Abstract
Aims/Introduction: The present study investigated the frequency of mild anemia, which is not an indication of intensive therapy using drugs, in Japanese patients with type 2 diabetes mellitus and the association of mild anemia with diabetic complications.
Materials and Methods: This is a cross‐sectional study of 1189 patients with type 2 diabetes mellitus. Anemia was defined as a hemoglobin level <13.5 g/dL in men and <12.0 g/dL in women. The patients with anemia were divided into two groups: (i) grade 1 anemia with a hemoglobin level ≥11.0 g/dL; and (ii) grade 2 anemia with a hemoglobin level <11.0 g/dL.
Results: The prevalence of anemia increased with the progression of the stage of diabetic nephropathy and chronic kidney disease. The frequencies of diabetic micro‐ and macroangiopathies increased with the progression of anemia among 798 patients without anemia, 300 with grade 1 anemia and 91 with grade 2 anemia. Both grade 1 and grade 2 anemia were associated with diabetic micro‐ and macroangiopathies. They remained independently associated with diabetic retinopathy, coronary heart disease and peripheral arterial disease after adjustment by age, sex, body mass index, use of angiotensin II receptor blocker, estimated glomerular filtration rate and stage of diabetic nephropathy.
Conclusions: Mild anemia is frequent and associated with micro‐ and macroangiopathies in patients with type 2 diabetes mellitus. It is important to carry out intensive examinations for the detection of diabetic micro‐ and macroangiopathies in addition to evaluating the causes of anemia when mild anemia is found in patients with diabetes mellitus. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00060.x, 2010)
Keywords: Anemia, eGFR, Diabetic nephropathy
Introduction
Chronic anemia is common in diabetic patients1–15. Although the definition of anemia differs among studies, the rate of anemia ranges from 14 to 17% and 8–66% in patients with type 1 and type 2 diabetes mellitus, respectively1–16. Several mechanisms have been discussed in diabetes mellitus‐associated anemia and an association of anemia with the progression of diabetic nephropathy is reported1–15,17. Vitamin deficiencies, such as folate and B12, are relatively unusual and the major causes of anemia are thought to be iron and erythropoietin (EPO) deficiencies, and hyporesponsiveness to the actions of EPO15,17.
Anemia is also recognized as an independent risk factor for hospitalization as a result of heart failure, because it exacerbates the cardiac ischemia results of decreased supply or increased demand for oxygen, such as in patients with underlying coronary heart disease, left ventricular hypertrophy or arrhythmia. Furthermore, anemia is accompanied by cardiovascular events9–12, mortality13 and mortality after acute myocardial infarction16 in patients with diabetes mellitus.
The correction of anemia is usually carried out with an iron supplement or erythropoiesis stimulating agents (ESA). The target level of hemoglobin is recommended to be 11.0–12.0 g/dL and not to exceed 13.0 g/dL in the guidelines by National Kidney Foundation (NKF), European Renal Association‐European Dialysis and Transplantation Association (ERA‐EDTA) and National Institute for Health18–20. Therefore, patients with mild anemia who have a hemoglobin level ≥11.0 g/dL are not indicated for intensive treatments using drugs in the clinical routine.
No study has yet determined whether mild anemia is associated with cardiovascular events in patients with type 2 diabetes mellitus. The present study investigated the frequency of mild anemia in Japanese patients with type 2 diabetes mellitus and the association of mild anemia with diabetic micro‐ and macroangiopathies.
Materials and Methods
A cross‐sectional study was carried out in a population of 1189 patients diagnosed with type 2 diabetes mellitus under consecutive evaluations of urinalysis, hemoglobin and serum creatinine levels attending the Department of Diabetes, Metabolism and Kidney Diseases of Edogawa Hospital, Tokyo, Japan, between April 2008 and March 2009. Patients with end‐stage renal disease receiving maintenance dialysis were excluded from the study. Subjects who underwent physical health examinations were also entered into the study as normal controls (age‐matched 112 men and 99 women). They did not have a history of diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease (CKD), myocardial infarction and/or cerebral infarction. They were confirmed to show no proteinuria or glucosuria with normal liver and kidney functions according to the findings of urine and blood examinations.
