Abstract
Fulminant type 1 diabetes is characterized by almost complete β‐cell destruction, resulting in scarce insulin secretion. In the present study, we aimed to clarify clinical features related to serum C‐peptide levels measured by a high sensitivity method, chemiluminescent enzyme immunoassay, in 12 patients with fulminant type 1 diabetes. Serum C‐peptide was detected (0.007–0.10 nmol/L) in four patients and was not detected in eight patients. A negative correlation was observed between serum C‐peptide levels and daily dosages of insulin (P < 0.01). The patients with detectable C‐peptide showed a significantly lower M‐value than those without (P = 0.01). In conclusion, our present results suggest that even very low levels of endogenous insulin secreting capacity can improve daily dosages of insulin and stabilize blood glucose levels. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.0059.x, 2010)
Keywords: Fulminant type 1 diabetes, Serum C‐peptide, M‐value
Introduction
Type 1 diabetes is characterized by insulin deficiency resulting from destruction of pancreatic β‐cells and subclassified into type 1A (autoimmune) and type 1B (idiopathic) diabetes1. We previously reported a novel subtype of type 1B diabetes that we referred to fulminant type 1 diabetes2. In fulminant type 1 diabetes, remarkably acute and almost complete β‐cell destruction occurs and nearly no insulin secretion remains, even just after the disease onset3.
Measurement of serum C‐peptide level is effective for assessing the ability of endogenous insulin secretions. In particular, it is valuable to presume residual β‐cell capability for type 1 diabetic patients. With a conventional method, enzyme immunoassays (EIA), low levels (usually <0.07 nmol/L [0.2 ng/mL]) of serum C‐peptide are difficult to detect. However, we can now detect up to 0.003 nmol/L (0.01 ng/mL) serum C‐peptide levels using a high sensitivity method, chemiluminescent enzyme immunoassay (CLEIA). It enables us to know more precise levels of serum C‐peptide in patients who have almost no insulin secretion, such as patients with fulminant type 1 diabetes4.
In the present study, we evaluated serum C‐peptide levels measured by CLEIA and clarified the clinical features of fulminant type 1 diabetic patients based on residual endogenous insulin secretions.
Materials and Methods
Patients and Samples
We studied 12 patients with fulminant type 1 diabetes (5 males and 7 females). These patients were diagnosed as having fulminant type 1 diabetes between 1988–2006, and had been followed in our hospital for more than 0.5 years since the disease onset. The diagnosis of fulminant type 1 diabetes was established according to the inclusion criteria proposed by the committee of the Japan Diabetes Society5. Namely: (i) the presence of ketosis or ketoacidosis within a week after the onset of hyperglycemic symptoms; (ii) urinary C‐peptide excretion <10 μg/day or fasting serum C‐peptide level <0.10 nmol/L (0.3 ng/mL) and peak serum C‐peptide level <0.17 nmol/L (0.5 ng/mL) after glucagon (1 mg) or a meal load soon after disease onset; and (iii) plasma glucose level ≥16.0 mmol/L (288 mg/dL) and HbA1c level <8.5% at first visit.
Patients’ characteristics were as follows: they were aged 22–78 years (44.2 ± 18.4), duration of the disease was 0.8–19 years (4.8 ± 5.2), body mass index (BMI) was 18.5–24.8 (20.8 ± 1.9), HbA1c was 5.7–10.4% (7.2 ± 1.5), and glycoalbumin was 19.0–32.5% (25.1 ± 4.1). GAD65 antibody was negative in all patients. Eight of the 12 patients were receiving multiple daily injections and three patients were receiving continuous subcutaneous insulin infusion. One patient was treated with biphasic insulin analog crystallized with protamine twice a day. Every patient was usually followed every month in our outpatient clinic and insulin doses were adjusted for targeting nearly normal glucose levels (HbA1c < 6.5%, fasting glucose level <5.6 mmol/L [100 mg/dL], 120‐min postprandial glucose level <7.8 mmol/L [140 mg/dL]).
