD, Jensen RA, Evans WP, Savino DA, Wells RV. MR imaging of the breast with rotating delivery of excitation off resonance: clinical experience with pathologic correlation. Radiology. 1993;187:493–501. doi: 10.1148/radiology.187.2.8475297. [DOI] [PubMed] [Google Scholar]
- 14.Mumtaz H, Hall-Craggs MA, Davidson T, Walmsley K, Thurell W, Kissin MW, Taylor I. Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol. 1997;169:417–424. doi: 10.2214/ajr.169.2.9242745. [DOI] [PubMed] [Google Scholar]
- 15.Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 16.Orel SG, Schnall MD, LiVolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic-pathologic correlation. Radiology. 1994;190:485–493. doi: 10.1148/radiology.190.2.8284404. [DOI] [PubMed] [Google Scholar]
- 17.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 18.Qayyum A, Birdwell RL, Daniel BL, Nowels KW, Jeffrey SS, Agoston TA, Herfkens RJ. MR imaging features of infiltrating lobular carcinoma of the breast: histopathologic correlation. AJR Am J Roentgenol. 2002;178:1227–1232. doi: 10.2214/ajr.178.5.1781227. [DOI] [PubMed] [Google Scholar]
- 19.Rodenko GN, Harms SE, Pruneda JM, Farrell RS, Evans WP, Copit DS, Krakos PA, Flamig DP. MR imaging in the management before surgery of lobular carcinoma of the breast: correlation with pathology. AJR Am J Roentgenol. 1996;167:1415–1419. doi: 10.2214/ajr.167.6.8956569. [DOI] [PubMed] [Google Scholar]
- 20.Amano G, Ohuchi N, Ishibashi T, Ishida T, Amari M, Satomi S. Correlation of three-dimensional magnetic resonance imaging with precise histopathological map concerning carcinoma extension in the breast. Breast Cancer Res Treat. 2000;60:43–55. doi: 10.1023/a:1006342711426. [DOI] [PubMed] [Google Scholar]
- 21.Hlawatsch A, Teifke A, Schmidt M, Thelen M. Preoperative assessment of breast cancer: sonography versus MR imaging. AJR Am J Roentgenol. 2002;179:1493–1501. doi: 10.2214/ajr.179.6.1791493. [DOI] [PubMed] [Google Scholar]
- 22.Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003;10:381–388. doi: 10.1245/aso.2003.03.085. [DOI] [PubMed] [Google Scholar]
- 23.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 24.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, Schild HH. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23:8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 25.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, Gilbert FJ, Griebsch I, Hoff RJ, Kessar P, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 26.Schelfout K, Van Goethem M, Kersschot E, Colpaert C, Schelfhout AM, Leyman P, Verslegers I, Biltjes I, Van Den Haute J, Gillardin JP, et al. Contrast-enhanced MR imaging of breast lesions and effect on treatment. Eur J Surg Oncol. 2004;30:501–507. doi: 10.1016/j.ejso.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Schelfout K, Van Goethem M, Kersschot E, Verslegers I, Biltjes I, Leyman P, Colpaert C, Thienpont L, Van den Haute J, Gillardin JP, et al. Preoperative breast MRI in patients with invasive lobular breast cancer. Eur Radiol. 2004;14:1209–1216. doi: 10.1007/s00330-004-2275-7. [DOI] [PubMed] [Google Scholar]
- 28.Biglia N, Mariani L, Sgro L, Mininanni P, Moggio G, Sismondi P. Increased incidence of lobular breast cancer in women treated with hormone replacement therapy: implications for diagnosis, surgical and medical treatment. Endocr Relat Cancer. 2007;14:549–567. doi: 10.1677/ERC-06-0060. [DOI] [PubMed] [Google Scholar]