Anemia was defined as a hemoglobin level <13.5 g/dL in men and <12.0 g/dL in women according to the guidelines by ERA‐EDTA19 and NKF21. Anemia was divided into two groups in the present study: (i) grade 1 anemia with a hemoglobin level ≥11.0 g/dL; and (ii) grade 2 anemia with a hemoglobin level <11.0 g/dL.
The estimated glomerular filtration rate (eGFR) was calculated using the formula reported by Matsuo et al.22 This equation originated from the MDRD study group23, arranged for Japanese individuals, and recommended by the Japanese Society of Nephrology:
The stages of CKD were based on the NKF K/DOQI clinical practice guidelines24. The UAE is presented as the albumin‐to‐creatinine ratio (ACR; mg/g creatinine). Diabetic nephropathy (DN) was staged according to an analysis of a spot urine sample as: DN stage I (normoalbuminuria), ACR <30 mg/g creatinine; DN stage II (microalbuminuria), 30 ≤ACR <300 mg/g creatinine; DN stage III (macroalbuminuria), ACR ≥300 mg/g creatinine (or dipstick urinalysis revealed 2+, 3+ or 4+) and eGFR ≥30 mL/min/1.73 m2; DN stage IV, ACR ≥300 mg/g creatinine (or dipstick urinalysis revealed 2+, 3+ or 4+) and eGFR <30 mL/min/1.73 m225. Individuals complicated with other kidney diseases, such as chronic glomerulonephritis and interstitial nephritis, were excluded from the study. Hypertension was defined as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg. Participants currently using antihypertensive medications were also classified as positive for hypertension. Hyperlipidemia was defined by serum concentrations of total cholesterol ≥220 mg/dL, low‐density cholesterol ≥140 mg/dL, or patients already treated with lipid‐lowering agents.
Diabetic retinopathy was defined as simple retinopathy or more severe conditions judged according to the results of funduscopic examinations carried out by expert ophthalmologists. Diabetic neuropathy was diagnosed by the presence of two or more components among clinical symptoms (bilateral spontaneous pain, hypoesthesia or paresthesia of the legs), absence of ankle tendon reflexes and decreased vibration sensations using a C128 tuning fork. Cerebrovascular disease was diagnosed by the physicians as a history of ischemic stroke using brain computed tomography or magnetic resonance imaging. Only patients with symptoms were classified as having cerebrovascular disease, and cases of silent brain infarction, transient ischemic attack or brain hemorrhage were excluded from the study. Coronary heart disease was diagnosed according to a previous history of myocardial infarction, angina pectoris, electrocardiogram abnormalities suggesting myocardial ischemia or interventions after coronary angiographic examination. Peripheral arterial disease was diagnosed by the absence of a pulse in the legs with ischemic symptoms, obstructive findings on ultrasonographic examination of the lower extremities or ankle‐brachial pressure index <0.9.
The hemoglobin level was measured using a Beckman/Coulter LH750 automated processor (Beckman Coulter, Fullerton, CA, USA).
Statistical Analysis
An analysis of variance (anova) and the χ2‐test were used for between‐group comparisons of continuous and categorical variables, respectively. A univariate analysis was carried out to investigate the correlations of two independent continuous variables. The odds ratio (OR) and respective 95% confidence interval (95% CI) were determined to examine the strength of the relationship between anemia and the clinical parameters by a multiple logistic regression analysis. The associations of the prevalence of diabetic micro‐ and macroangiopathies with anemia were also evaluated by logistic regression analyses. Differences with P < 0.05 (two‐tailed) were considered to be statistically significant. The statistical software package jmp, version 8.0 (SAS Institute, Cary, NC, USA), was used to carry out all analyses.
