Summary
Using a novel murine model, we demonstrate that silicotic inflammation increases susceptibility to lung cancer initiated by exposure to NNK, a potent carcinogen in cigarettes. Cancer susceptibility is reduced to background by blocking this inflammation through treatment with immunosuppressive oligonucleotides.
Abstract
Silicosis is an inflammatory lung disease induced by the inhalation of silica-containing dust particles. There is conflicting data on whether patients with silicosis are more susceptible to lung cancer induced by cigarette smoke. To examine this issue experimentally, a model was developed in which one of the most abundant and potent carcinogens present in cigarette smoke [4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK)] was administered to mice at the peak of silica-induced pulmonary inflammation. Results show that the incidence of lung tumors in silicotic mice treated with NNK was significantly increased compared with mice exposed to silica or NNK alone. Synthetic oligonucleotides (ODN) containing repetitive TTAGGG motifs can block pathologic inflammation. We therefore examined whether treatment with these suppressive (Sup) ODN could block silica-induced pulmonary inflammation and thereby reduce susceptibility to lung cancer. Results show that Sup (but not control) ODN inhibit pulmonary fibrosis and other inflammatory manifestations of chronic silicosis. Of greater import, Sup ODN reduced lung tumor incidence and multiplicity in silicotic mice exposed to NNK. These findings establish an experimental model for examining the role of silicotic inflammation in cancer susceptibility and demonstrate that Sup ODN represent a novel therapy for chronic silicosis.
Introduction
The World Health Organization estimates that more than 1 million workers are occupationally exposed to silica dust annually in the USA (1). These inhaled particles are taken up by lung macrophages via scavenger receptors and trigger the production of proinflammatory cytokines [including tumor necrosis factor alpha (TNFα) and interleukin-1β (IL-1β)], chemokines and reactive oxygen species (2). Patients with silicosis typically present with pulmonary infiltrates and fibrosis (3–9). Epidemiological studies suggest that silicosis increases an individual’s susceptibility to lung tumors initiated by exposure to cigarette smoke although data on this issue is inconsistent (10–13). The broader literature shows that inflammatory processes contribute to the development and/or progression of many types of cancer (14).
Cigarette smoke contains a number of carcinogens of which 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is among the most abundant and potent (15). Exposure to NNK induces the formation of DNA adducts that cause mutations in key genes including K-ras and p53 (15,16). Although cellular genes are mutated by exposure to cigarette smoke, inflammation (such as that induced by silica particles) promotes the growth of these mutagenized cells and thus supports cancer development (17–19). Limited data suggest that treatment with anti-inflammatory agents can reduce host susceptibility to cancer (14,20).
Our group has been studying the ability of suppressive oligonucleotides (Sup ODN) to downregulate pathologic inflammatory responses. These ODN express repetitive TTAGGG motifs patterned after the immunosuppressive domains present in mammalian telomeres (21). Previous studies showed that TTAGGG motifs (released by injured host cells) block Th1 and proinflammatory cytokine production in vitro and downmodulate overexuberant/pathologic immune responses in vivo (such as those found in septic shock and autoimmune diseases) (21–26). The activity of Sup ODN is linked to their ability to inhibit elements of the STAT1 and STAT4 stimulatory cascades (26–28).
A novel murine model was developed to explore whether Sup ODN could limit the chronic inflammation caused by silicosis and thereby reduce susceptibility to NNK-initiated lung cancer. Silicosis similar to that observed in human miners was elicited by instilling crystalline silica into the lungs of mice (29). When these animals were exposed to NNK, lung tumor multiplicity increased. This increased susceptibility to lung cancer was reversed by treatment with Sup ODN. These findings provide the first experimental evidence that (i) silicotic inflammation increases lung cancer susceptibility and (ii) treatment with Sup ODN can protect against this increased tumorigenesis.
Materials and methods
ODN and reagents
Phosphorothioate ODN were synthesized at the Core Facility of the Center for Biologics Evaluation and Research facility, Food and Drug Administration (Bethesda, MD). The following ODN were used: suppressive ODN, TTAGGGTTAGGG TTAGGGTTAGGG; control ODN TTCAAATTCAAA TTCAAATTCAAA. All ODN were free of detectable protein or endotoxin contamination. Crystalline silica (mean particle size 1.7 μm) was obtained from US Silica (Berkeley Springs, WV) and was sterilized at 200°C for 2 h before intratracheal instillation to inactivate any potential endotoxin contamination. NNK was purchased from Sigma–Aldrich (St. Louis, MO).
Animals
Female A/J mice (Charles River Laboratories, Frederick, MD) were maintained on an AIN-76A diet (TestDiet, Richmond, IN). Animals were treated starting from 6 weeks of age. All studies were performed according to National Institutes of Health guidelines for the use and care of live mice and were approved by the Animal Care and Use Committee of the NCI in Frederick.