- 29.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 30.Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008;107:1–14. doi: 10.1007/s10549-007-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selinko VL, Middleton LP, Dempsey PJ. Role of sonography in diagnosing and staging invasive lobular carcinoma. J Clin Ultrasound. 2004;32:323–332. doi: 10.1002/jcu.20052. [DOI] [PubMed] [Google Scholar]
- 32.Paramagul CP, Helvie MA, Adler DD. Invasive lobular carcinoma: sonographic appearance and role of sonography in improving diagnostic sensitivity. Radiology. 1995;195:231–234. doi: 10.1148/radiology.195.1.7892476. [DOI] [PubMed] [Google Scholar]
- 33.Menezes GL, van den Bosch MA, Postma EL, El Sharouni MA, Verkooijen HM, van Diest PJ, Pijnappel RM. Invasive ductolobular carcinoma of the breast: spectrum of mammographic, ultrasound and magnetic resonance imaging findings correlated with proportion of the lobular component. Springerplus. 2013;2:621. doi: 10.1186/2193-1801-2-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann RM, Veltman J, Barentsz JO, Wobbes T, Blickman JG, Boetes C. The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol. 2008;34:135–142. doi: 10.1016/j.ejso.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Mann RM, Loo CE, Wobbes T, Bult P, Barentsz JO, Gilhuijs KG, Boetes C. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2010;119:415–422. doi: 10.1007/s10549-009-0616-6. [DOI] [PubMed] [Google Scholar]
- 36.McGhan LJ, Wasif N, Gray RJ, Giurescu ME, Pizzitola VJ, Lorans R, Ocal IT, Stucky CC, Pockaj BA. Use of preoperative magnetic resonance imaging for invasive lobular cancer: good, better, but maybe not the best? Ann Surg Oncol. 2010;17 Suppl 3:255–262. doi: 10.1245/s10434-010-1266-y. [DOI] [PubMed] [Google Scholar]
- 37.Esserman LJ, Kumar AS, Herrera AF, Leung J, Au A, Chen YY, Moore DH, Chen DF, Hellawell J, Wolverton D, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. 2006;24:4603–4610. doi: 10.1200/JCO.2005.04.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SH, Cha ES, Park CS, Kang BJ, Whang IY, Lee AW, Song BJ, Park J. Imaging features of invasive lobular carcinoma: comparison with invasive ductal carcinoma. Jpn J Radiol. 2011;29:475–482. doi: 10.1007/s11604-011-0584-8. [DOI] [PubMed] [Google Scholar]
- 39.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 40.Teller P, Kramer RK. Management of the asymptomatic BRCA mutation carrier. Appl Clin Genet. 2010;3:121–131. doi: 10.2147/TACG.S8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American College of Radiology (ACR). ACR practice guideline for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. 2013. Available from: http: //www.acr.org. [DOI] [PubMed] [Google Scholar]
- 42.Passaperuma K, Warner E, Causer PA, Hill KA, Messner S, Wong JW, Jong RA, Wright FC, Yaffe MJ, Ramsay EA, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107:24–30. doi: 10.1038/bjc.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, Fourquet A, Borger J, Jager J, Hoogenraad W, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 44.Elkhuizen PH, van de Vijver MJ, Hermans J, Zonderland HM, van de Velde CJ, Leer JW. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 45.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, White E. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 47.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 48.Brem RF, Hoffmeister JW, Rapelyea JA, Zisman G, Mohtashemi K, Jindal G, Disimio MP, Rogers SK. Impact of breast density on computer-aided detection for breast cancer. AJR Am J Roentgenol. 2005;184:439–444. doi: 10.2214/ajr.184.2.01840439. [DOI] [PubMed] [Google Scholar]
