Abstract
Background:
Intraosseous Angiolipoma of the skull bone (IOAL) is a very rare bony lesion of the calvarium. This lesion occurs most commonly in the soft, subcutaneous tissue of the trunk. Only a single case of angiolipoma of the skull has been previously reported. The authors report the first case of giant IOAL of the calvarium evaluated by 3D CTS, MRI and full histopathological staining in a young lady treated surgically and with 23 months of follow up.
Case Description:
A 41-year-old female was admitted because of a prominent bulging on her right parietal region. Three dimensional CT and CT angiographic reconstruction of the cranium elucidated the geographical pattern of the lesion. MRI revealed a huge intraosseous right frontotemporoparietooccipital expansile lesion, nonhomogeneous but mostly hyperintense, in T1W images. In T2W images, the lesion was nonhomogeneously hyperintense and trabeculated with no perilesional edema. In the FLAIR-images, the lesion was trabeculated and nonhomogeneously hypointense. The lesion was excised totally followed by skull reconstruction and no recurrence after 23 months.
Conclusion:
We hypothesize that the possible pathogenesis of IOAL may be a kind of mutation or dedifferentiation of either a primary intradiploic hemangioma or lipoma changing its growth pattern with possibly more aggressive behavior.
Keywords: Hemangioma, intradiploic, intraosseous, lipoma, skull bone
INTRODUCTION
Angiolipomas are benign, slow growing, soft tissue tumors, which may occur anywhere in the body especially in the subcutaneous region. The intraosseous lesions frequently occur in the long bones and in the spinal epidural space.[2] In our review of the literature, we have only found seven such cases reported occurring; four in the mandible,[4,6,7,10] two in the ribs,[3,8] and one in the parietal bone of skull.[12] We intend to report a giant intraosseous angiolipoma (IOAL) involving the frontoparietotemporal and part of the occipital bones in a 41-year-old female, expanding both into the cranial cavity with remarkable out bulging.
According to our knowledge, this is the first extra-large and radiologically invasive IOAL of the skull reported in the literature. There is only one other report describing a midsize, parietal osseous angiolipoma[12] in which the magnetic resonance imaging (MRI) and full histopathological characteristics were not described. In our case, we will add these findings and try summarize the possible theories regarding the genesis of the lesion according to the literature, and present MRI and immunohistochemical (IHC) characteristics of such lesions for the first time.
CASE REPORT
A 41-year-old female was admitted complaining from a bulging on the right parietal region, which had grown up during the previous 2 years and became tender recently. There was no history of head trauma. She was suffering from mild generalized headache of about 4 months duration without nausea or vomiting or any attack of epilepsy. On physical examination, cranial nerves were all intact, no papilledema was detected and no hemiparesis. The head was asymmetric and bulged in the right parietal region and was a bit tender on palpation. Plain X-ray showed a wide lucent trabeculated area expanding the bone [Figure 1]. Computed tomography scanning (CTS) performed with and without contrast enhancement revealed a densely trabeculated lesion containing both lucent and densely calcified components. The lesion extended alongside the diploe in some parts and expanded both internally and externally, destroying both the internal and external tables in different places. Remarkable extradural compression on the brain was present in the right temporoparietal region. There was no significant enhancement after contrast material injection [Figure 2]. Three dimensional CT and CT angiographic reconstruction of the cranium elucidated the geographical pattern of the lesion in relation with the whole skull and the remarkable vascular markings all around the lesion [Figure 3]. MRI revealed a huge intraosseous right frontotemporoparietooccipital expansile lesion with remarkable mass effect upon the adjacent dura. It was trabeculated in shape and nonhomogenous in all sequences. In T1W images, the lesion was nonhomogeneous but mostly hyperintense, projecting both intra- and extra-cranially, and containing foci of both decreased and increased signal intensity filling up all the bulk of the lesion. In T2W images, the lesion was nonhomogeneously hypeintense and trabeculated with no perilesional edema [Figure 4]. There was no remarkable enhancement after contrast material injection. In the FLAIR-images, the lesion was trabeculated and nonhomogeneously hypointense [Figure 5]. Both CT and MR-angiographies showed mild increased vascularity in the region of the tumor.
Figure 1.