Results
Table 1 shows the clinical characteristics and laboratory parameters of the patients included in the present study. The hemoglobin level was significantly lower in the study subjects than in the normal controls (14.5 ± 1.5 g/dL in men and 12.9 ± 1.3 g/dL in women, P < 0.01 and P = 0.04, respectively). The frequencies of anemia were significantly higher in the patients (37% in men and 27% in women) than in the normal controls (17% in men and 16% in women, P < 0.01 and P = 0.01, respectively). Individuals with grade 1 anemia were found in 15% of both men and women in the controls.
Table 1. Clinical characteristics and laboratory parameters of the patients.
| %/Mean (SD) | Number estimated (%) | |
|---|---|---|
| Age (years) | 64 (12) | 1189 (100) |
| Men | 60 | 1189 (100) |
| Duration of diabetes mellitus (years) | 10 (10) | 869 (73) |
| Current plus past cigarette smoking | 58 | 726 (61) |
| Therapeutic method | 1189 (100) | |
| Diet only/OHA/Insulin | 10/58/33 | |
| Body mass index (kg/m2) | 24.4 (4.1) | 1172 (99) |
| Hypertension | 77 | 1189 (100) |
| ACEi | 16 | 186 |
| ARB | 45 | 538 |
| Hyperlipidemia | 70 | 1189 (100) |
| HbA1c (%) | 7.0 (1.4) | 1119 (94) |
| Hemoglobin (g/dL) | 13.3 (1.6) | 1189 (100) |
| Men | 13.8 (1.6) | 709 |
| Women | 12.6 (1.4) | 480 |
| Diabetic nephropathy | 1189 (100) | |
| Stage I | 58 | 691 |
| Stage II | 19 | 226 |
| Stage III + IV | 23 | 272 |
| Estimated GFR (mL/min/1.73 m2) | 61.5 (19.4) | 1189 (100) |
| CKD stage | 1189 (100) | |
| 1 | 6 | 73 |
| 2 | 50 | 595 |
| 3 | 38 | 447 |
| 4 + 5 | 6 | 74 |
| Diabetic retinopathy | 43 | 801 (67) |
| Diabetic neuropathy | 81 | 845 (71) |
| Cerebrovascular disease | 15 | 1186 (100) |
| Coronary heart disease | 19 | 1187 (100) |
| Peripheral arterial disease | 4 | 1187 (100) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; GFR, glomerular filtration rate; OHA, oral hypoglycemic agent.
Figure 1 shows the frequency of anemia according to the stages of CKD and diabetic nephropathy graded by albuminuria. Anemia increased with the progression of renal dysfunction and diabetic nephropathy. Grade 1 anemia accounted for the majority of patients in each group except for stage CKD 4 + 5 plus DN stage III + IV.
Figure 1.
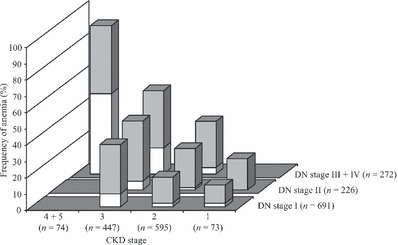
Frequency of anemia according to the stages of chronic kidney disease (CKD) and diabetic nephropathy (DN). Grey and open bars indicate grade 1 and grade 2 anemia, respectively.
Comparisons of the clinical features among the 798 (67%) patients without anemia, 300 (25%) with grade 1 anemia and 91 (8%) with grade 2 anemia are shown in Table 2. The patient’s age, the duration of diabetes mellitus, the need to receive insulin therapy, hypertension and use of angiotensin II receptor blocker (ARB) increased with the progression of anemia. The subjects with a smoking history, the body mass index, HbA1c level and eGFR decreased along with the progression of anemia.