- 49.Bird RE, Wallace TW, Yankaskas BC. Analysis of cancers missed at screening mammography. Radiology. 1992;184:613–617. doi: 10.1148/radiology.184.3.1509041. [DOI] [PubMed] [Google Scholar]
- 50.Park CS, Jung NY, Kim K, Jung HS, Sohn KM, Oh SJ. Detection of breast cancer in asymptomatic and symptomatic groups using computer-aided detection with full-field digital mammography. J Breast Cancer. 2013;16:322–328. doi: 10.4048/jbc.2013.16.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnall MD, Blume J, Bluemke DA, Deangelis GA, Debruhl N, Harms S, Heywang-Köbrunner SH, Hylton N, Kuhl CK, Pisano ED, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol. 2005;92:32–38. doi: 10.1002/jso.20381. [DOI] [PubMed] [Google Scholar]
- 52.Kristoffersen Wiberg M, Aspelin P, Sylvan M, Boné B. Comparison of lesion size estimated by dynamic MR imaging, mammography and histopathology in breast neoplasms. Eur Radiol. 2003;13:1207–1212. doi: 10.1007/s00330-002-1718-2. [DOI] [PubMed] [Google Scholar]
- 53.Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G, Lattanzio V, Del Maschio A. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in Fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol. 2004;183:1149–1157. doi: 10.2214/ajr.183.4.1831149. [DOI] [PubMed] [Google Scholar]
- 54.Peters NH, Borel Rinkes IH, Mali WP, van den Bosch MA, Storm RK, Plaisier PW, de Boer E, van Overbeeke AJ, Peeters PH. Breast MRI in nonpalpable breast lesions: a randomized trial with diagnostic and therapeutic outcome - MONET - study. Trials. 2007;8:40. doi: 10.1186/1745-6215-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, Diepstraten SC, Weits T, Westenend PJ, Stapper G, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. Eur J Cancer. 2011;47:879–886. doi: 10.1016/j.ejca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 56.Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, Hanby A, Manca A, Napp V, Sculpher M, et al. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE) Health Technol Assess. 2010;14:1–182. doi: 10.3310/hta14010. [DOI] [PubMed] [Google Scholar]
- 57.Stehouwer BL, Klomp DW, van den Bosch MA, Korteweg MA, Gilhuijs KG, Witkamp AJ, van Diest PJ, Houwert KA, van der Kemp WJ, Luijten PR, et al. Dynamic contrast-enhanced and ultra-high-resolution breast MRI at 7.0 Tesla. Eur Radiol. 2013;23:2961–2968. doi: 10.1007/s00330-013-2985-9. [DOI] [PubMed] [Google Scholar]
- 58.Stehouwer BL, Klomp DW, Korteweg MA, Verkooijen HM, Luijten PR, Mali WP, van den Bosch MA, Veldhuis WB. 7 T versus 3T contrast-enhanced breast magnetic resonance imaging of invasive ductulolobular carcinoma: first clinical experience. Magn Reson Imaging. 2013;31:613–617. doi: 10.1016/j.mri.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Lehman CD. Diffusion weighted imaging (DWI) of the breast: ready for clinical practice? Eur J Radiol. 2012;81 Suppl 1:S80–S81. doi: 10.1016/S0720-048X(12)70032-3. [DOI] [PubMed] [Google Scholar]
- 60.Klomp DW, van de Bank BL, Raaijmakers A, Korteweg MA, Possanzini C, Boer VO, van de Berg CA, van de Bosch MA, Luijten PR. 31P MRSI and 1H MRS at 7 T: initial results in human breast cancer. NMR Biomed. 2011;24:1337–1342. doi: 10.1002/nbm.1696. [DOI] [PubMed] [Google Scholar]
- 61.Partridge SC. Future applications and innovations of clinical breast magnetic resonance imaging. Top Magn Reson Imaging. 2008;19:171–176. doi: 10.1097/RMR.0b013e31818a4090. [DOI] [PubMed] [Google Scholar]