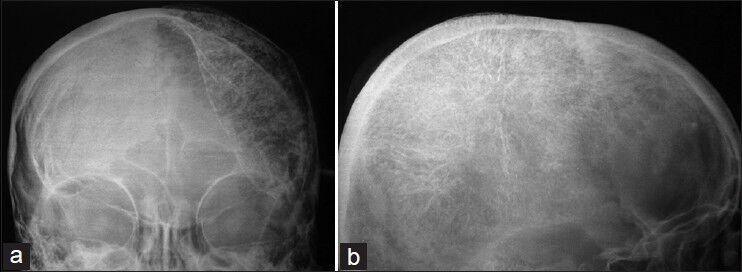
Plain X-rays showing large lenticular shaped expansion of a calvarial lesion in AP view, with irregular margins expanding the bone from in front of the coronal suture anteriorly, adjacent to the petrous base inferiorly, behind the lambdoid suture posteriorly and juxta to the sagittal suture in the midline
Figure 2.
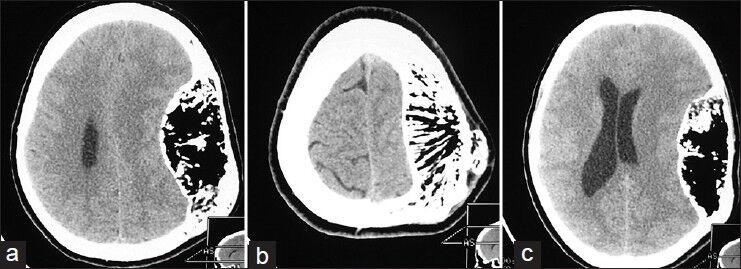
Horizontal nonenhanced sections, a multicompartmental, trabeculated mass expanding the calvarial bone in all directions is visible. The density of the lesion in the hypodense regions varied between -50 and -100. These hypodensities were separated by osseous and calcified septations and foci
Figure 3.
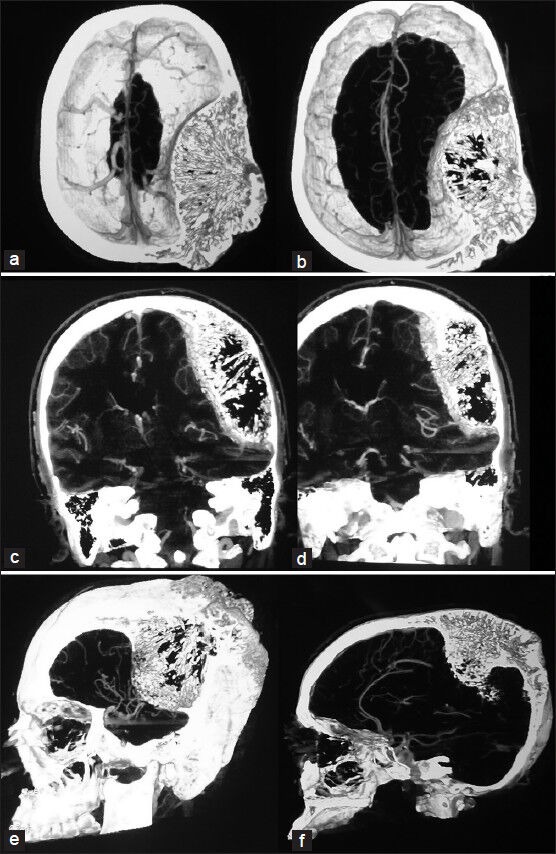
Horizontal, coronal and sagittal angio-enhancing, standard, and 3D sections showing mild vascularity of the lesion and its interface with the dura mater
Figure 4.
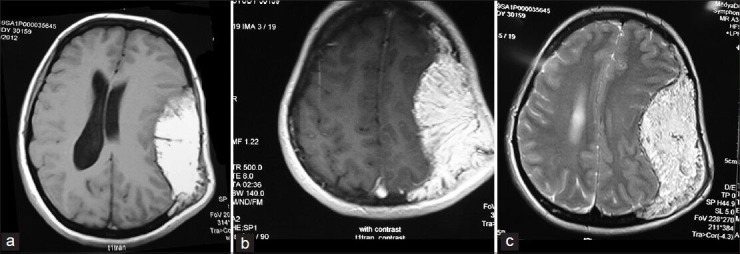
Axial T1W and T2W MR images showing hyperintense trabeculated lesion expanding the calvarial bone both intra- and extra-cranially. The tumoral tissue extended within the diploe of the adjacent frontal, temporal, occipital bones, and toward the midline suture
Figure 5.