Table 2. Clinical characteristics and laboratory parameters of the diabetic patients without and with anemia.
| %/Mean (SD) | ||||
|---|---|---|---|---|
| Anemia (−) (n = 798) | Grade 1 anemia (n = 300) | Grade 2 anemia (n = 91) | P | |
| Age (years) | 62 (12) | 68 (10) | 70 (11) | <0.01 |
| Men | 56 | 76 | 37 | <0.01 |
| Duration of diabetes mellitus (years) | 9 (9) | 14 (11) | 14 (10) | <0.01 |
| Current plus past cigarette smoking | 61 | 59 | 37 | <0.01 |
| Therapeutic method | ||||
| Diet only/OHA/ Insulin | 10/63/28 | 8/54/38 | 13/30/57 | <0.01 |
| Body mass index (kg/m2) | 24.8 (4.2) | 23.5 (3.7) | 24.0 (4.1) | <0.01 |
| Hypertension | 75 | 80 | 88 | <0.01 |
| ACEi | 16 | 15 | 16 | 0.97 |
| ARB | 40 | 54 | 58 | <0.01 |
| Hyperlipidemia | 72 | 66 | 64 | 0.01 |
| HbA1c (%) | 7.1 (1.4) | 6.8 (1.4) | 6.4 (0.8) | <0.01 |
| Hemoglobin (g/dL) | 14.1 (1.1) | 12.3 (0.7) | 9.9 (0.9) | <0.01 |
| Diabetic nephropathy | <0.01 | |||
| Stage I | 66 | 46 | 29 | |
| Stage II | 19 | 22 | 7 | |
| Stage III + IV | 15 | 32 | 64 | |
| Estimated GFR (mL/min/1.73 m2) | 66.5 (16.2) | 56.0 (19.8) | 36.1 (19.6) | <0.01 |
| CKD stage | <0.01 | |||
| 1 | 8 | 3 | 1 | |
| 2 | 59 | 38 | 12 | |
| 3 | 32 | 49 | 38 | |
| 4 + 5 | 1 | 10 | 40 | |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; GFR, glomerular filtration rate; OHA, oral hypoglycemic agent.
The frequencies of diabetic micro‐ and macroangiopathies significantly increased with the progression of anemia (Figure 2).
Figure 2.
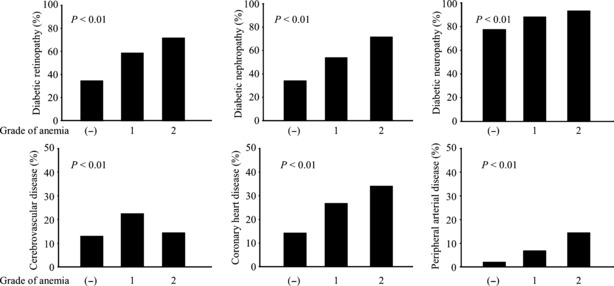
Frequencies of diabetic micro‐ and macroangiopathies among the grades of anemia in patients with type 2 diabetes mellitus. Diabetic nephropathy includes Stage II + III + IV. All diabetic complications increased with the progression of anemia (P < 0.01, χ2‐test).
Univariate analyses showed the hemoglobin level to negatively correlate with age (r = −0.358, P < 0.01) and the duration of diabetes mellitus (r = −0.217, P < 0.01), whereas it positively correlated with body mass index (r = 0.156, P < 0.01) and eGFR (r = 0.440, P < 0.01). A multiple logistic regression analysis was carried out after the patients were divided into two groups with anemia (n = 391) and without anemia (n = 798). The following factors were entered as independent variables: age, sex (men vs women), duration of diabetes mellitus, cigarette smoking (current plus past smoking vs none), therapeutic method (insulin vs diet only plus OHA), body mass index, hypertension (present vs absent), use of ARB (yes vs no), hyperlipidemia (present vs absent), eGFR and the stage of diabetic nephropathy (DN stage II + III + IV vs DN stage I). Age (Wald χ2‐score = 11.8, OR = 14.01, 95% CI = 3.18–64.67, P < 0.01), sex (Wald χ2‐score = 26.7, OR = 0.23, 95% CI = 0.13–0.40, P < 0.01), body mass index (Wald χ2‐score = 8.8, OR = 0.08, 95% CI = 0.01–0.40, P < 0.01), eGFR (Wald χ2‐score = 31.6, OR = 0.003, 95% CI = 0.000–0.023, P < 0.01) and the stage of diabetic nephropathy (Wald χ2‐score = 8.5, OR = 1.99, 95% CI = 1.25–3.18, P < 0.01) were all factors significantly associated with anemia. Age (Wald χ2‐score = 14.5, OR = 22.04, 95% CI = 4.61–112.39, P < 0.01), sex (Wald χ2‐score = 40.4, OR = 0.11, 95% CI = 0.06–0.22, P < 0.01), body mass index (Wald χ2‐score = 5.9, OR = 0.10, 95% CI = 0.01–0.60, P = 0.02), use of ARB (Wald χ2‐score = 4.0, OR = 1.73, 95% CI = 1.02–2.99, P = 0.04), eGFR (Wald χ2‐score = 12.5, OR = 0.02, 95% CI = 0.003–0.18, P < 0.01), and the stage of diabetic nephropathy (Wald χ2‐score = 9.0, OR = 2.15, 95% CI = 1.31–3.55, P < 0.01) also showed a significant association with anemia when the study subjects were divided into two groups with grade 1 anemia (n = 300) and without anemia (n = 798) after exclusion of grade 2 anemia.
Table 3 shows the odds ratios for diabetic micro‐ and macroangiopathies with anemia by logistic regression analyses. Both grade 1 and grade 2 anemia were significantly associated with all diabetic micro‐ and macroangiopathies, except the relationship between grade 2 anemia and cerebrovascular disease. Grade 1 anemia remained significantly associated with diabetic retinopathy, coronary heart disease and peripheral vascular disease after adjusting for age, sex, body mass index, the duration of diabetes mellitus, smoking, hypertension, hyperlipidemia, use of ARB, eGFR and stage of diabetic nephropathy.
Table 3. Odds ratios for diabetic micro‐ and macroangiopathies with anemia by a logistic regression analysis.
| Grade 1 anemia | Grade 2 anemia | Total (grade 1 + 2) anemia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wald χ2 score | OR (95% CI) | P | Wald χ2 score | OR (95% CI) | P | Wald χ2 score | OR (95% CI) | P | |
| Unadjusted | |||||||||
| Nephropathy | 33.5 | 2.22 (1.70–2.91) | <0.01 | 40.2 | 4.60 (2.90–7.48) | <0.01 | 59.1 | 2.65 (2.07–3.40) | <0.01 |
| Retinopathy | 28.4 | 2.70 (1.94–3.77) | <0.01 | 34.6 | 4.77 (2.73–8.67) | <0.01 | 51.9 | 3.07 (2.27–4.17) | <0.01 |
| Neuropathy | 10.1 | 2.12 (1.35–3.42) | <0.01 | 8.6 | 4.01 (1.74–11.6) | <0.01 | 16.6 | 2.43 (1.61–3.78) | 0.01 |
| CVD | 15.3 | 1.98 (1.40–2.78) | <0.01 | 0.4 | 1.22 (0.64–2.17) | 0.52 | 11.8 | 1.76 (1.27–2.42) | <0.01 |
| CHD | 22.7 | 2.20 (1.58–3.03) | <0.01 | 23.4 | 3.21 (1.98–5.12) | <0.01 | 33.4 | 2.40 (1.78–3.22) | <0.01 |
| PAD | 13.7 | 3.56 (1.82–7.07) | <0.01 | 27.6 | 8.93 (4.16–19.0) | <0.01 | 23.3 | 4.49 (2.48–8.47) | <0.01 |
| Adjusted* | |||||||||
| Retinopathy | 11.4 | 1.93 (1.31–2.82) | <0.01 | 3.8 | 1.97 (1.00–3.98) | 0.049 | 13.1 | 1.93 (1.35–2.77) | <0.01 |
| Neuropathy | 0.4 | 1.18 (0.71–2.00) | 0.53 | 2.8 | 2.56 (0.95–9.00) | 0.09 | 1.4 | 1.35 (0.83–2.23) | 0.23 |
| CVD | 0.8 | 1.20 (0.81–1.75) | 0.36 | 1.7 | 0.62 (0.29–1.23) | 0.19 | 0.2 | 1.07 (0.74–1.55) | 0.72 |
| CHD | 7.4 | 1.64 (1.15–2.34) | <0.01 | 8.1 | 2.28 (1.29–3.98) | <0.01 | 10.6 | 1.74 (1.25–2.45) | <0.01 |
| PAD | 4.1 | 2.13 (1.02–4.52) | 0.04 | 6.0 | 3.50 (1.30–9.19) | 0.01 | 5.6 | 2.33 (1.17–4.78) | <0.01 |
| Adjusted** | |||||||||
| Retinopathy | 10.1 | 2.13 (1.34–3.42) | <0.01 | 0.1 | 0.93 (0.41–2.16) | 0.86 | 8.4 | 1.92 (1.24–2.98) | <0.01 |
| Neuropathy | 0.0 | 1.02 (0.581.81) | 0.95 | 1.3 | 2.07 (0.67–9.11) | 0.26 | 0.1 | 1.10 (0.64–1.91) | 0.73 |