Mouse lung tumor model
Study 1.
Preliminary experiments were performed to clarify the effect of the dose and timing of NNK and silica delivery on tumor formation. Doses of silica ranging from 0.5 to 2.5 mg per mouse were delivered from 2 weeks before to 2 weeks after doses of NNK ranging from 125 to 2000 μg. The crystalline silica was administered intratracheally to mice anesthetized with a mixture of ketamine (80mg/kg) plus xylazine (10mg/kg, both from Sigma–Aldrich) in 50 μl of sterile saline using a high-pressure syringe (MicroSprayer Aerolizer, Penn-Century, Philadelphia, PA). The NNK was injected intraperitoneally (i.p.) in 200 μl of sterile saline. A total amount of 55 mice were used, with 15 mice each in the groups treated with NNK alone, NNK plus silica and untreated, whereas 10 were treated with silica alone. Results of these preliminary studies supported the use of 1mg of silica, followed 1 week later by 500 μg of NNK. The effect of treatment was studied for up to 6 months. When mice were killed, their lungs were inflated and fixed in 10% neutral-buffered formalin. Tumors visible on the surface of the lung were quantified by two independent and blinded observers.
Study 2.
One milligram of crystalline silica was instilled intratracheally into A/J mice, followed 1 week later by 500 μg of NNK i.p. Animals were treated by i.p. injection of 300 μg of suppressive or control ODN on day 0 and weekly thereafter for five total treatments. A total number of 52 mice were used; of which, 20 were treated with Sup ODN and the remaining 32 with even phosphate-buffered saline (PBS) or control ODN. Animal health and body weight were assessed weekly and were killed at 16 weeks. Messenger RNA (mRNA) was extracted from the right upper lobe using RNAlater (Quiagen, Valencia, CA). Histology was performed on lung tissue that was inflated and fixed in 10% neutral-buffered formalin. Total soluble collagen was measured by homogenizing lung tissue in 0.5M acetic acid with 0.1mg/ml pepsin (Sigma–Aldrich) using a Polytron PA 1200 (Kinematica AG, Lucerne, Switzerland).
Histopathologic analysis
Formalin fixed lungs were embedded in paraffin and sections taken serially every 100 microns. The lung sections from all 52 mice in study 2 were stained with hematoxylin and eosin (H&E) and evaluated histologically in a blinded fashion by a board-certified veterinary pathologist.
Bronchoalveolar lavage
Bronchoalveolar lavage fluid was collected from mice by repeatedly instilling and removing 1ml of PBS using a 22-gauge catheter as described previously (30). Cell differentials were performed on cytocentrifuge preparations of bronchoalveolar lavage after methanol fixation and staining with Diff-Quik (Dade Behring, Newark, DE). Total leukocyte counts were determined using a Sysmex KX-21N cell counter (Sysmex, Lincolnshire, IL).
RNA isolation and quantitative real-time PCR
Total RNA was isolated from lung tissue homogenates using the QIAshredder homogenizer column and RNeasy Mini Kit (both Quiagen). Complementary DNA was synthesized using a QuantiTect Reverse Transcription kit according to the manufacturer’s instructions (Applied Biosystems, Carlsbad, CA). Gene expression levels (normalized to glyceraldehyde-3-phosphate dehydrogenase) were analyzed using the StepOnePlus RT–PCR system (Applied Biosystems). All reagents and probes used in these studies were purchased from Applied Biosystems. The following TaqMan assays were used: IL-1β (Mm00434228_m1), TNFα (Mm00443260_g1) and IL-10 (Mm00439614-m1).
Collagen assay
Collagen levels were measured in lung homogenates from mice using the Sircol collagen assay according to the manufacturer’s instructions (Biocolor, Northern Ireland, UK). Briefly, Sirius Red Reagent was added to the homogenates and incubated for 30min. The collagen-dye complex was pelleted by centrifugation and the precipitated material dissolved in 0.5M NaOH. Optical density at 555nm was measured using a microplate reader.
Statistical analysis
Results are expressed as arithmetic means + standard error of the mean, unless otherwise noted. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA) and the R Statistical Language and Environment (31). Data were analyzed using Student’s t-test, two-way analysis of variation, chi square test and generalized linear models. The association of IL-1β gene expression with tumor multiplicity was evaluated using Poisson’s regression analysis. Probability values less than 0.05 were considered significant.