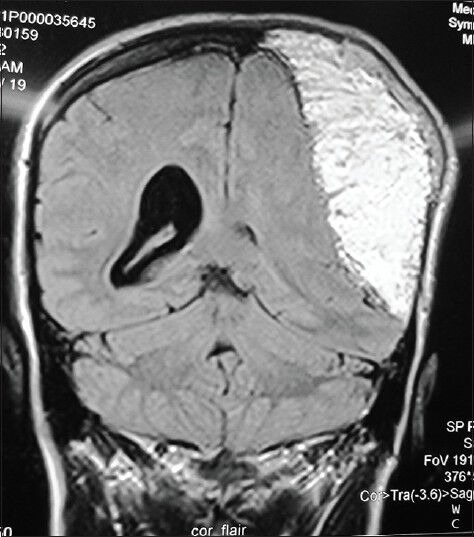
FLAIR sequence of MR images in coronal view showing mixed hypo- and hyper-intensities compatible with mixed lipoid and hemosiderin/clot filled compartments in the vicinity of the tumor
To excise the lesion totally, we had to make a large skin incision from frontal to the occipital region exposing the low temporal region and passing the midline to the left side in the parietal region. The tumor did not infiltrate the skin but periosteum was hardly removable from the external surface of the lesion and did not appear to be intact. Multiple burr holes were placed around the tumor lump and the entire lesion was excised. There was no tumor infiltration to the adjacent dural layer. There was no tumor infiltration to the adjacent dural layer. A large molded titanium mesh was used to repair the bone defect and the postoperative result was aesthetically satisfactory.
Macroscopically, the bone was expanded by a multinodular lesion with soft yellow and red cut surface. It was a yellowish lipomatous bone with cancellous and vascular components [Figure 6]. The hematoxylin and eosin (H and E) staining examination of the specimen showed bony trabeculae admixed with large fragments of mature adipose tissue and numerous delicate vessels, some of which contained fibrin thrombi. No cellular atypia or mitotic figures were seen. The result of IHC staining of the specimen was negative S100 staining for glial and Schwann cells and positive CD34 staining for the endothelial cells of blood vessels. The Sudan black stain was positive for neutral lipid cells in the tissue [Figure 6]. The diagnosis was “Angiolipoma”. The surgical margins were all free of pathological tissue.
Figure 6.
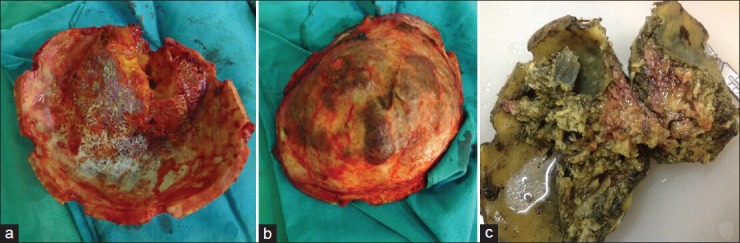
a and b, demonstrating the lesional flap involving the thickened bone measuring 20 × 13 × 6 cm regarding the greatest diameter of the lesion. Both the outer and inner cortical surfaces were irregular, and the overlying periosteum was discolored. The vascular markings of the inner surface of the bone were remarkable. The cut surface of the lesion was shiny-yellow with fat bobbles. 6c, showing the specimen after formalin fixation and before decalcification containing yellow-brown lesion with varying consistencies
The postoperative period was uneventful and there was no sign of tumor recurrence when visited after 23 months.
DISCUSSION
Intraosseous hemangiomas are rarely seen in the calvarium and their frequency in this region is 0.2% of all bone neoplasms.[5,6] Intraosseous lipomas are another uncommon tumor of the skeleton reported as 0.1% of all bone tumors, and less than 10 cases reported in the calvarium.[1,9,10] The IOAL are only anecdotally reported in the literature and to the best of our knowledge, we report the first fully described case of giant IOAL of the calvarium in a middle-aged lady.
Involutional changes are well documented to occur spontaneously within intraosseous lipomas going from stage 1 to 3 according to the histological and radiological changes.[1,9,10] In our case, the calcified trabecular and expanded bone was present with marrow spaces occupied by small and large caliber blood vessels and adipose tissue. These vessels were separated by fibrous stroma. The vessel wall thickness was also variable and so was the size of the lumen. It may be hypothesized that the IOAL may thus represent either hyperplasia of fat, with an associated increase in the vascular channels, or a true neoplasm. Although progesterone, estrogen, and androgen are reported to be secreted from lipoma cells, their own receptors are negative in this regard in the reported cases[11] and we could not evaluate these receptors in our specimen. The presence of both the proliferation of collagen tissue in the lipoma and development of the angiomatous components led us to suspect that they stemmed from a common cellular element. We were not able to identify the receptors or the genetic pattern of the lesion because of the technical limitations but the hypothesis will stay for further elucidations.
