Abstract
BackgroundThe objectives of this study were to evaluate the association between varicella-zoster virus (VZV)–specific humoral and cell-mediated immunity (CMI) to herpes zoster (HZ) and protection against HZ morbidity and to compare immune responses to HZ and zoster vaccine
MethodsIn 981 elderly persons who developed HZ during a zoster vaccine efficacy trial (321 vaccinees and 660 placebo recipients) and 1362 without HZ (682 vaccinees and 680 placebo recipients), CMI was measured by VZV responder cell frequency and interferon-γ enzyme-linked immunospot, and antibodies were measured by VZV enzyme-linked immunosorbent assay against affinity-purified VZV glycoproteins (gpELISA)
ResultsRobust VZV CMI at HZ onset correlated with reduced HZ morbidity, whereas VZV gpELISA titers did not. Three weeks after HZ onset, gpELISA titers were highest in those with more severe HZ and were slightly increased in placebo recipients (compared with zoster vaccine recipients) and in older individuals. VZV CMI responses to HZ were similar in zoster vaccine and placebo recipients and were not affected by demographic characteristics or antiviral therapy, except for responder cell frequency at HZ onset, which decreased with age. When responses to zoster vaccine and HZ could be compared, VZV CMI values were similar, but antibody titers were lower
ConclusionsHigher VZV CMI at HZ onset was associated with reduced HZ severity and less postherpetic neuralgia. Higher antibody titers were associated with increased HZ severity and occurrence of postherpetic neuralgia. HZ and zoster vaccine generated comparable VZV CMI
Herpes zoster (HZ) is the clinical manifestation of varicella-zoster virus (VZV) reactivation. HZ typically affects individuals with decreased cell-mediated immunity (CMI), including elderly persons [1–7]. Severe pain in HZ and the occurrence of postherpetic neuralgia (PHN) are correlated with increasing age [8–12]. An association between decreased VZV CMI and severity of HZ is likely, but to our knowledge, it has not been previously demonstrated
In the absence of overt immunosuppression, one attack of HZ decreases the risk of subsequent episodes [10], suggesting that a boost in VZV CMI protects against HZ. Indeed, a randomized, double-blind, placebo-controlled trial in 38,546 subjects ⩾60 years of age, US Department of Veterans Affairs (VA) Cooperative Study 403 (Shingles Prevention Study [SPS]), demonstrated that a live, attenuated VZV vaccine (zoster vaccine) that boosts VZV immunity protects against HZ [13, 14]. Although a unique immune correlate with protection against HZ conferred by zoster vaccine was not identified, the boost in VZV CMI was deemed crucial, based on previous studies showing that the magnitude of VZV CMI correlated with an increased likelihood of HZ [14–16]
In this study, we evaluated the association between immune responses to HZ and both HZ disease severity and the occurrence of PHN, as well as the effect of zoster vaccine and of key demographics on immune responses to HZ. We also defined the kinetics of the immune response to HZ and compared the immune responses to zoster vaccine with those to HZ
Methods
Subject population and study designThe SPS was a randomized, double-blind, placebo-controlled trial in subjects ⩾60 years of age, who received zoster vaccine or placebo and were observed for the development of HZ [13]. Subjects with suspected HZ underwent clinical and laboratory evaluations [13] and were offered antiviral therapy. HZ-associated pain was quantified using an HZ severity-of-illness score, which measures the area under the curve for pain severity over time, and by the occurrence of PHN, defined as pain graded ⩾3 on a scale of 0–10, present >90 days after the onset of HZ rash [13, 17, 18]
In subjects with suspected HZ, VZV antibody and CMI were measured 1, 3, and 6 weeks and 1, 2, and 3 years after rash onset. Blood samples for immunologic assays were processed at the study’s central immunology laboratories (ILs) in Denver, Colorado, and San Diego, California [14]. In addition, between February 2000 and September 2001, all subjects enrolled in the SPS in Denver and San Diego were asked to participate in the Immunology Substudy. A total of 1395 subjects who agreed to participate had VZV-specific immunity measured before vaccine or placebo administration, after 6 weeks, and after 1, 2, and 3 years
Immunologic assessmentsVZV-specific responder cell frequency (RCF) assay, interferon-γ–enzyme-linked immunospot (ELISPOT) assay, and enzyme-linked immunosorbent assay against affinity-purified VZV glycoproteins (gpELISA) were performed as described elsewhere [14]. For RCF, the frequency of circulating VZV-specific CD4+ T cells was measured by adding a limiting dilution step to a lymphoproliferative assay [19]. Two-fold dilutions of peripheral blood mononuclear cells (PBMCs) between 50,000 and 1563 cells/well were added to 24 microtiter wells containing VZV-infected human lung fibroblast lysate or mock-infected control antigens. Responding wells were defined as VZV-stimulated wells with tritiated thymidine–measured proliferation at least 3 standard deviations (SDs) above the median for mock-stimulated wells at the same PBMC concentration or 3-fold higher than that median if it was <100 counts per minute. On the basis of the Poisson distribution, the RCF was defined as the cell concentration at which 37% of wells were nonresponders and was expressed as the number of VZV-responding cells per 105 PBMCs. The analytic sensitivity of this test has been conventionally considered ⩾1 responding cell/105 PBMCs. A mathematical model predicted a sensitivity of ⩾0.2 responding cells/105 PBMCs. Statistical analyses using either of these thresholds showed similar results. Here, we report the results of analyses that used the conservative threshold of 1 responding cell/105 PBMCs. Values below this limit were recorded as 0.5 responding cell/105 PBMCs to minimize the potential for introducing biases due to biologically insignificant assay variability