Results
Silica-induced pulmonary inflammation promotes NNK-dependent tumor development
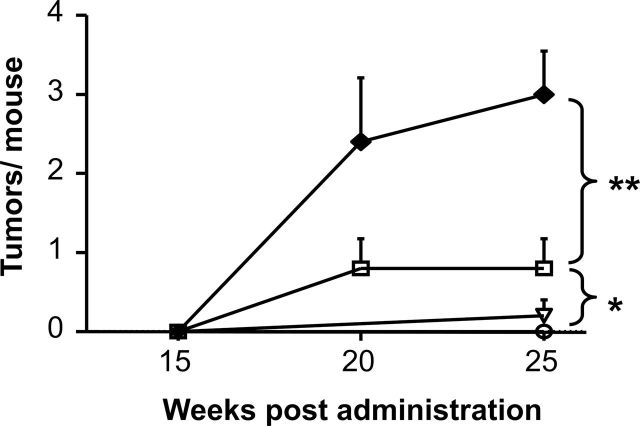
Preliminary experiments were conducted to establish a murine model capable of examining the effect of silicosis on NNK-initiated lung tumor development. These initial studies showed that a dose of 500 μg of NNK induced a low but statistically significant increase in lung tumor incidence, rising from 10% in untreated A/J mice to 60% in recipients of NNK (P = 0.02, chi square). This was associated with an increase in tumor multiplicity (Figure 1; P < 0.05), consistent with previous published results (32). Silicosis was elicited by instilling 1mg of crystalline silica intratracheally into these animals. The pulmonary inflammation induced by silica peaked after 1 week, at which time the mice were injected i.p. with 500 μg of NNK. Consistent with the hypothesis that pulmonary inflammation promotes the growth of tumors, tumor multiplicity increased significantly when mice were treated with both silica plus NNK (to greater than 3 tumors per animal, P < 0.01 versus NNK alone; Figure 1). Tumor incidence also increased from 60% to 93% (P = 0.04) although the diameter of the tumors was similar between groups (1.00±0.32 versus 0.88±0.39mm).
Fig. 1.
Mice treated with NNK plus silica develop lung tumors. Naive A/J mice (○, N = 15) were treated with 1mg of crystalline silica alone (▿, N = 10), 500 μg of NNK alone (□, N = 15) or silica followed by NNK (◆, N = 15, see Materials and methods for details). Lungs were fixed in formalin and analyzed for tumor formation 15–25 weeks posttreatment. Results represent the mean + standard error of the mean (SEM) of all animals per group. Statistic significance was determined by two-way analysis of variation. *P < 0.05; **P < 0.01.
Silica-induced IL-1β mRNA as a biomarker of inflammation-driven tumor susceptibility
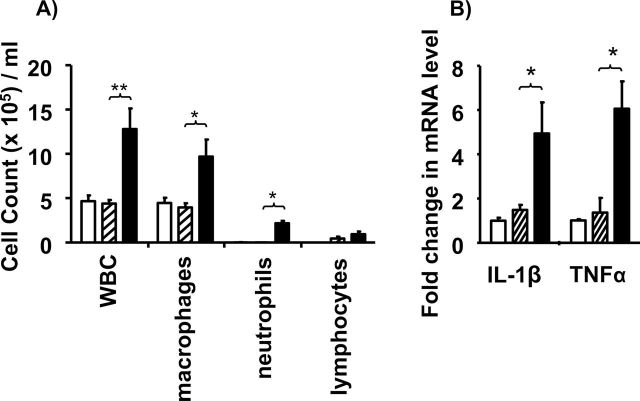
Mice treated with NNK alone showed no signs of inflammation (Figure 2 and refs 33,34). In contrast, animals treated with silica developed pulmonary inflammation characterized by the accumulation of leukocytes (P < 0.01), macrophages (P < 0.05) and neutrophils (P < 0.05; Figure 2A). The expression of genes encoding inflammatory cytokines (including TNFα and IL-1β) also remained elevated although they declined by more than 75% from peak values (Figure 2B; P < 0.05 and data not shown).
Fig. 2.
Effect of NNK plus silica on pulmonary inflammation. Naive A/J mice (open bar) were exposed to NNK (cross-hatched bar) or NNK plus silica (solid bar). Bronchoalveolar lavage and lung tissue were collected 16 weeks later. (A) The accumulation of inflammatory leukocytes in bronchoalveolar lavage was determined histologically. (B) The expression of genes encoding proinflammatory cytokines in lung tissue homogenates was quantified by reverse transcription–PCR (B). Relative cytokine mRNA levels were determined by comparison with untreated controls after normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Results represent the combined mean ± SEM of 2–3 independent experiments involving 5–9 mice per group. *P < 0.05; **P < 0.01.