The findings in X-rays are nonspecific as was in our case. These changes are commonly seen in other skull bone lesions, such as bone cyst, hemangioma, inclusion tumors, eosinophilic granuloma, intraosseous meningioma, Paget's disease, osteoma, osteosarcoma, chondrosarcoma, chondromyxoid fibroma, osteoblastoma, fibrous dysplasia, metastatic lesions, or multiple myeloma.[1,4,6,8,9,10]
CT scan shows diffuse nonhomogeneous hypodense lesion expanding the bone in different directions both intra- and extra-cranially. Counting the Hounsfield number of the hypodense compartment can be of paramount importance for differential diagnosis of skull bone pathologies. Enhancement of the lesional parenchyma depends upon the accompanying vascular component, which was minimal in our case [Figures 2 and 3].
According to the best of our knowledge, there has been no previous report about the MRI characteristics of IOAL of the calvarium in the literature. The MRIs of our case showed mixed hyperintensity both in T1W and T2W images, with trabeculated pattern. The heterogeneously increased signals on MRI indicate the presence of hemosiderin, methemoglobin, or oxy- and deoxy-hemoglobin within the diploic space. These hyperintensities extended all along the adjacent diploe in different directions and distances. Either the internal or external table was intact in some parts or burst out by the tumor invasion. There was no remarkable enhancement after contrast material injection on T1W images. Fat suppression images were not taken in the preoperative evaluations. On FLAIR sequences, mixed hypo- and hyper-intensities keeping filiform pattern everywhere were also compatible with mixed fatty and vascular tissue components [Figure 5]. The images were all suggestive of infiltration of the lesion to the periosteum. Both CT angiogram and time of flight MR angiogram were suggestive of moderate hypervascularity of the lesion (only partially presented in Figure 3 because of space limitations).
En bloc resection has always been the recommended procedure for such lesions. We encountered moderate amount of bleeding during surgery without preoperative embolization of the lesion as suggested for some cases of bone hemangiomas.[6,7] The cut surface of the lesion showed both remarkable fat infiltration and channels filled with blood clot. In microscopic examination of the specimen, mature lipocytes and fibrocytes with missing a fibrous capsule were separated by septae composed of loose connective tissue and moderate amount of collagen fibers. Numerous endothelium lined spaces and remarkable amount of vascular channels containing erythrocyte and clots in several sections were also visualized. The endothelial cells lacked any mitotic activity. There were no inflammatory cells or hematopoietic bone marrow detected in the preparations. These characteristics resembled those of angiolipomas in the extremities. Reviewing the whole specimen and the IHC markers negative for glial cells and positive for adipose tissue and endothelial cells of the vessels [Figure 7], it appeared that the tumor fulfilled the criteria of IOAL.
Figure 7.
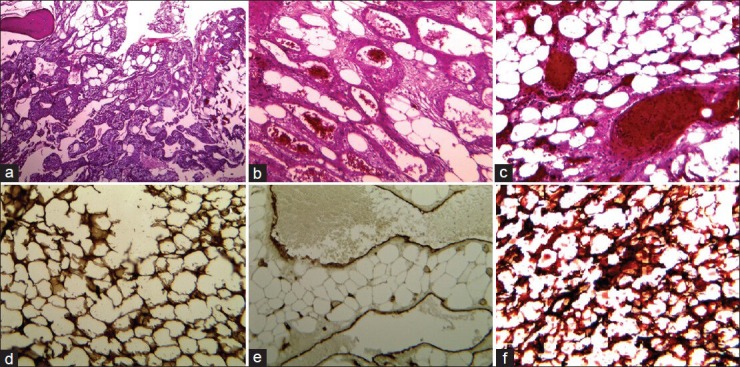
a-c, H and E staining of the specimen with different magnifications, showing mature adipose tissue with a prominent vascular component. The lipomatous components were surrounded by bands of fibrous connective sheaths containing foamy macrophages and scattered mast cells ‘chicken wire appearance’. Bone spicules remained within the lesion contained osteocytes but no osteoclast and osteoblast cells. The vascular components consisted of thin-walled vessels of different sizes and small groups of capillaries containing erythrocytes and thrombus formations. d and e, The IHC staining using antibodies for CD34 and S100 protein showing the endothelial vascular sheaths and foamy lipomatous compartments. d: Sudan III staining without mounting, demonstrating neutral lipids component in the tissue
The limitations of this report are: (a) our patient did not undergo bone survey X-ray examination or bone isotope scanning for depiction of possible multiplicity of the lesion, (b) we did not have the feasibility to prepare a patient-specific implant to be fashioned out of a contourable material matching the defect before operation, (c) the tests for finding the hormone receptors and the genetic studies needed for supporting our hypothesis were lacking, and (d) the follow up period is still short for detection the possible recurrence of the lesion.