ELISPOT assay was used to measure the frequency of VZV-specific interferon-γ–producing PBMCs, expressed as spot-forming cells (SFCs) per 106 PBMCs [20]. Previously cryopreserved PBMCs at 500,000 cells/well were stimulated in triplicate wells with VZV lysate, mock-infected control, or phytohemagglutinin. After overnight incubation, SFCs were counted with an ImmunoSpot Analyzer (Cellular Technology). Valid assays were defined as those with ⩾500 phytohemagglutinin-stimulated SFCs/106 PBMCs. VZV-specific SFCs were calculated as SFCs in VZV-stimulated wells minus SFCs in control-stimulated wells. Values <1 SFC/106 PBMCs were recorded as 0.5 SFC/106 PBMCs [14]
VZV-gpELISA was used to measure the VZV-specific antibody concentration [21]. This assay measures antibodies against affinity-purified VZV glycoproteins. At baseline, all subjects had titers >5 gpELISA units/mL, the level considered seroprotective against varicella in varicella vaccine recipients [22]. ELISPOT assay and gpELISA were performed at Merck Research Laboratories, and RCF assay was performed at ILs
Statistical analysisVZV-specific immune responses were summarized as geometric means with 95% confidence intervals (CIs) and/or geometric mean fold rises from the first week after onset of HZ rash to specified follow-up times. Comparisons of these responses between groups were performed using an analysis of covariance (ANCOVA) model that included log-transformed VZV-response or fold rise as the response variable and the group-defining characteristic as the independent variable. When appropriate, cohort, treatment, location of laboratory, age, and sex were included as covariates in ANCOVA models. To account for laboratory-specific effects, statistical analyses included the laboratory site as a covariate
The correlations between immune responses were examined using Spearman&rank correlations. The association between immune responses and severity of HZ was evaluated with an ANCOVA model that included the log-transformed immune response as the response variable and the HZ severity-of-illness score as the independent variable adjusted by age. Similar analyses were performed for the incidence of PHN
Results
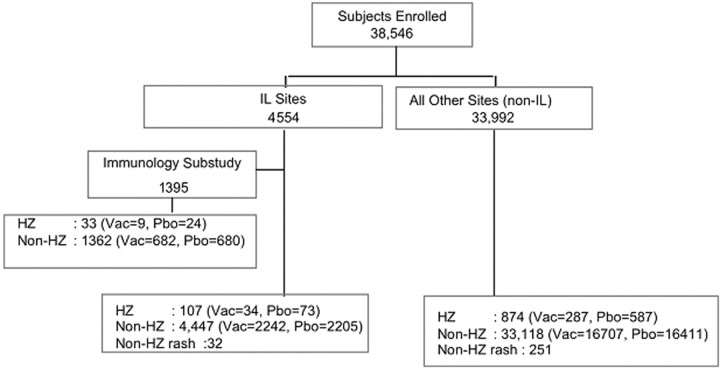
Subject groups and demographic characteristics of subjects analyzed for VZV-specific immune responsesAnalyses of the VZV-specific immune responses used samples from specific groups of subjects enrolled in the SPS that best addressed a specific analytical question. Overall, 981 subjects had confirmed HZ, 283 had rashes with other causes, and 1395 were enrolled in the SPS Immunology Substudy with longitudinal VZV immune response data (Figure 1). Subjects with HZ had a mean age (±SD) of 70±6 years; 57% were male, and 97% were white. Subjects without HZ had a mean age of 69±6 years. Other demographic characteristics were similar between the 2 groups
Figure 1.
Diagram of the Shingles Prevention Study, Immunology Substudy, and distribution of herpes zoster (HZ) cases. The Immunology Substudy included a group of subjects at clinical sites where an immunology laboratory (IL) was located. Subjects with HZ enrolled in the Immunology Substudy are also included in the total count of HZ cases at the IL sites. Likewise, subjects with non-HZ rashes at IL or non-IL sites are also included in the total number of subjects without HZ (non-HZ) at their respective sites; non-IL sites are clinical sites at locations distant from the ILs, which shipped samples overnight to the ILs for immune response assays. Of the 32 subjects with non-HZ rash at IL sites, 14 were vaccine (Vac) and 18 were placebo (Pbo) recipients. Of the 251 subjects with non-HZ rash at non-IL sites, 124 were vaccine and 127 were placebo recipients
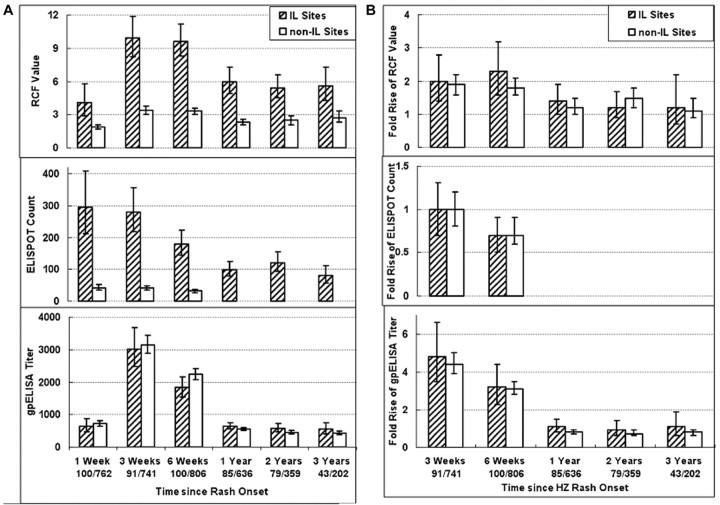
Effect of delayed isolation of PBMCs on VZV CMI measurementsVZV CMI responses of subjects at non-IL sites were consistently 50%–86% lower than those of subjects at IL sites (Figure 2A). This likely reflected the difference in the interval from collection to processing of blood, because specimens at the IL sites were processed on the same day they were obtained, whereas specimens at the non-IL sites were shipped overnight to the IL sites and processed ∼24 h after collection. Similar decrements in CMI responses have been reported elsewhere with delayed processing [23–25]. Antibodies are stable for 24 h at room temperature; thus, gpELISA titers were similar for subjects at IL and non-IL sites (Figure 2A)
Figure 2.