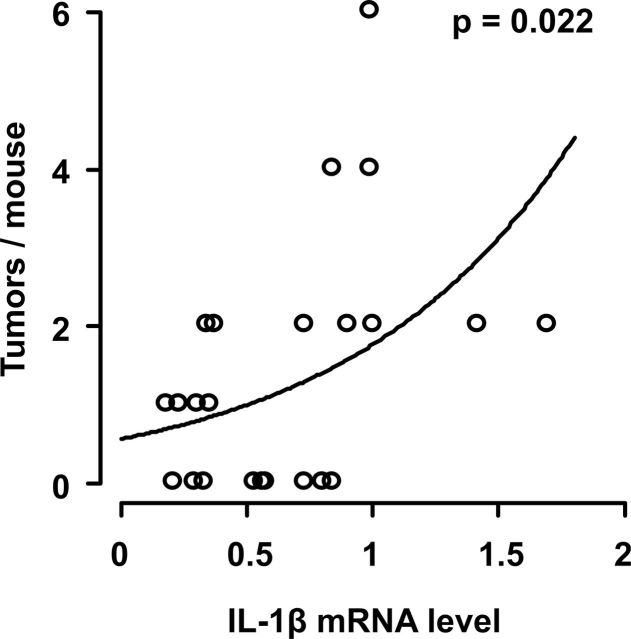
Since the expression of proinflammatory genes and susceptibility to lung cancer both increased in mice with silicosis, a Poisson’s regression analysis was performed to determine whether cytokine gene expression might provide a biomarker for tumor susceptibility. Results show that IL-1β mRNA levels varied as a function of tumor multiplicity in silicotic mice exposed to NNK (Figure 3; P = 0.022). No such association was observed in mice treated with NNK alone, a model in which inflammation plays no role.
Fig. 3.
Association between IL-1β gene expression and lung tumor development. Mice were treated with NNK plus silica. At 16 weeks, the incidence of lung tumors was quantified histologically, whereas cytokine mRNA levels were measured by reverse transcription–PCR in lung tissue homogenates. Tumor multiplicity versus IL-1β mRNA levels (normalized to GAPDH) from mice in four independent experiments (N = 23) is shown. The solid trend line and P value were calculated using the maximum likelihood estimate derived from a Poisson linear analysis. Note that IL-1β mRNA levels did not vary as a function of tumor multiplicity in mice treated with NNK alone (a model independent of pulmonary inflammation).
Suppressing inflammation reduces susceptibility to NNK- initiated tumors
If pulmonary inflammation increases susceptibility to NNK-initiated lung cancer, then reducing inflammation by treatment with Sup ODN might lower tumor susceptibility. As expected, proinflammatory gene expression was reduced to near background levels by the administration of Sup ODN (Figure 4, P < 0.05). In contrast, no effect was observed when mice exposed to silica plus NNK were treated with PBS or control (non-CpG) ODN (Figure 4).
Fig. 4.

Effect of Sup ODN on proinflammatory cytokine production in mice treated with NNK plus silica. Mice were treated with NNK plus silica as described in Figure 1. They were then injected i.p. with PBS (N = 16), 300 μg of Sup ODN (N = 20) or 300 μg of control ODN (N = 16) once per week for 4 weeks, starting on day 0. TNFα and IL-1β mRNA levels were measured by reverse transcription–PCR on tissue homogenates at 4 months. Bars show cytokine mRNA levels relative to naive mice (all values normalized to GAPDH). Results from the PBS and control ODN-treated groups were indistinguishable and thus combined to increase statistical power. Each bar represents the mean ± SEM of data combined from three independent experiments. *P < 0.05, **P < 0.01.
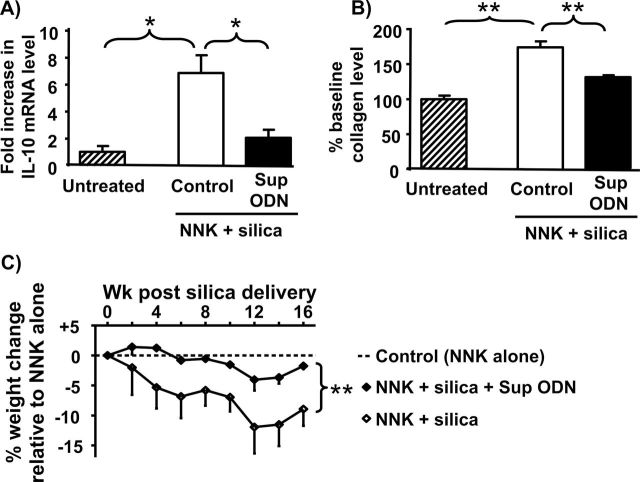
Sup ODN also reduced the clinical changes associated with chronic silicosis (35,36). Pulmonary fibrosis was evaluated by measuring the accumulation of collagen and IL-10 (a profibrotic cytokine) in the lungs (37,38). Consistent with previous reports, pulmonary IL-10 mRNA levels rose significantly in mice exposed to silica + NNK (more than 7-fold; P < 0.05) as did collagen protein levels (more than 75%; P < 0.01), compared with naive animals or mice exposed to NNK alone (Figure 5A and B and data not shown). When silicotic animals were treated with Sup ODN, IL-10 mRNA expression and collagen deposition fell by 50–70% (Figure 5A and B; P < 0.05). These effects were specific as control ODN and/or PBS had no significant impact on these markers of fibrosis. In addition, mice treated with NNK plus silica lost 10–15% of their body weight. This weight loss was significantly reduced by treatment with Sup ODN (Figure 5C; P < 0.01).