CONCLUSION
Even though the risk of malignant changes in IOAL is considered to be very low, we suggest en bloc resection of the skull lesions with similar CTS/MRI features when the diagnosis is reasonably certain.
Notification: This case has been presented at the 2012, Interim Meeting of The Iranian Association of Neurological Surgeons (IANS), Kermanshah, Iran. A very short abstract of it has been included in J Inj Violence Res. 2012 Nov; 4 (3 Suppl 1). doi: pii: Paper No. 40.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/50/130773
Contributor Information
Abbas Amirjamshidi, Email: abamirjamshidi@yahoo.com.
Babak Ghasemi, Email: babak.ghassemi@gmail.com.
Kazem Abbasioun, Email: dr.abbassioun@gmail.com.
REFERENCES
- 1.Arslan G, Karaali K, Cubuk M, Senol U, Luleci E. Intraossous lipoma of the frontal bone. A case report. Acta Radiol. 2000;41:320–1. doi: 10.1080/028418500127345578. [DOI] [PubMed] [Google Scholar]
- 2.Anson JA, Cybulski GR, Reyes M. Spinal extradural angiolipoma: A report of two cases review of the literature. Surg Neurol. 1990;34:173–8. doi: 10.1016/0090-3019(90)90069-2. [DOI] [PubMed] [Google Scholar]
- 3.Hall FM, Cohen RB, Grumbach K. Case report 377. Intraosseous lipoma (angiolipoma) of the third rib. Skeletal Radiol. 1986;15:401–3. doi: 10.1007/BF00348872. [DOI] [PubMed] [Google Scholar]
- 4.Hemavathy S, Roy S, Kiresur A. Intraosseous angiolipoma of the mandible. J Oral Maxillofac Pathol. 2012;16:283–7. doi: 10.4103/0973-029X.99091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanam H, Lipper MH, Wolff CL, Lopes MB. Calvarial hemangiomas: Report of two cases and review of the literature. Surg Neurol. 2001;55:63–7. doi: 10.1016/s0090-3019(00)00268-8. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DM, Brannon RB, Isaksson B, Larsson I. Intraosseous angiolipoma of the mandible. Oral Surg Oral Med Oral Pathol. 1980;50:156–9. doi: 10.1016/0030-4220(80)90204-2. [DOI] [PubMed] [Google Scholar]
- 7.Mangarano AM, Hammond HL, Williams TP. lntraosseous angiolipoma of the Mandible: A case report and review of the literature. J Oral Maxillofac Surg. 1994;52:767–9. doi: 10.1016/0278-2391(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 8.Mardi K, Sharma J. Intraosseous angiolipoma of the rib. Indian J Pathol Microbiol. 2007;50:606–7. [PubMed] [Google Scholar]
- 9.Nahles G, Schaeper F, Bier J, Klein M. An intraosseous lipoma in the frontal bone. A case report. Int J Oral Maxillofac Surg. 2004;33:408–10. doi: 10.1016/j.ijom.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Polte HW, Kolodny SC, Hooker SP. Intraosseous angiolipoma of the mandible. Oral Surg Oral Med Oral Pathol. 1976;41:637–43. doi: 10.1016/0030-4220(76)90316-9. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Kimura M, Kinoshita A, Hasegawa H, Yamashita J. Meningioma associated with intraosseous lipoma. Case report. Clin Neurol Neurosurg. 2003;105:221–4. doi: 10.1016/s0303-8467(03)00014-3. [DOI] [PubMed] [Google Scholar]
- 12.Yu K, Van Dellen J, Idaewor P, Roncaroli F. Intraosseous angiolipoma of the cranium: Case report. Neurosurgery. 2009;64:E189–90. doi: 10.1227/01.NEU.0000335785.12401.8C. [DOI] [PubMed] [Google Scholar]