- 62.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, Mahankali S, Gao JH. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–178. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 63.Woodhams R, Matsunaga K, Iwabuchi K, Kan S, Hata H, Kuranami M, Watanabe M, Hayakawa K. Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr. 2005;29:644–649. doi: 10.1097/01.rct.0000171913.74086.1b. [DOI] [PubMed] [Google Scholar]
- 64.Plana MN, Carreira C, Muriel A, Chiva M, Abraira V, Emparanza JI, Bonfill X, Zamora J. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: systematic review of diagnostic accuracy and meta-analysis. Eur Radiol. 2012;22:26–38. doi: 10.1007/s00330-011-2238-8. [DOI] [PubMed] [Google Scholar]
- 65.Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, Irwig L. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 66.El Khouli RH, Louie A. Case of the season: a giant fibroadenoma in the guise of a phyllodes tumor; characterization role of MRI. Semin Roentgenol. 2009;44:64–66. doi: 10.1053/j.ro.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009;193:1716–1722. doi: 10.2214/AJR.08.2139. [DOI] [PubMed] [Google Scholar]
- 68.Tan SL, Rahmat K, Rozalli FI, Mohd-Shah MN, Aziz YF, Yip CH, Vijayananthan A, Ng KH. Differentiation between benign and malignant breast lesions using quantitative diffusion-weighted sequence on 3 T MRI. Clin Radiol. 2014;69:63–71. doi: 10.1016/j.crad.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol. 2007;17:2646–2655. doi: 10.1007/s00330-007-0621-2. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693. doi: 10.1186/1471-2407-10-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yabuuchi H, Matsuo Y, Sunami S, Kamitani T, Kawanami S, Setoguchi T, Sakai S, Hatakenaka M, Kubo M, Tokunaga E, et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol. 2011;21:11–17. doi: 10.1007/s00330-010-1890-8. [DOI] [PubMed] [Google Scholar]
- 72.Partridge SC, Demartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J Magn Reson Imaging. 2010;31:562–570. doi: 10.1002/jmri.22078. [DOI] [PubMed] [Google Scholar]
- 73.Rahbar H, Partridge SC, Eby PR, Demartini WB, Gutierrez RL, Peacock S, Lehman CD. Characterization of ductal carcinoma in situ on diffusion weighted breast MRI. Eur Radiol. 2011;21:2011–2019. doi: 10.1007/s00330-011-2140-4. [DOI] [PubMed] [Google Scholar]
- 74.Park SH, Moon WK, Cho N, Song IC, Chang JM, Park IA, Han W, Noh DY. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2010;257:56–63. doi: 10.1148/radiol.10092021. [DOI] [PubMed] [Google Scholar]
- 75.Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22:104–113. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 76.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–847. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Mountford CE, Stanwell P, Lin A, Ramadan S, Ross B. Neurospectroscopy: the past, present and future. Chem Rev. 2010;110:3060–3086. doi: 10.1021/cr900250y. [DOI] [PubMed] [Google Scholar]
- 78.Kurhanewicz J, Vigneron DB. Advances in MR spectroscopy of the prostate. Magn Reson Imaging Clin N Am. 2008;16:697–710, ix-x. doi: 10.1016/j.mric.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolan PJ. Magnetic resonance spectroscopy of the breast: current status. Magn Reson Imaging Clin N Am. 2013;21:625–639. doi: 10.1016/j.mric.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tozaki M, Sakamoto M, Oyama Y, Maruyama K, Fukuma E. Predicting pathological response to neoadjuvant chemotherapy in breast cancer with quantitative 1H MR spectroscopy using the external standard method. J Magn Reson Imaging. 2010;31:895–902. doi: 10.1002/jmri.22118. [DOI] [PubMed] [Google Scholar]