Fig. 5.
Effect of Sup ODN on chronic silicosis. Mice were treated with NNK plus silica and then injected with PBS, Sup or control ODN as described in Figure 4. Lung tissue collected at wk 16 was homogenized and examined for (A) IL-10 mRNA levels by reverse transcription–PCR and (B) collagen levels by the Sircol collagen assay. (C) Changes in body weight were measured over 16 weeks. Data represent the combined mean ± SEM of two independent experiments involving greater than or equal to 10 mice per group. Results from the PBS and control ODN-treated groups were indistinguishable and thus combined to increase statistical power. Differences in body weight between groups were analyzed by two-way analysis of variation. *P < 0.05, **P < 0.01
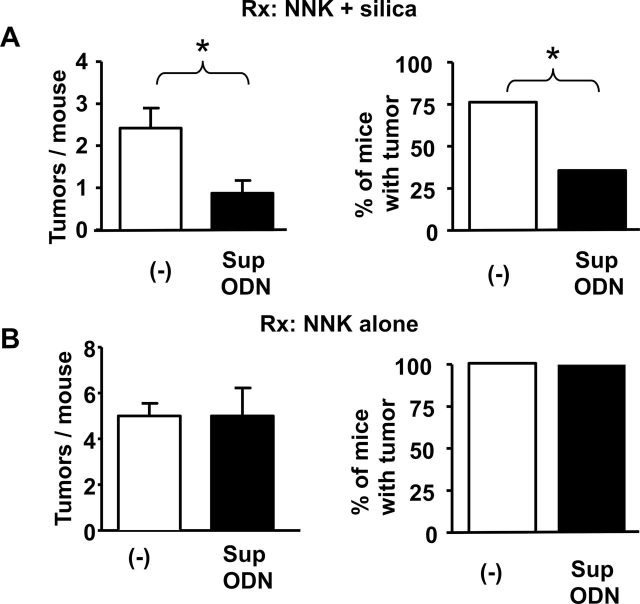
To evaluate the effect of Sup ODN on tumor susceptibility, mice were given NNK plus silica and were randomly assigned to Sup ODN-treated versus control groups. After 16 weeks, lungs were removed and analyzed histologically by a veterinary pathologist. Sup ODN treatment reduced the incidence and multiplicity of tumors by 50 and 67%, respectively, compared with the controls (P < 0.05, Figure 6A). Indeed, tumor multiplicity in animals exposed to NNK plus silica and then treated with Sup ODN fell to that of control mice exposed to NNK alone (compare Figure 2 with Figure 6). The tumors in these two groups of mice were also similar: 94/95 were histologically confirmed as adenomas and expressed mutations in the K-ras gene, a hallmark of NNK-induced tumors (16) (Supplementary Figures 1 and 2, available at Carcinogenesis Online).
Fig. 6.
Effect of Sup ODN on lung tumor development in mice treated with NNK + silica. (A) Mice were treated with NNK plus silica and then injected with PBS (N = 16), Sup ODN (N = 20) or control ODN (N = 16) as described in Figure 4. (B) Mice were treated with 2000 μg of NNK alone and then injected with PBS (N = 10), Sup ODN (N = 20) or control ODN (N = 10). At 16 weeks, all lungs were analyzed histologically for the presence of tumors. Results from mice treated with PBS or control ODN were indistinguishable and thus combined to increase statistical power. Statistical analysis of tumor incidence was performed using chi square test. *P < 0.05.
To verify that the decrease in tumor incidence and multiplicity observed in mice treated with Sup ODN was due to a reduction in silicotic inflammation rather than some nonspecific antitumor effect, Sup ODN were administered to mice treated with NNK alone. In these experiments, animals were injected with 2000 ug (rather than 500 ug) of NNK to increase tumor incidence in the absence of silica. As seen in Figure 6B, Sup ODN treatment did not reduce either tumor incidence or multiplicity in mice exposed to NNK alone.
Discussion
This report provides the first experimental evidence that the pulmonary inflammation induced by silica dust promotes the growth of tumor cells mutagenized by exposure to NNK, an abundant and potent carcinogen in cigarette smoke. This model supports epidemiologic data indicating that miners who inhale silica dust are at increased risk of developing smoking-induced lung cancer (10–13). These results further demonstrate that silica-induced tumor susceptibility can be reversed by treatment with immunosuppressive ODN.