| CVD | 0.2 | 1.10 (0.67–1.81) | 0.70 | 1.6 | 0.57 (0.22–1.31) | 0.19 | 0.0 | 0.98 (0.60– .55) | 0.89 |
| CHD | 5.5 | 1.82 (1.09–2.71) | 0.02 | 2.2 | 1.70 (0.83–3.37) | 0.14 | 7.7 | 1.88 (1.19–2.80) | <0.01 |
| PAD | 4.1 | 2.18 (1.03–6.11) | 0.04 | 5.2 | 2.74 (1.16–8.27) | 0.03 | 4.2 | 2.44 (1.02–6.34) | 0.04 |
CHD, coronary heart disease; CI, confidence interval; CVD, cerebrovascular disease; OR, odds ratio; PAD, peripheral arterial disease.
*Adjusted by age, sex, body mass index, use of angiotensin II receptor blocker, estimated glomerular filtration rate and stage of diabetic nephropathy (DN stage I, II and III + IV).
**Adjusted by age, sex, body mass index, duration of diabetes mellitus, current plus past smoking, hypertension, hyperlipidemia, use of ARB, estimated glomerular filtration rate and stage of diabetic nephropathy (DN stage I, II, and III + IV).
Discussion
The current study showed that anemia was more frequent in patients with type 2 diabetes than in normal controls, and that mild (grade 1) anemia as well as grade 2 or total (grade 1 and grade 2) anemia were associated with the prevalence of diabetic micro‐ and macroangiopathies. Although anemia is recognized to be a risk factor for cardiovascular events9–12,14,15 and diabetic retinopathy26, no study has so far investigated whether mild anemia, which does not require supplement therapy using iron or ESA, is associated with diabetic complications.
Anemia, including mild anemia, was related to many clinical parameters in the patients with type 2 diabetes mellitus of the present study. In particular, anemia increased with the progression of the stage of diabetic nephropathy and CKD consistent with previous studies1–15,17. Diabetic micro‐ and macroangiopathies increased with the progression of anemia. The presence of anemia remained a significant variable associated with diabetic retinopathy, coronary heart disease and peripheral arterial disease after adjustment for age, sex, body mass index, the duration of diabetes mellitus, smoking, hypertension, hyperlipidemia, the use of ARB, eGFR and stage of diabetic nephropathy. Recently, Zoppini et al.27 reported that anemia predicts the all‐cause and cardiovascular mortality in type 2 diabetic patients, independently of the presence of CKD, similar to the present study.
Although anemia is more frequent in women than in men in the general population, the incidence of mild anemia was higher in men with type 2 diabetes mellitus in the current series. This is interesting, because the cardiovascular diseases frequently occur in diabetic males, although the reason for the increase in male anemia was unclear. Because the patients with macroalbuminuria were significantly more frequently men than women in the present study (data are not shown), this phenomenon might therefore be dependent on the patients’ background alone. Mild anemia might therefore be an additional marker that expresses micro‐ and macrovascular damage dependent on multiple factors, such as aging, hypertension, hyperglycemia and kidney dysfunction.