Varicella-zoster virus (VZV)–specific immune responses over time in subjects with herpes zoster (HZ). A Bars indicate geometric means and 95% confidence intervals (CIs) for absolute responder cell frequency (RCF) values, measured as responder cells per 105 peripheral blood mononuclear cells (PBMCs); enzyme-linked immunospot (ELISPOT) counts, measured as spot-forming cells per 106 PBMCs; and titers for enzyme-linked immunosorbent assay against affinity-purified VZV glycoproteins (gpELISA), measured as gpELISA units per milliliter. Data for ELISPOT responses were not available in subjects from clinical sites at locations distant from immunology laboratories (ILs) (non-IL sites) beyond week 6 after the onset of HZ rash. RCF and ELISPOT values were significantly lower in subjects from non-IL than in those from IL sites (P<.05). B Bars indicate geometric means and 95% CIs of the fold change in value for each assay at the indicated time point relative to the value measured 1 week after HZ rash onset. Numbers indicate the numbers of subjects who contributed samples at each time point at IL or non-IL sites. Fold change comparisons are not provided for ELISPOT responses beyond week 6 after HZ rash onset, because of the lack of data in the subjects from non-IL sites. RCF and ELISPOT fold changes were similar in subjects from IL and non-IL sites
The impact of processing differences on VZV CMI results was consistent across all samples, such that the relative change in responses between the first visit after HZ rash onset and subsequent visits was similar for subjects at IL and non-IL sites (P>.1 at each time point) (Figure 2B). Furthermore, analyses of RCF or ELISPOT results across time points when no change in CMI was expected, such as weeks 3 and 6 after rash onset in subjects with non-HZ rashes, showed similar degrees of concordance (0.46–0.82) at IL and non-IL sites, validating the use of CMI results from subjects at non-IL sites. For VZV CMI analyses, subjects from IL and non-IL sites were considered separately. For serologic analyses, subjects from all sites were considered together
Immune correlates of protection against HZ morbidityWe correlated immune responses with HZ severity-of-illness scores in placebo recipients who developed HZ (71 subjects from IL sites and 571 from non-IL sites) at the visits 1 and 3 weeks after HZ rash onset (Figure 3) and compared the immune responses of subjects who developed PHN with those of subjects who did not. At 1 week after HZ rash onset (Figure 3A), RCF values were higher in subjects with lower HZ severity-of-illness scores at both non-IL (P=.002) and IL sites (P=.09). The difference did not reach statistical significance in the IL group, because of the smaller number of subjects. Among subjects from non-IL sites, RCF results were also significantly higher in the 516 who did not develop PHN than in the 71 who did (P=.002) (Figure 3G). Only 9 subjects at the IL sites had PHN, which precluded a formal analysis. At 3 weeks after HZ rash onset, there were no significant correlations between RCF values and HZ severity-of-illness scores (Figure 3B) or development of PHN (Figure 3G)
Figure 3.
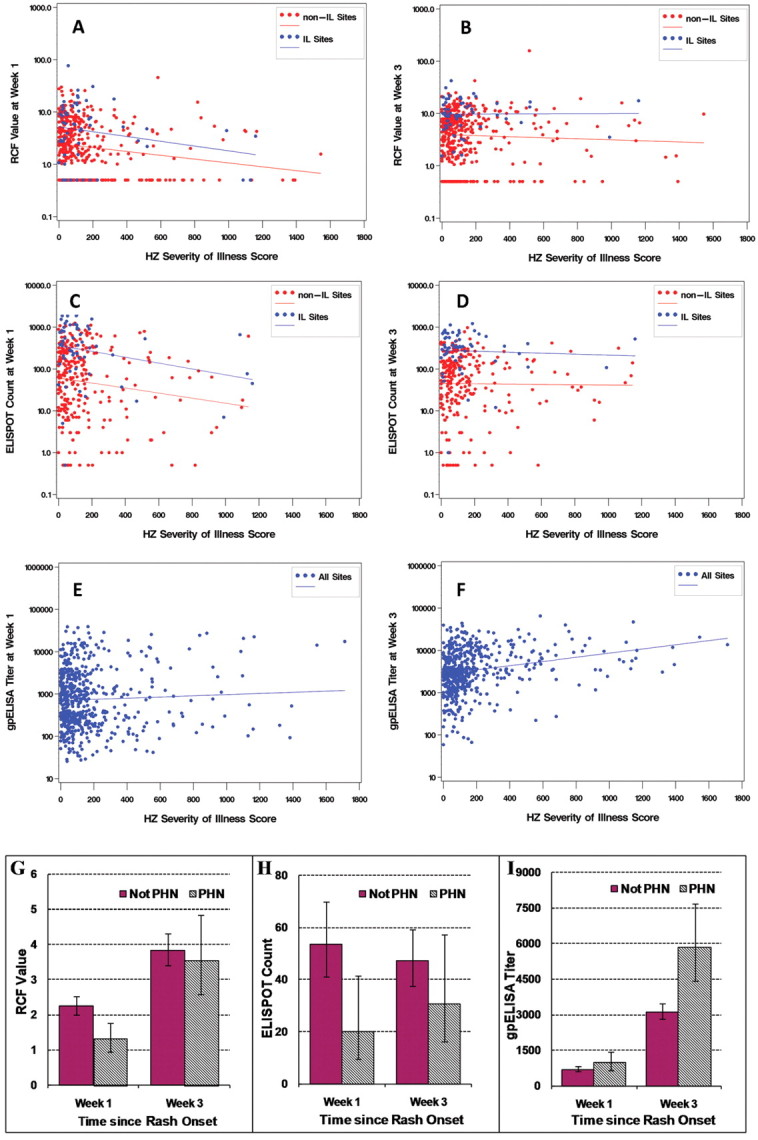
Immune correlates with severity of illness for herpes zoster (HZ) and occurrence of postherpetic neuralgia (PHN). Data were derived from placebo recipients who developed HZ. HZ severity-of-illness scores (area under the curve for pain severity by time) were calculated using a validated HZ-specific assessment tool, the Zoster Brief Pain Inventory [17]. A and B Observed responder cell frequency (RCF) values, measured as responder cells per 105 peripheral blood mononuclear cells (PBMCs) 1 week and 3 weeks, respectively, after the onset of HZ rash, by HZ severity-of-illness scores and corresponding