Cancer is a multistage process in which cells mutagenized by exposure to carcinogens are driven to proliferate by factors in the environment (39). Numerous studies show that an inflammatory milieu can support the survival, proliferation and metastasis of tumor cells (20,40,41). Silica dust creates such an environment by attracting leukocytes, macrophages and neutrophils to the lungs and stimulating them to produce proinflammatory cytokines and chemokines (29). Consistent with silica dust promoting NNK-initiated tumors, the malignancies present mice exposed to NNK plus silica carried the same K-ras mutations and were histologically indistinguishable from those induced by NNK alone (16). These results suggest that silicosis promotes but does not initiate carcinogenesis as mice exposed to silica alone (even at near-lethal doses up to 2.5 mg per mouse) showed no increase in tumor incidence.
The Sup ODN used in this work contain repetitive TTAGGG motifs identical to those found at high frequency in mammalian telomeres. Previous studies established that Sup ODN prevent inflammation in murine models of septic shock and autoimmune disease (22,26,28,42,43). Current findings demonstrate that Sup ODN also limit the fibrosis, weight loss and proinflammatory cytokine production caused by chronic silicosis. Of particular importance, Sup ODN decreased the incidence and multiplicity of lung tumors in mice exposed to silica plus NNK but not NNK alone. This leads us to conclude that Sup ODN reduce tumor susceptibility by controlling inflammation rather than any direct effect on mutagenized cells (32). The therapeutic benefit of Sup ODN was sequence specific as no protection was conferred by control ODN lacking the TTAGGG motif.
Previous studies showed that Sup ODN exert pleotropic effects on multiple inflammatory pathways (21,27,44). Based on the observation that the Nalp3 inflammasome may contribute to silica mediated inflammation (45), we examined the effect of Sup ODN on the activation of the Nalp3 inflammasome. Although silica activation of this pathway was observed, no inhibition by Sup ODN occurred (data not shown). This outcome was not entirely unexpected as recent findings indicate that Nalp3 activation is not required for silica to induce an inflammatory response in vivo (46). Thus, the molecular mechanism by which silica particles drive inflammation and how this process is blocked by Sup ODN remains uncertain.
Cox postulated that the tumor-promoting activity of silica exhibited a ‘threshold effect’ (47). Consistent with that prediction, we found that both the amount and timing of silica influenced tumor susceptibility. Preliminary studies showed that increased tumorigenesis was observed only when NNK was administered at the peak of silica-induced inflammation and that a minimum of 1mg of silica was required. By comparison, Yokohira et al. (48) failed to promote tumor formation by exposing A/J mice to only 0.1mg of silica several weeks after (rather than 1 week before) NNK. Several observations suggest that the tumor-promoting effect of silica was short lived: (i) tumor promotion was only observed at the peak of silica-induced inflammation, (ii) this promoting effect was reversed by early treatment with Sup ODN and (iii) long-term low-level silicotic inflammation yielded no increase in tumor size.
IL-1β mRNA levels provided a reliable metric of the effect of Sup ODN on tumor susceptibility. Although it is unlikely that a single cytokine is the primary mediator of carcinogenesis in this model, results suggest that IL-1β represent a useful biomarker of silica and/or inflammation dependent tumor promotion. Consistent with this hypothesis, IL-1β and TNFα mRNA levels in the lungs of tumor bearing mice treated with NNK alone did not differ from normal controls, indicating that tumors did not induce the production of these cytokines. Similarly, Sup ODN had no effect on tumor incidence or IL-1β mRNA levels in mice treated with NNK alone, a model in which chronic inflammation played no role.
This work provides the first experimental evidence that silicotic inflammation increases susceptibility to NNK-initiated lung cancer. It further shows that reducing inflammation through treatment with Sup ODN interferes with this processes, thereby prevents the development of such malignancies. Epidemiologic studies indicate that prolonged use of anti-inflammatory agents (such as aspirin or cyclooxygenase-2 inhibitors) may reduce the risk of humans developing certain tumors (40,49,50). That prediction is consistent with murine models showing that susceptibility to chemically induced tumors is reduced by anti-inflammatory agents (51–57). Current findings demonstrate that synthetic ODN specifically designed to inhibit immune activation lower cancer susceptibility of mice with inflammatory lung disease. Preclinical testing shows that these Sup ODN (which are patterned after and recapitulate the anti-inflammatory activity of human telomeric DNA) are safe when administered repeatedly to mice and nonhuman primates (reviewed in ref. 58). Our results support their testing in patients with silicosis at high risk of developing lung cancer, with ongoing studies being directed toward determining their impact in models of inflammation-induced cancer metastasis.
Supplementary material
Supplementary Figures 1–2 can be found at http://carcin.oxford journals.org/.
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Supplementary Material
Acknowledgements
We thank Mr Octavio Quinones for his support in the statistical analysis of this work. We also thank Mr Olfert Landt for technical and material support in the K-ras mutation analysis. The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the National Cancer Institute at large.