The main limitation of the current study was that the results were obtained from a single centre and with a cross‐sectional design. These findings are inherently limited by an inability to eliminate causal relationships between mild anemia and diabetic micro‐ and macroangiopathies. Therefore, it is impossible to clarify whether mild anemia causes diabetic vascular complications. However, the present study was considered to be sufficient to show that a high incidence of mild anemia in patients with type 2 diabetes mellitus was associated with diabetic micro‐ and macroangiopathies in a large number of subjects. The prospective study is necessary to investigate the incidence of diabetic complications among the groups divided by the grade of anemia. The other limitation was that anemia was evaluated using only the hemoglobin level. The etiology could not be discussed in the current study, because the reticulocyte count, serum iron, ferritin, transferrin, erythropoietin and C‐reactive protein concentrations were not measured. Furthermore, subjects with occult malignant disease or chronic gastrointestinal bleeding might not be completely excluded from the study. Almoznino‐Sarafian et al.15 reported anemia in diabetic patients to be caused by multiple factors, such iron deficiency (38%), and vitamin B12 and/or folate (12%) deficiency.
The results of the present study suggest that the current clinical strategy for mild anemia might be insufficient to prevent diabetic complications, especially macroangiopathies. However, it is doubtful whether mild anemia should be corrected using ESA in patients with type 2 diabetes mellitus from the viewpoint of the medical cost and the possibility of causing increased hypertension. Correcting the target hemoglobin to a level of 13.0–15.0 g/dL using epoetin beta in patients with diabetes and anemia does not improve the left ventricular mass index according to echocardiographic measurements28. Furthermore, an earlier complete correction of anemia has been reported to show no reduction in the risk for cardiovascular events29, whereas it increased hospitalization as a result of cardiovascular causes in the patients with CKD30. The administration of darbepoetin alpha that aimed to elevate the hemoglobin level to 13.0 g/dL increased the onset of stroke; whereas it did not reduce the risk of end‐stage renal disease and death in 4038 patients with diabetes mellitus, CKD and anemia31. Therefore, it is important to carry out intensive examinations for the detection of diabetic micro‐ and macroangiopathies, in addition to the evaluation of treating some of the easily correctable causes of anemia, such as nutritional deficiency, medications, occult bleeding, inflammatory diseases, hemolysis, thyroid and other disorders when mild anemia is found in patients with type 2 diabetes mellitus.
Acknowledgements
The authors thank Ms Sakura Yamamoto and Ms Tomoko Koyanagi from the secretarial section of Edogawa Hospital for their valuable help in the data collection. There is no conflict of interest in all authors listed.