regression lines. Results are shown separately for subjects at clinical sites where an immunology laboratory (IL) was located (IL sites; blue) and subjects at non-IL sites (red) who developed HZ. RCF values 1 week after HZ rash onset were significantly higher in subjects from non-IL sites with less severe disease (P=.002) and also tended to be higher in subjects with less severe disease among the fewer subjects from the IL sites who developed HZ (P=.09). C and D Observed enzyme-linked immunospot (ELISPOT) counts, measured as spot-forming cells per 106 PBMCs at 1 and 3 weeks, respectively, after HZ rash onset, by HZ severity-of-illness scores and corresponding regression lines. Results are shown separately for subjects at IL and non-IL sites who developed HZ. ELISPOT values during the first week after HZ rash onset were significantly higher in subjects from IL (P=.05) or non-IL (P=.009) sites with less severe disease. E and F Titers for enzyme-linked immunosorbent assay (ELISA) against affinity-purified varicella-zoster virus (VZV) glycoproteins (gpELISA), measured as ELISA units per milliliter, by HZ severity-of-illness scores and corresponding regression lines during the first and third weeks, respectively, after HZ rash onset. gpELISA titers are shown for subjects at all sites. G–I Geometric means and 95% confidence intervals for RCF, ELISPOT, and gpELISA values, respectively, during the first and third weeks after HZ onset in subjects who did not develop PHN (solid bars) and in those who did (hatched bars). For ELISPOT assays and RCF, data were derived from 71 subjects with PHN and 516 subjects without PHN from the non-IL sites (there were only 9 subjects with PHN at the IL sites). For gpELISA, data are shown from both IL and non-IL sites combined. gpELISA values during the third week after HZ rash onset were significantly higher in subjects with more severe disease (P<.001)
ELISPOT counts 1 week after HZ onset (Figure 3C) were significantly higher in subjects with lower HZ severity-of-illness scores from non-IL (P=.009) and IL (P=.05) sites and were significantly higher in subjects from non-IL sites who did not develop PHN than in those from non-IL sites who did (P=.01) (Figure 3H). At 3 weeks after HZ onset, there were no significant correlations between ELISPOT counts and HZ severity-of-illness scores (Figure 3D) or development of PHN (Figure 3H)
gpELISA titers from subjects at IL and non-IL sites 1 week after HZ onset did not correlate with HZ severity-of-illness scores (Figure 3E) or development of PHN. At 3 weeks after HZ onset, gpELISA titers were significantly higher in subjects with more severe disease as indicated by HZ severity-of-illness scores (P<.001) (Figure 3F) or by development of PHN (P<.001) (Figure 3I)
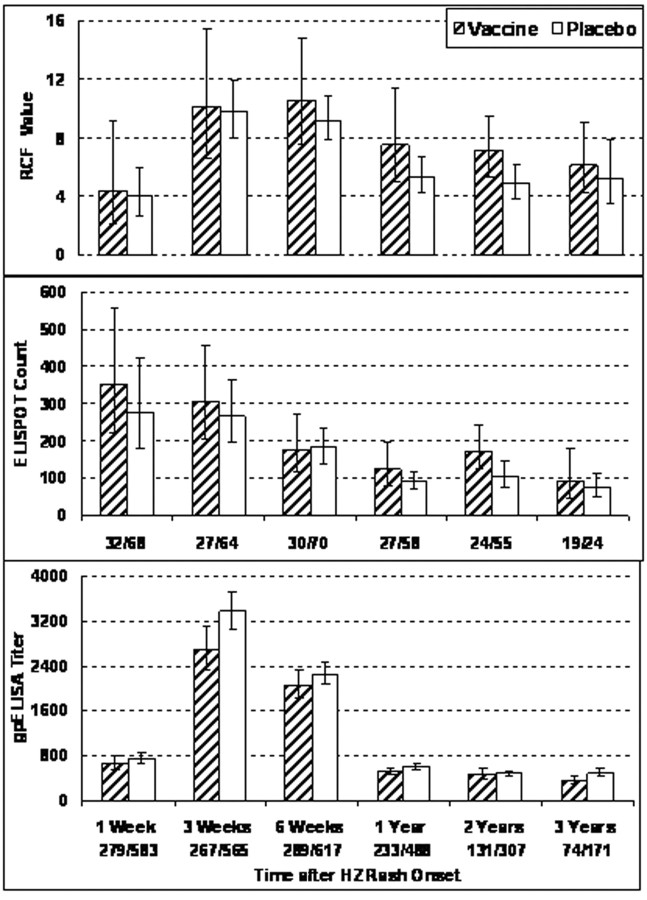
Effects of zoster vaccine, demographic characteristics, and antiviral treatment on immune responses to HZOur analyses included subjects with HZ from IL (n=107) and non-IL (n=874) sites. To determine how prior administration of the zoster vaccine affected the immune response to HZ—particularly 1 week after onset of rash, when VZV CMI correlated with protection against the severity of HZ—we compared the immune responses to HZ of vaccinees and placebo recipients. VZV CMI responses were analyzed separately in subjects with HZ from IL sites (Figure 4) and non-IL sites (not depicted). There were no significant differences in VZV CMI after onset of HZ between zoster vaccine and placebo recipients at IL or non-IL sites. gpELISA titers after HZ onset were higher in placebo recipients (n=660) than in vaccine recipients (n=321) (Figure 4). The differences were small but reached statistical significance at 3 weeks and at 1 and 3 years after HZ onset (P=.002, .025, and .012, respectively)
Figure 4.