Conflict of Interest Statement: D.M.K. and members of his laboratory hold or have applied for patents concerning the activity of suppressive ODN, including their use in preventing/treating tumors. The rights to all such patents have been transferred to the United States government.
Glossary
Abbreviations:
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IL
interleukin
- NNK
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone
- PBS
phosphate-buffered saline
- SEM
standard error of the mean
- Sup ODN
suppressive oligodeoxynucleotides
- TNFα
tumor necrosis factor alpha.
References
- 1. Greenberg M.I., et al. (2007). Silicosis: a review. Dis. Mon., 53, 394–416 [DOI] [PubMed] [Google Scholar]
- 2. Beamer C.A., et al. (2005). Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am. J. Physiol. Lung Cell. Mol. Physiol., 289, L186–L195 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. (2007). Silicosis. WHO Media Centre, Geneva, Switzerland [Google Scholar]
- 4. Verma D.K., et al. (2002) Translating evidence about occupational conditions into strategies for prevention. Occup. Environ. Med., 59, 205–13; quiz 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fubini B., et al. (2003). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med., 34, 1507–1516 [DOI] [PubMed] [Google Scholar]
- 6. Davis G.S., et al. (2000). Interferon-gamma production by specific lung lymphocyte phenotypes in silicosis in mice. Am. J. Respir. Cell Mol. Biol., 22, 491–501 [DOI] [PubMed] [Google Scholar]
- 7. Huaux F., et al. (2002). A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J. Immunol., 169, 2653–2661 [DOI] [PubMed] [Google Scholar]
- 8. Van Z.M., et al. (2000). Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity. Toxicol. Appl. Pharmacol., 168, 131–139 [DOI] [PubMed] [Google Scholar]
- 9. Brown J.M., et al. (2004). Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal. Toxicol., 16, 133–139 [DOI] [PubMed] [Google Scholar]
- 10. Kurihara N., et al. (2004). Silicosis and smoking strongly increase lung cancer risk in silica-exposed workers. Ind. Health, 42, 303–314 [DOI] [PubMed] [Google Scholar]
- 11. Lacasse Y., et al. (2009). Dose-response meta-analysis of silica and lung cancer. Cancer Causes Control, 20, 925–933 [DOI] [PubMed] [Google Scholar]
- 12. Brown T. (2009). Silica exposure, smoking, silicosis and lung cancer–complex interactions. Occup. Med. (Lond)., 59, 89–95 [DOI] [PubMed] [Google Scholar]
- 13. De M.S., et al. (2012). Impact of occupational carcinogens on lung cancer risk in a general population. Int. J. Epidemiol., 41, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balkwill F., et al. (2001). Inflammation and cancer: back to Virchow? Lancet, 357, 539–545 [DOI] [PubMed] [Google Scholar]
- 15. Hecht S.S. (1998). Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol., 11, 559–603 [DOI] [PubMed] [Google Scholar]
- 16. Belinsky S.A., et al. (1989). Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res., 49, 5305–5311 [PubMed] [Google Scholar]
- 17. Karoor V., et al. (2012). Alveolar hypoxia promotes murine lung tumor growth through a VEGFR-2/EGFR-dependent mechanism. Cancer Prev. Res. (Phila)., 5, 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Catassi A., et al. (2008). Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat. Res., 659, 221–231 [DOI] [PubMed] [Google Scholar]
- 19. Hanahan D., et al. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 20. Coussens L.M., et al. (2002). Inflammation and cancer. Nature, 420, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gursel I., et al. (2003). Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J. Immunol., 171, 1393–1400 [DOI] [PubMed] [Google Scholar]
- 22. Dong L., et al. (2004). Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum., 50, 1686–1689 [DOI] [PubMed] [Google Scholar]
- 23. Zeuner R.A., et al. (2002). Reduction of CpG-induced arthritis by suppressive oligodeoxynucleotides. Arthritis Rheum., 46, 2219–2224 [DOI] [PubMed] [Google Scholar]
- 24. Zeuner R.A., et al. (2003). Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum., 48, 1701–1707 [DOI] [PubMed] [Google Scholar]
- 25. Shirota H., et al. (2004). Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J. Immunol., 173, 5002–5007 [DOI] [PubMed] [Google Scholar]
- 26. Shirota H., et al. (2005). Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J. Immunol., 174, 4579–4583 [DOI] [PubMed] [Google Scholar]
- 27. Shirota H., et al. (2004). Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J. Immunol., 173, 5002–5007 [DOI] [PubMed] [Google Scholar]
- 28. Cheng X., et al. (2008). Suppressive oligodeoxynucleotides inhibit atherosclerosis in ApoE(-/-) mice through modulation of Th1/Th2 balance. J. Mol. Cell. Cardiol., 45, 168–175 [DOI] [PubMed] [Google Scholar]
- 29. Rimal B., et al. (2005). Basic pathogenetic mechanisms in silicosis: current understanding. Curr. Opin. Pulm. Med., 11, 169–173 [DOI] [PubMed] [Google Scholar]