- 82.Begley JK, Redpath TW, Bolan PJ, Gilbert FJ. In vivo proton magnetic resonance spectroscopy of breast cancer: a review of the literature. Breast Cancer Res. 2012;14:207. doi: 10.1186/bcr3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haddadin IS, McIntosh A, Meisamy S, Corum C, Styczynski Snyder AL, Powell NJ, Nelson MT, Yee D, Garwood M, Bolan PJ. Metabolite quantification and high-field MRS in breast cancer. NMR Biomed. 2009;22:65–76. doi: 10.1002/nbm.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mountford C, Ramadan S, Stanwell P, Malycha P. Proton MRS of the breast in the clinical setting. NMR Biomed. 2009;22:54–64. doi: 10.1002/nbm.1301. [DOI] [PubMed] [Google Scholar]
- 85.Baltzer PA, Dietzel M. Breast lesions: diagnosis by using proton MR spectroscopy at 1.5 and 3.0 T--systematic review and meta-analysis. Radiology. 2013;267:735–746. doi: 10.1148/radiol.13121856. [DOI] [PubMed] [Google Scholar]
- 86.Katz-Brull R, Lavin PT, Lenkinski RE. Clinical utility of proton magnetic resonance spectroscopy in characterizing breast lesions. J Natl Cancer Inst. 2002;94:1197–1203. doi: 10.1093/jnci/94.16.1197. [DOI] [PubMed] [Google Scholar]
- 87.Shin HJ, Baek HM, Cha JH, Kim HH. Evaluation of breast cancer using proton MR spectroscopy: total choline peak integral and signal-to-noise ratio as prognostic indicators. AJR Am J Roentgenol. 2012;198:W488–W497. doi: 10.2214/AJR.11.7292. [DOI] [PubMed] [Google Scholar]
- 88.Meisamy S, Bolan PJ, Baker EH, Bliss RL, Gulbahce E, Everson LI, Nelson MT, Emory TH, Tuttle TM, Yee D, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy--a pilot study at 4 T. Radiology. 2004;233:424–431. doi: 10.1148/radiol.2332031285. [DOI] [PubMed] [Google Scholar]
- 89.Jacobs MA, Stearns V, Wolff AC, Macura K, Argani P, Khouri N, Tsangaris T, Barker PB, Davidson NE, Bhujwalla ZM, et al. Multiparametric magnetic resonance imaging, spectroscopy and multinuclear (²³Na) imaging monitoring of preoperative chemotherapy for locally advanced breast cancer. Acad Radiol. 2010;17:1477–1485. doi: 10.1016/j.acra.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsougos I, Svolos P, Kousi E, Athanassiou E, Theodorou K, Arvanitis D, Fezoulidis I, Vassiou K. The contribution of diffusion tensor imaging and magnetic resonance spectroscopy for the differentiation of breast lesions at 3T. Acta Radiol. 2014;55:14–23. doi: 10.1177/0284185113492152. [DOI] [PubMed] [Google Scholar]
- 91.Wijnen JP, van der Kemp WJ, Luttje MP, Korteweg MA, Luijten PR, Klomp DW. Quantitative 31P magnetic resonance spectroscopy of the human breast at 7 T. Magn Reson Med. 2012;68:339–348. doi: 10.1002/mrm.23249. [DOI] [PubMed] [Google Scholar]
- 92.Korteweg MA, Veldhuis WB, Visser F, Luijten PR, Mali WP, van Diest PJ, van den Bosch MA, Klomp DJ. Feasibility of 7 Tesla breast magnetic resonance imaging determination of intrinsic sensitivity and high-resolution magnetic resonance imaging, diffusion-weighted imaging, and (1)H-magnetic resonance spectroscopy of breast cancer patients receiving neoadjuvant therapy. Invest Radiol. 2011;46:370–376. doi: 10.1097/RLI.0b013e31820df706. [DOI] [PubMed] [Google Scholar]
- 93.American College of Radiology (ACR). ACR practice guideline for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. 2013 [cited 2013 December]. Available from: http: //www.acr.org. [Google Scholar]
- 94.Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, Helbich T, Heywang-Köbrunner SH, Kaiser WA, Kerin MJ, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 95.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]