The data of HbA1c, glycoalbumin, bodyweight and daily dosages of insulin were measured every 9 months (from January to September in 2006) and expressed as mean of 9 months’ measurements. Seven‐point (fasting and 120 min postprandial for each meal and bedtime) capillary blood glucose concentrations were measured in seven of the 12 patients for at least two days, and mean M‐value was calculated in each patient. M‐value is a logarithmic transformation of the deviation of blood glucose from a selected standard. We used 5.6 mmol/L (100 mg/dL) as the selected standard. The deviation index (δ) of each individual glucose value (γ) is first calculated as δ = (10 × log[γ/5.0])3. M‐value is the average of all the individually calculated deviation indices (∑δ/n)6.
Serum C‐peptide levels were measured by CLEIA (LUMIPULSE f, lumipulse C‐peptide; Fuji‐revio, Tokyo, Japan) and two different EIA, AIA‐21 (E test C‐peptide; TOSOH, Tokyo, Japan) and ECLusys 2010 (eclusis C‐peptide; Roche Diagnostics, Basel, Switzerland) simultaneously by using the same serum samples. Serum C‐peptide level was also followed for 9 months by CLEIA, and the maximal value was determined in each patient.
The present study was carried out with patients’ approvals and informed consent was obtained from every patient.
Statistical Analysis
The significance of differences between the two groups was evaluated by Pearson’s correlation coefficient and M‐value was evaluated by Mann–Whitney U‐test. Data are presented as mean ± SD. P < 0.05 was considered statistically significant.
Results
Maximal concentrations of serum C‐peptide evaluated by CLEIA were 0.02–0.10 nmol/L (0.06–0.29 ng/mL) in four patients and <0.003 nmol/L (0.01 ng/mL) in eight patients.
Serum C‐peptide concentrations of the former four patients ranged from 0.007 to 0.10 nmol/L (0.02–0.29 ng/mL) and those of the latter eight patients were always less than 0.003 nmol/L throughout the study period (Figure 1). In contrast, serum C‐peptide was detectable in just two patients (0.09–0.10 nmol/L, respectively) by conventional EIA.
Figure 1.
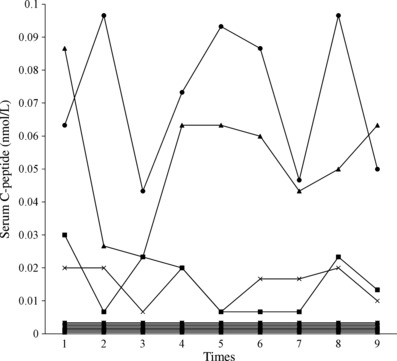
Transition of serum C‐peptide levels during the study period. Detectable serum C‐peptide levels in 4 patients (•, ▲, ■, ×) ranged from 0.007 to 0.10 nmol/L, however serum C‐peptide levels in eight patients were always <0.003 nmol/L.
Patients who kept more serum C‐peptide levels needed significantly fewer daily dosages of insulin (P < 0.01; Figure 2). The patients with detectable serum C‐peptide levels showed a significantly lower M‐value (median 2.94, range 0.96–6.15, n = 4) than those without detectable serum C‐peptide levels (median 16.62, range 5.36–27.10, n = 3; P = 0.01).
Figure 2.
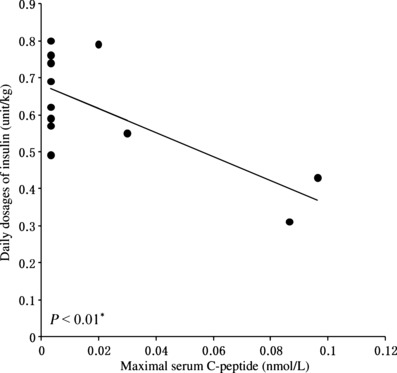
Relationship between maximal serum C‐peptide level(nmol/L) and daily dosages of insulin (unit/kg). Residual insulin secreting capacity shown as serum C‐peptide level and daily dosages of insulin indicated a significant negative correlation (*P < 0.01).