Effect of the zoster vaccine on varicella-zoster virus (VZV)–specific immune responses to herpes zoster (HZ). VZV cell-mediated immunity results are shown for vaccine and placebo recipients who were enrolled at clinical sites where an immunology laboratory was located and who developed HZ; titers for enzyme-linked immunosorbent assay (ELISA) against affinity-purified VZV glycoproteins (gpELISA) are for subjects with HZ at all sites. Bars indicate geometric means and 95% confidence interval (CI) at each visit of the absolute responder cell frequency (RCF) values, measured as responder cells per 105 peripheral blood mononuclear cells (PBMCs); enzyme-linked immunospot (ELISPOT) counts, measured as spot-forming cells per 106 PBMCs; and gpELISA titers, measured as ELISA units per milliliter. Numbers indicate the numbers of subjects contributing samples at each time point. There were no significant differences in RCF or ELISPOT values between vaccine and placebo recipients. gpELISA titers were significantly lower in vaccine recipients at 3 weeks and at 1 and 3 years after onset of HZ (P=.002, .025, and .012, respectively)
The effects of demographic characteristics and treatment were also examined (data not depicted). At 1 week after HZ onset, the RCF correlated inversely with age in subjects from both IL and non-IL sites (P<.05). However, the week 1 RCF values reflected, in part, the level of preexisting immunity to VZV (see below), which was heavily influenced by age [14]. An effect of age on RCF was not observed at subsequent time points. There was no effect of age on ELISPOT responses to HZ at any time points. gpELISA titers did not vary with age 1 week after HZ onset, but they increased with age at weeks 3 and 6 (P⩽.003). This increase in gpELISA response paralleled the age-associated increase in severity of HZ. Sex, race, and administration of antivirals before the first visit after HZ onset had no appreciable effects on immune responses to HZ
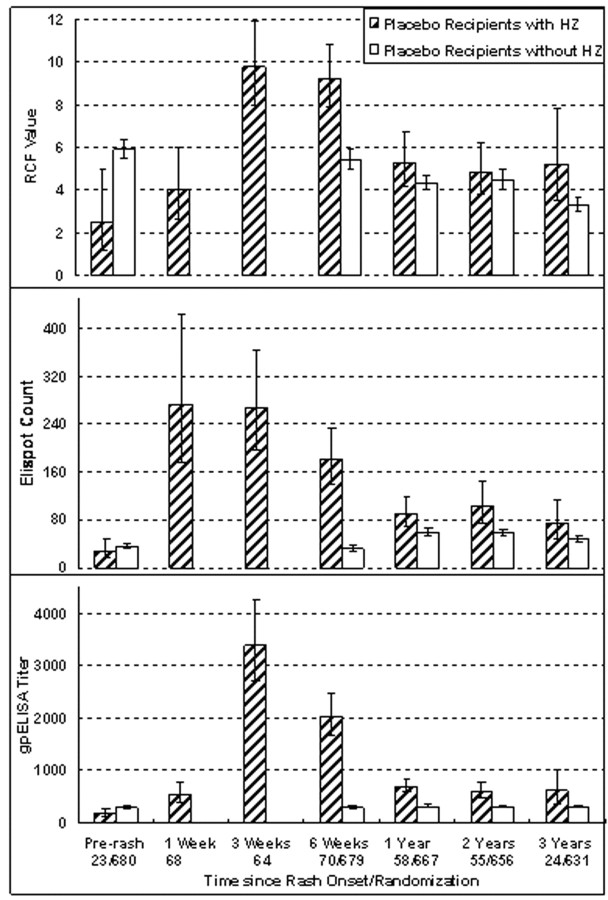
Kinetics of the immune responses to HZWe compared changes in VZV CMI over time in 73 placebo recipients with HZ who were observed at IL sites with those in 680 placebo recipients without HZ who were observed at the same sites in the Immunology Substudy (Figure 5). Also shown are immune responses obtained 1–12 months before HZ onset (mean, 200 days) in 23 of the 73 subjects with HZ in the Immunology Substudy for whom these data were available. VZV RCF values in subjects with HZ increased between the last visit before and the first visit after HZ rash onset (P=.02). The RCF values 1 week after HZ onset and at the last visit before HZ onset were moderately correlated (ρ=0.30). VZV RCF peaked between 3 and 6 weeks after HZ onset, decreased by year 1, and plateaued over the remaining 2 years of follow-up. The kinetics were consistent with memory CMI. Pre-HZ RCF values of subjects who developed HZ were significantly lower than baseline values of subjects who did not develop HZ (P=.001), whereas the opposite was true at 6 weeks after HZ onset
Figure 5.
Comparison of varicella-zoster virus (VZV)–specific immune responses of placebo recipients who developed herpes zoster (HZ) with placebo recipients who did not develop HZ. Data were derived from 73 subjects who received placebo at clinical sites where an immunology laboratory (IL) was located, developed HZ, and were followed up at those sites, as well as from 680 placebo recipients without HZ who were enrolled in the Immunology Substudy and were also followed up at the IL sites. Bars indicate geometric means and 95% confidence intervals (CIs) for the absolute responder cell frequency (RCF) values, measured as responder cells per 105 peripheral blood mononuclear cells (PBMCs); enzyme-linked immunospot (ELISPOT) counts, measured as spot-forming cells per 106 PBMCs; and titers for enzyme-linked immunosorbent assay (ELISA) against affinity-purified VZV glycoproteins (gpELISA), measured as ELISA units per milliliter. Levels before rash onset for subjects without HZ were those measured at enrollment. Levels at other time points were measured after onset of HZ rash in the subjects with HZ or after enrollment in the subjects without HZ. Numbers indicate the numbers of subjects contributing samples at each time point. Subjects who developed HZ had significantly lower values for RCF (P=.001) and gpELISA titers (P=.02) but not for ELISPOT counts at the last visit before HZ onset, compared with subjects who did not develop HZ. HZ significantly increased all immune responses
The analysis of the ELISPOT responses was complicated by the fact that the baseline and 6-week samples of the Immunology Substudy subjects were tested in a single batch of assays, whereas samples from later time points were tested in many different batches along with samples from subjects who developed HZ. The ELISPOT counts observed in placebo recipients at baseline and week 6 were 30–50 SFCs/106 PBMCs lower than subsequent measurements, indicating a change in the characteristics of the ELISPOT assay. This precluded a formal comparison of ELISPOT values between the last visit before and the first visit after HZ onset. The ELISPOT results did not change between weeks 1 and 3 after HZ onset, declined at 6 weeks and 1 year, and did not change during the subsequent 2 years. The kinetics were consistent with effector CMI. At the last visit before HZ onset, there were no significant ELISPOT count differences between subjects with HZ and those without HZ. At 6 weeks, ELISPOT counts were higher in placebo recipients with HZ than in those without HZ, even after accounting for technical differences. At years 1 and 2, ELISPOT counts were significantly higher in subjects with HZ than in those without HZ (P⩽.01), but at year 3, the difference was no longer significant