References
- 1.Thomas MC, MacIsaac RJ, Tsalamandris C, et al. Unrecognized anemia in patients with diabetes: a cross‐sectional survey. Diabetes Care 2003; 26: 1164–1169 [DOI] [PubMed] [Google Scholar]
- 2.Thomas MC, MacIsaac RJ, Tsalamandris C, et al. Anemia in patients with type 1 diabetes. J Clin Endocrinol Metab 2004; 89: 4359–4363 [DOI] [PubMed] [Google Scholar]
- 3.Al‐Khoury S, Afzali B, Shah N, et al. Anaemia in diabetic patients with chronic kidney disease‐‐prevalence and predictors. Diabetologia 2006; 49: 1183–1189 [DOI] [PubMed] [Google Scholar]
- 4.Wolf G, Müller N, Hunger‐Battefeld W, et al. Hemoglobin concentrations are closely linked to renal function in patients with type 1 or 2 diabetes mellitus. Kidney Blood Press Res 2008; 31: 313–321 [DOI] [PubMed] [Google Scholar]
- 5.New JP, Aung T, Baker PG, et al. The high prevalence of unrecognized anaemia in patients with diabetes and chronic kidney disease: a population‐based study. Diabet Med 2008; 25: 564–569 [DOI] [PubMed] [Google Scholar]
- 6.Ezenwaka CE, Jones‐Lecointe A, Nwagbara E, et al. Anaemia and kidney dysfunction in Caribbean type 2 diabetic patients. Cardiovasc Diabetol 2008; 7: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adetunji OR, Mani H, Olujohungbe A, et al. ‘Microalbuminuric anaemia’– The relationship between haemoglobin levels and albuminuria in diabetes. Diabetes Res Clin Pract 2009; 85: 179–182 [DOI] [PubMed] [Google Scholar]
- 8.Goldhaber A, Ness‐Abramof R, Ellis MH. The prevalence of anemia among unselected adults with diabetes mellitus and normal creatinine levels. Endocr Pract 2009; 20: 1–20 [DOI] [PubMed] [Google Scholar]
- 9.Thomas MC, MacIsaac RJ, Tsalamandris C, et al. The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross‐sectional audit. Nephrol Dial Transplant 2004; 19: 1792–1797 [DOI] [PubMed] [Google Scholar]
- 10.Thomas MC, Tsalamandris C, MacIsaac RJ, et al. The epidemiology of hemoglobin levels in patients with type 2 diabetes. Am J Kidney Dis 2006; 48: 537–545 [DOI] [PubMed] [Google Scholar]
- 11.Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all‐cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol 2005; 16: 3403–3410 [DOI] [PubMed] [Google Scholar]
- 12.Tong PC, Kong AP, So WY, et al. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in Chinese patients with type 2 diabetes. Diabetes Care 2006; 29: 2439–2444 [DOI] [PubMed] [Google Scholar]
- 13.Joss N, Patel R, Paterson K, et al. Anaemia is common and predicts mortality in diabetic nephropathy. QJM 2007; 100: 641–647 [DOI] [PubMed] [Google Scholar]
- 14.McGill JB, Bell DS. Anemia and the role of erythropoietin in diabetes. J Diabetes Complications 2006; 20: 262–272 [DOI] [PubMed] [Google Scholar]
- 15.Almoznino‐Sarafian D, Shteinshnaider M, Tzur I, et al. Anemia in diabetic patients at an internal medicine ward: clinical correlates and prognostic significance. Eur J Intern Med 2010; 21: 91–96 [DOI] [PubMed] [Google Scholar]
- 16.Shu DH, Ransom TP, O’Connell CM, et al. Anemia is an independent risk for mortality after acute myocardial infarction in patients with and without diabetes. Cardiovasc Diabetol 2006; 5: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care 2009; 32: 1320–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.KDOQI . KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50: 471–530 [DOI] [PubMed] [Google Scholar]
- 19.Locatelli F, Covic A, Eckardt KU, et al. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant 2009; 24: 348–354 [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Clinical Excellence . Anaemia management in people with chronic kidney disease. NICE clinical guideline 39, 2006.
- 21.National Kidney Foundation . KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 2006; 47: S1–S146 [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population. Am J Kidney Dis 2003; 41: 1–12 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089–2100 [DOI] [PubMed] [Google Scholar]
- 25.Japan Diabetes Society . Treatment Guide for Diabetes 2007. Bunkodo, Tokyo, 2007. [Google Scholar]
- 26.Qiao Q, Keinänen‐Kiukaanniemi S, Läärä E. The relationship between hemoglobin levels and diabetic retinopathy. J Clin Epidemiol 1997; 50: 153–158 [DOI] [PubMed] [Google Scholar]
- 27.Zoppini G, Targher G, Chonchol M, et al. Anaemia, independent of chronic kidney disease, predicts all‐cause and cardiovascular mortality in type 2 diabetic patients. Atherosclerosis 2010; 210: 575–580 [DOI] [PubMed] [Google Scholar]
- 28.Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49: 194–207 [DOI] [PubMed] [Google Scholar]
- 29.Drüeke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 30.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]