There were no significant correlations between HbA1c, glycoalbumin, BMI, duration of disease and serum C‐peptide levels.
Discussion
In the present study, we clarified a significant negative correlation between serum C‐peptide levels and daily dosages of insulin. The patients who had more residual endogenous insulin secreting capacity needed fewer daily dosages of insulin injection. In addition, the patients whose serum C‐peptide levels were <0.003 nmol/L had a significantly higher M‐value than the patients whose serum C‐peptide levels were 0.007–0.10 nmol/L. These results suggest that even very low endogenous insulin secreting capacity would improve the doses of required insulin and stability of blood glucose controls in patients with fulmi‐nant type 1 diabetes. Fukuda et al.7 have reported a negative correlation between endogenous insulin secreting capacity and degree of blood glucose instability in 20 patients with type 1 diabetes whose fasting serum C‐peptide levels were 0.01–0.13 nmol/L. In the present study, compared with the report from Fukuda et al., not fasting but maximal postprandial C‐peptide levels were 0.02–0.10 nmol/L or less, showing that a negative correlation was observed even in the patients with lower levels of serum C‐peptide.
Second, serum C‐peptides were detectable and ranged from 0.007 to 0.10 nmol/L in four patients with fulminant type 1 diabetes throughout the follow‐up period of 9 months. Pathophysiology of fulminant type 1 diabetes is known as almost complete β‐cell destruction around the disease onset, resulting in nearly no C‐peptide secretion. However, our results also suggest that endogenous insulin secreting capacity would be preserved by intensive insulin therapy, as shown in data from the Diabetes Control and Complications Trial8, even in patients with fulminant type 1 diabetes. It is important because preserving β‐cell function, even at a low level, could help to stabilize blood glucose levels in patients with fulminant type 1 diabetes.
In conclusion, our present results suggest that even very low levels of serum C‐peptide could reduce daily dosages of insulin and stabilize blood glucose controls in fulminant type 1 diabetes.
Acknowledgements
We thank Michiyo Horie, Kazuhito Ueda (Central Laboratory, Osaka Medical College Hospital), and Hiroaki Matsumoto and Tetsuya Hiraiwa (First Department of Internal Medicine, Osaka Medical College) for their excellent assistance. This study was supported in part by a Grant‐in Aid from the Japanese Society for the Promotion of Science (KAKENHI 19790641, 19591087, 19591069, 21591184) and Labour Sciences Grants on Research on Intractable Diseases by the Ministry of Health, Labour and Welfare. The authors declare that there is no conflict of interest associated with this manuscript.
References
- 1.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31: S55–S60 [DOI] [PubMed] [Google Scholar]
- 2.Imagawa A, Hanafusa T, Miyagawa J, et al. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes‐related antibodies. N Engl J Med 2000; 342: 301–307 [DOI] [PubMed] [Google Scholar]
- 3.Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 2003; 26: 2345–2352 [DOI] [PubMed] [Google Scholar]
- 4.Horie M, Ueda K, Shibasaki S, et al. The assessment of low‐dose serum C‐peptide secretion clarified by chemiluminescent enzyme immunoassay and its clinical effectiveness. J Japan Diab Soc 2008; 51: 639–644 (in Japanese). [Google Scholar]
- 5.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab 2007; 3: 36–45 [DOI] [PubMed] [Google Scholar]
- 6.Murase Y, Imagawa A, Hanafusa T, et al. Fulminant type 1 diabetes as a high risk group for diabetic microangiopathy – a nationwide 5‐year‐study in Japan. Diabetologia 2007; 50: 531–537 [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Tanaka A, Tahara Y, et al. Correlation between minimal secretory capacity of pancreatic beta‐cells and stability of diabetic control. Diabetes 1988; 37: 81–88 [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group . Effect of intensive therapy on residual beta‐cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998; 128: 517–523 [DOI] [PubMed] [Google Scholar]