The gpELISA titers of subjects with HZ increased between the last visit before and the first visit after HZ rash onset (P<.001). This analysis used data from the 23 subjects with HZ in the Immunology Substudy. Titers before and 1 week after HZ onset were significantly correlated (ρ=0.68; P=.001). Antibody titers increased another 5-fold 3 weeks after HZ onset, decreased significantly at 6 weeks and 1 year, and plateaued during the next 2 years. Pre-HZ antibody titers were slightly lower in subjects with HZ than in placebo recipients without HZ (P=.02). After HZ, antibody titers were significantly higher in subjects with HZ than in those without HZ at all time points (P<.001 at 6 weeks and 1 and 2 years; P=.04 at 3 years). CMI responses to HZ measured by RCF and ELISPOT were moderately correlated in subjects from IL sites at all time points except year 3 (ρ=0.29–0.69). gpELISA and CMI responses were not correlated at any time point, except for a weak correlation of gpELISA titers with ELISPOT counts at 1 week after HZ onset (ρ=0.27)
Comparison of the immune responses to zoster vaccine and to HZTo evaluate the immunologic responses induced by the zoster vaccine versus those induced by HZ, we compared the VZV immunity of 73 placebo recipients at the IL sites who developed HZ with that of 680 vaccine recipients at these sites in the Immunology Substudy who did not develop HZ (Figure 6). The VZV RCF values were similar in placebo recipients with HZ and in zoster vaccine recipients at all time points. Because of the technical problems with the ELISPOT assay mentioned above, formal statistical analyses were not performed at week 6. However, the 6-week ELISPOT response to HZ exceeded the response to zoster vaccine by substantially more than the 30–50 SFCs/106 PBMCs that appear to have been contributed by the change in assay characteristics. At years 1, 2, and 3, ELISPOT values were similar in subjects with HZ and zoster vaccine recipients. gpELISA titers were significantly higher at all time points in placebo recipients with HZ than in zoster vaccine recipients (P<.001)
Figure 6.
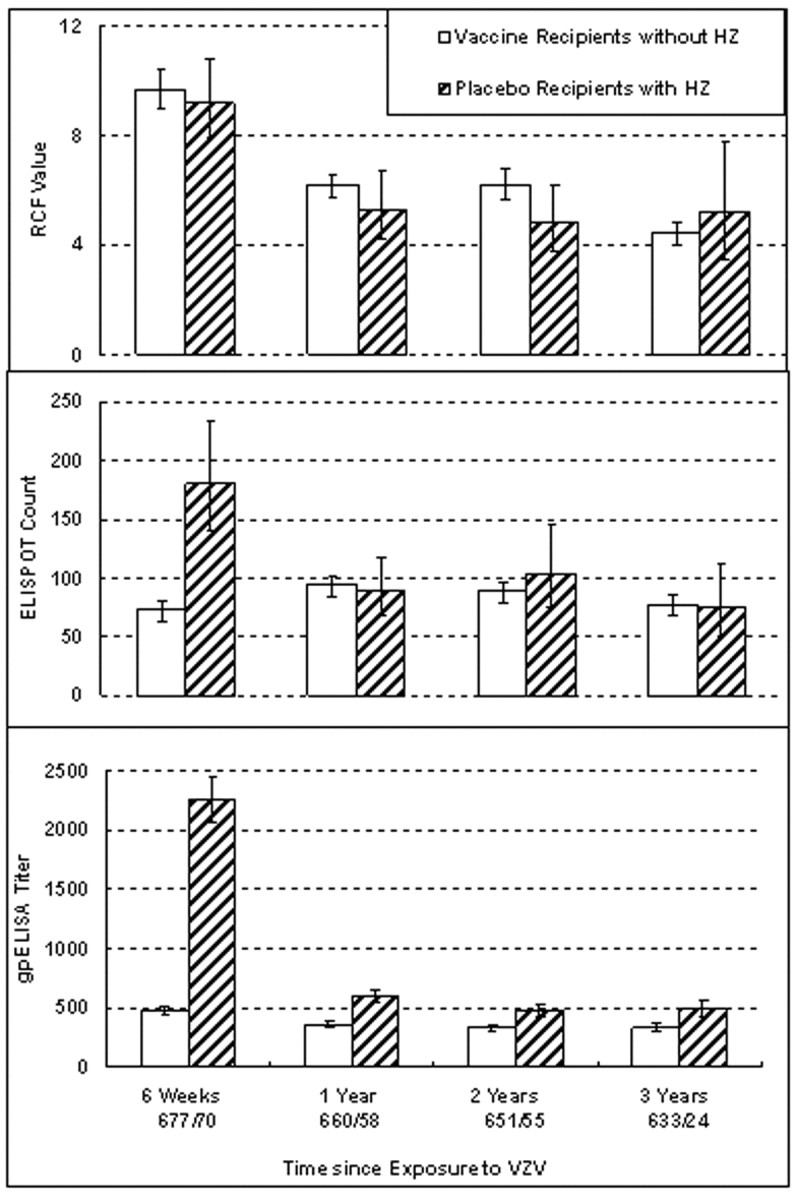
Comparison of the varicella-zoster virus (VZV)–specific immune responses to herpes zoster (HZ) and to zoster vaccine. Data were derived from subjects with HZ at clinical sites where an immunology laboratory (IL) was located and from recipients of zoster vaccine without HZ who were enrolled in the Immunology Substudy and were also followed up at the IL sites. Bars indicate geometric means and 95% confidence intervals for absolute responder cell frequency (RCF) values, measured as responder cells per 105 peripheral blood mononuclear cells (PBMCs); enzyme-linked immunospot (ELISPOT) counts, measured as spot-forming cells per 106 PBMCs; and titers for enzyme-linked immunosorbent assay (ELISA) against affinity-purified VZV glycoproteins (gpELISA), measured as ELISA units per milliliter. Numbers indicate the numbers of subjects contributing samples at each time point. RCF values were similar at all time points after vaccination and HZ onset; the analysis of ELISPOT responses at 6 weeks was complicated by technical problems, but at 1, 2 and 3 years, responses to vaccine and HZ were similar. gpELISA titers were significantly higher after HZ onset at all time points (P<.001)
Discussion
This study showed that greater VZV CMI responses in the first week after HZ rash onset, as measured by RCF and ELISPOT assays, correlated with decreased severity of disease and with lower occurrence of PHN, suggesting a protective effect of CMI against the morbidity of HZ. ELISPOT responses peaked during the first week after HZ onset, whereas RCF responses peaked between 3 and 6 weeks after HZ onset, by which time they no longer correlated with the severity of HZ. This indicates that high levels of VZV CMI at early time points were more important for protection against HZ and PHN than the magnitude of the peak response [26, 27]
In contrast to CMI, VZV antibody responses during the first week of HZ did not correlate with protection against severity of HZ or PHN. At 3 weeks after HZ onset, gpELISA titers were greater in subjects with more severe disease. The correlation between the magnitude of the antibody response to HZ and the severity of HZ also explains our observation that post-HZ gpELISA titers correlated with age. Increasing age is associated with more severe HZ and an increased incidence of PHN, implying more extensive VZV replication and consequent antigenic stimulation. This was confirmed by a multivariate analysis (not shown) of the association of antibody responses with age in subjects with HZ, in which the HZ severity-of-illness score was the only independent determinant of the antibody response. The observation that vaccine recipients who developed HZ had lower week 3 antibody titers than placebo recipients who developed HZ was also consistent with the association between post-HZ gpELISA titers and severity of disease; we showed elsewhere that zoster vaccine decreased HZ severity-of-illness scores in subjects who developed HZ [13]