- 30. Sato T., et al. (2008). Suppressive oligodeoxynucleotides inhibit silica-induced pulmonary inflammation. J. Immunol., 180, 7648–7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. R Development Core Team. (2011). Statisticalanalysis: anintroduction using R/R basics. In R Foundation for Statistical Computing (ed.) R: A language and environment for statistical computing. WikiBooks, Vienna, Austria [Google Scholar]
- 32. Hecht S.S., et al. (1989). Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis, 10, 1901–1904 [DOI] [PubMed] [Google Scholar]
- 33. Keohavong P., et al. (2011). K-ras mutations in lung tumors from NNK-treated mice with lipopolysaccharide-elicited lung inflammation. Anticancer Res., 31, 2877–2882 [PubMed] [Google Scholar]
- 34. Therriault M.J., et al. (2003). Immunomodulatory effects of the tobacco-specific carcinogen, NNK, on alveolar macrophages. Clin. Exp. Immunol., 132, 232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leung C.C., et al. (2012). Silicosis. Lancet, 379, 2008–2018 [DOI] [PubMed] [Google Scholar]
- 36. Huaux F. (2007). New developments in the understanding of immunology in silicosis. Curr. Opin. Allergy Clin. Immunol., 7, 168–173 [DOI] [PubMed] [Google Scholar]
- 37. Barbarin V., et al. (2005). The role of pro- and anti-inflammatory responses in silica-induced lung fibrosis. Respir. Res., 6, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huaux F., et al. (1998). Role of interleukin-10 in the lung response to silica in mice. Am. J. Respir. Cell Mol. Biol., 18, 51–59 [DOI] [PubMed] [Google Scholar]
- 39. Abel E.L., et al. (2009). Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat. Protoc., 4, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mantovani A., et al. (2008). Cancer-related inflammation. Nature, 454, 436–444 [DOI] [PubMed] [Google Scholar]
- 41. Apetoh L., et al. (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med., 13, 1050–1059 [DOI] [PubMed] [Google Scholar]
- 42. Dong L., et al. (2005) Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB x NZW mice. Arthritis Rheum., 52, 651–658 [DOI] [PubMed] [Google Scholar]
- 43. Fujimoto C., et al. (2009). A suppressive oligodeoxynucleotide inhibits ocular inflammation. Clin. Exp. Immunol., 156, 528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaminski J.J., et al. (2013). Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J. Immunol., 191, 3876–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dostert C., et al. (2008). Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science, 320, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kono H., et al. (2012). The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J. Immunol., 189, 3734–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cox L.A., Jr (2011). An exposure-response threshold for lung diseases and lung cancer caused by crystalline silica. Risk Anal., 31, 1543–1560 [DOI] [PubMed] [Google Scholar]
- 48. Yokohira M., et al. (2009). Lack of Modifying Effects of Intratracheal Instillation of Quartz or Dextran Sulfate Sodium (DSS) in Drinking Water on Lung Tumor Development Initiated with 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in Female A/J Mice. J. Toxicol. Pathol., 22, 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baron J.A., et al. (2000). Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu. Rev. Med., 51, 511–523 [DOI] [PubMed] [Google Scholar]
- 50. García-Rodríguez L.A., et al. (2001). Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology, 12, 88–93 [DOI] [PubMed] [Google Scholar]
- 51. Kawamori T., et al. (1998). Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res., 58, 409–412 [PubMed] [Google Scholar]
- 52. Grubbs C.J., et al. (2000). Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res., 60, 5599–5602 [PubMed] [Google Scholar]
- 53. Rioux N., et al. (1998). Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res., 58, 5354–5360 [PubMed] [Google Scholar]
- 54. Müller-Decker K., et al. (1998). Localization of prostaglandin H synthase isoenzymes in murine epidermal tumors: suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Mol. Carcinog., 23, 36–44 [DOI] [PubMed] [Google Scholar]
- 55. Fischer S.M., et al. (1999). Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol. Carcinog., 25, 231–240 [PubMed] [Google Scholar]
- 56. Pentland A.P., et al. (1999). Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis, 20, 1939–1944 [DOI] [PubMed] [Google Scholar]
- 57. Fürstenberger G., et al. (1989). Eicosanoids and multistage carcinogenesis in NMRI mouse skin: role of prostaglandins E and F in conversion (first stage of tumor promotion) and promotion (second stage of tumor promotion). Carcinogenesis, 10, 91–96 [DOI] [PubMed] [Google Scholar]
- 58. Klinman D.M., et al. (2005). Therapeutic potential of oligonucleotides expressing immunosuppressive TTAGGG motifs. Ann. N. Y. Acad. Sci., 1058, 87–95 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.