The lack of a protective effect of VZV-specific antibodies against HZ severity-of-illness scores may seem to contradict the reported correlation between antibody responses to the zoster vaccine and protection against the incidence of HZ [14]. However, a unifying hypothesis is that antibody titers increase in response to VZV antigenic stimulation, resulting either from zoster vaccine or from HZ. In the case of HZ, the extent of VZV replication determines both the severity of the disease and the magnitude of the antigenic stimulation (which is sufficient to induce substantial antibody and CMI responses). In the case of zoster vaccine, limited replication of the less pathogenic live, attenuated virus is insufficient to cause disease in seropositive recipients but still sufficient to induce VZV antibody and CMI responses
The model that emerges from these observations is that higher levels of VZV CMI at and/or soon after VZV reactivation result in reduced viral replication and a lower incidence of complications, such as pain, discomfort, and PHN. Conversely, a weak VZV CMI response allows the reactivated virus to replicate unchecked, resulting in higher morbidity as well as greater antigenic stimulation of VZV immune responses. The relationship between the extent of antigenic stimulation and immune response to HZ is less evident for CMI than for antibodies, because higher levels of CMI limit virus replication, creating a negative feedback loop
Memory VZV CMI, measured by RCF, emerged as the strongest immunologic predictor of protection against severity and development of HZ in this study, as evidenced by the following: (1) RCF values of subjects who did not develop HZ were higher than pre-HZ RCF values of subjects with HZ; (2) pre-HZ RCF values predicted the RCF values in the first week of HZ, which in turn was correlated with protection against severity of HZ; (3) after HZ onset, RCF values in subjects with HZ remained higher than those in controls without HZ during the entire follow-up period, which mirrors the clinical observation that a single episode of HZ protects against subsequent attacks
The VZV RCF responses to the zoster vaccine and to HZ were similar in magnitude and duration. The comparability of memory VZV CMI between subjects with HZ and zoster vaccine recipients suggests that the protective effect of the zoster vaccine may also be comparable to that induced by an episode of HZ. A clinical study is in progress to determine the duration of protection conferred by the zoster vaccine
Age is an important determinant of the risk of HZ and PHN [6, 8–10, 13, 28]. Therefore, the effects of age on the immune response to HZ and to zoster vaccine were analyzed in detail. Although VZV CMI responses to the zoster vaccine declined with increasing age, the VZV CMI responses to HZ were generally unaffected by age. This difference in the effect of age on VZV-specific immune responses to HZ and to zoster vaccine are probably related to the extent of viral replication, which is significantly greater in HZ than after vaccination. The replication of the attenuated vaccine virus is efficiently controlled in vaccine recipients independent of age, as indicated by the equal distribution of vaccine-associated rashes across all age groups in the SPS and other studies [13, 29]. In contrast, wild-type VZV reactivations are controlled by older individuals less well than by younger ones, resulting in an increase in antigenic stimulation and, consequently, a greater boost in the VZV immune responses. These observations suggest that the attenuating effect of age on the VZV CMI response to zoster vaccine might be overcome by increasing the potency or the number of vaccine doses. Both strategies deserve to be further studied in elderly individuals
Financial Support
Cooperative Studies Program, Office of Research and Development, Department of Veterans Affairs; Merck (grant to the VA Cooperative Studies Program); National Institute of Child Health and Human Development (grants N01-HD-33162 to A.W. and N01-HD-3-3345 to M.J.L.); National Institute of Allergy and Infectious Diseases (support to multiple clinical sites, grants 1R21AI073121–01A2 and N01-AI-40029 to A.W., grant U01 AI068632 to M.J.L.); National Institute of Diabetes and Digestive and Kidney Diseases (grant U01 KD61055-03 to A.W.); Health Resources and Services Administration (grant H12HA00070 to M.J.L); National Institutes of Health (grants R01 HL079955, R01 AG026364, R01 CA 10014152, T32MH19925, P60 AG 10415, M01-RR00865, R01 AG026006-01, R01 NR009228, R01 AR049840, and R01 MH 55253 to M.R.I.); Cousins Center for Psychoneuroimmunology (M.R.I.); James R. and Jesse V. Scott Fund for Shingles Research (M.N.O.)
VA Cooperative Studies Program Shingles Prevention Study Investigators
The Shingles Prevention Study was planned and/or administered by a planning/executive committee: M.N.O. (chair), R.D.A., Patricia Barry, Chris Beisel, Kathy D. Boardman, Cindy L. Colling, L.E.D., Lawrence Gelb, A.A.G., A.R.H., M.R.I., G.R.J., M.J.L., Peter N. Peduzzi, K.E.S., Michael S. Simberkoff, S.E.S., A.W., H.M.W., Jeffrey L. Silber, Paula Annunziato, and Christina Y. Chan. I.S.F.C. Study investigators include L.E.D. (Albuquerque, New Mexico); C.A.K. (Ann Arbor, Michigan); S. K. Keay (Baltimore, Maryland); A. R. Marques, N. E. Soto, and P. Brunell (Bethesda, Maryland); J. W. Gnann (Birmingham, Alabama); R. Serrao, D. J. Cotton, R. P. Goodman, and R.D.A. (Boston, Massachusetts); C. T. Pachucki (Hines, Illinois); M.J.L. (Denver, Colorado); K.E.S. (Durham, North Carolina); W. A. Keitel (Houston, Texas); R. N. Greenberg (Lexington); V.A.M. (Minneapolis, Minnesota); P. F. Wright and M. R. Griffin (Nashville, Tennessee); M. S. Simberkoff (New York, New York); S. S. Yeh and Z. Lobo (Northport, New York); M. Holodniy and J. Loutit (Palo Alto, California); R. F. Betts (Rochester, New York); L. D. Gelb (St. Louis, Missouri); G. E. Crawford (San Antonio, Texas); J. Guatelli and P. A. Brooks (San Diego, California); K. M. Neuzil (Seattle, Washington); and J. F. Toney (Tampa, Florida)
Acknowledgments
Laboratory assistance for the RCF assay was provided at the San Diego site by P. Jordan and at the Denver site by L. Enomoto, G. Pott, E. Ponnuraj, D. Thrasher, and M. Borakove. Clinical assistance was provided at the San Diego site by P. McCook, D. Beck, J. Guatelli, P. Brooks, and A. Kendall; at the Denver site by N. Lang and D. Barber; and at Merck by M. E. Thompson, R. Rutledge, and N. Bundick. Data management and analysis support at West Haven Cooperative Studies Program Coordinating Center was provided by K. Dellert. Statistical assistance at Merck was provided by W. W. B. Wang, J. Xu, J. Heyse, and H. Matthews. Clinical administration at Merck was provided by F.S., J. Sadoff, S. Manoff, C. J. White, and R. Vessey. Laboratory assistance at Merck was provided by R. Kaufhold, M. Wooters, J. Field, H. Joseph for ELISPOT and by O. Hammond, P. Stump, M. Norris, M. Santo, C. Moyer, M. Constanzer, and J. Kessler for gpELISA
Footnotes
Potential conflicts of interest: A.W., A.A.G., and M.J.L. receive research funds or consultation fees from Merck or are on its speakers bureau. M.J.L. claims intellectual property in a Merck patent on the use of varicella-zoster virus vaccine to prevent HZ. I.S.F.C., M.J.C., J.G.S., J.C., F.S. , and R.D.M. are employees of Merck. I.S.F.C., R.H., and H.M.W. hold Merck stock or stock options (less than $10,000)
Financial support is listed at the end of the text
Presented in part: 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, 25–28 October 2008 (abstract G408)
Deceased
Study group members are listed at the end of the text
References
- 1.Berger R, Florent G, Just M. Decrease of the lymphoproliferative response to varicella-zoster virus antigen in the aged. Infect Immun. 1981;32:24–7. doi: 10.1128/iai.32.1.24-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–6. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 3.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–3. [PubMed] [Google Scholar]
- 4.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 5.Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30:582–7. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 6.Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32:1481–6. doi: 10.1086/320169. [DOI] [PubMed] [Google Scholar]
- 7.Berman JN, Wang M, Berry W, Neuberg DS, Guinan EC. Herpes zoster infection in the post-hematopoietic stem cell transplant pediatric population may be preceded by transaminitis: an institutional experience. Bone Marrow Transplant. 2006;37:73–80. doi: 10.1038/sj.bmt.1705191. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–51. doi: 10.1016/0304-3959(96)03122-3. [DOI] [PubMed] [Google Scholar]
- 9.Gnann JW, Jr, Whitley RJ. Clinical practice: herpes zoster. N Engl J Med. 2002;347:340–6. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- 10.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Kost RG, Straus SE. Postherpetic neuralgia: pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 13.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 14.Levin MJ, Oxman MN, Zhang JH, et al. VZV-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 16.Patterson-Bartlett J, Levin MJ, Lang N, Schodel FP, Vessey R. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine. 2007;25:7087–93. doi: 10.1016/j.vaccine.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 17.Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–56. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 19.Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 20.Smith JG, Liu X, Kaufhold RM, Clair J, Caulfield MJ. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin Diagn Lab Immunol. 2001;8:871–9. doi: 10.1128/CDLI.8.5.871-879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond O, Wang Y, Green T, et al. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78:1679–87. doi: 10.1002/jmv.20754. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Chan IS, Matthews H, et al. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J. 2002;21:337–42. doi: 10.1097/00006454-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg A, Betensky RA, Zhang L, Ray G. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol. 1998;5:804–7. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betensky RA, Connick E, Devers J, et al. Shipment impairs lymphocyte proliferative responses to microbial antigens. Clin Diagn Lab Immunol. 2000;7:759–63. doi: 10.1128/cdli.7.5.759-763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JG, Levin M, Vessey R, et al. Measurement of cell-mediated immunity with a varicella-zoster virus-specific interferon-gamma ELISPOT assay: responses in an elderly population receiving a booster immunization. J Med Virol. 2003;70(Suppl 1):S38–41. doi: 10.1002/jmv.10318. [DOI] [PubMed] [Google Scholar]
- 26.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J Exp Med. 2005;201:1555–65. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinlivan ML, Ayres K, Ran H, et al. Effect of viral load on the outcome of herpes zoster. J Clin Microbiol. 2007;45:3909–14. doi: 10.1128/JCM.00874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- 29.Levin MJ, Barber D, Goldblatt E, et al. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis. 1998;178(Suppl 1):S109–12. doi: 10.1086/514264. [DOI] [PubMed] [Google Scholar]