Abstract
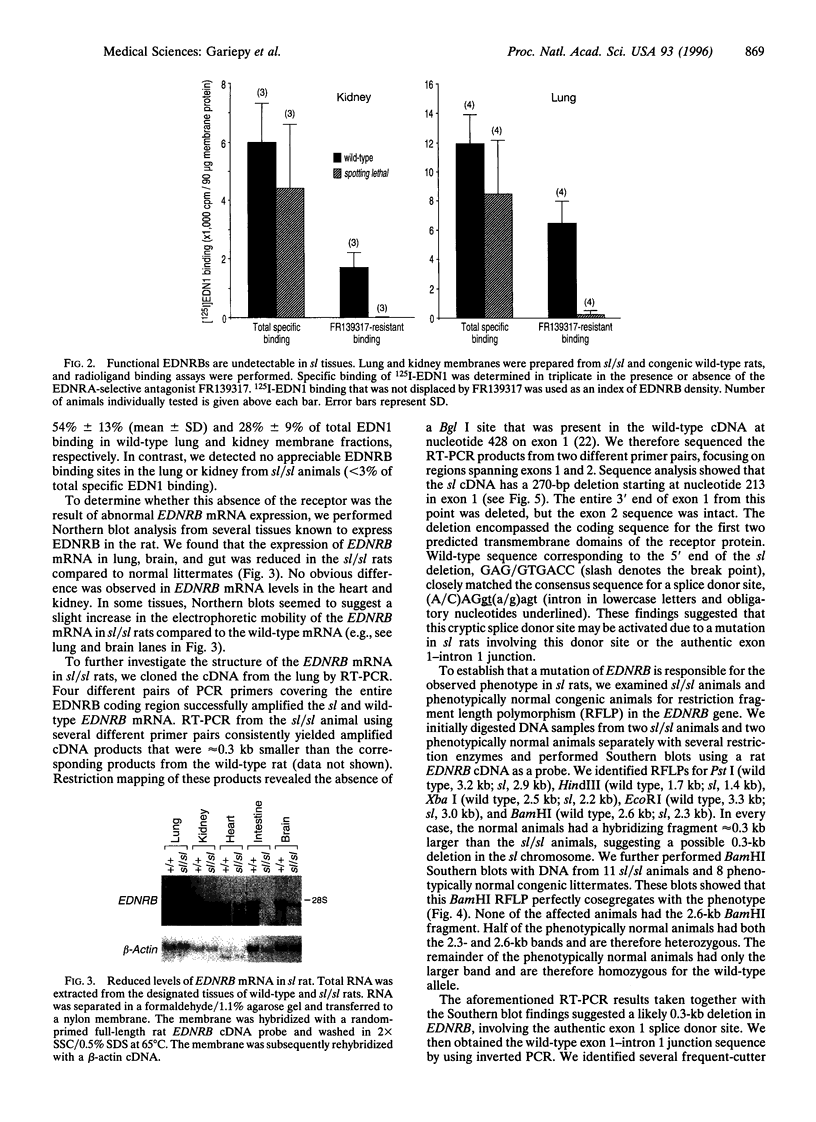
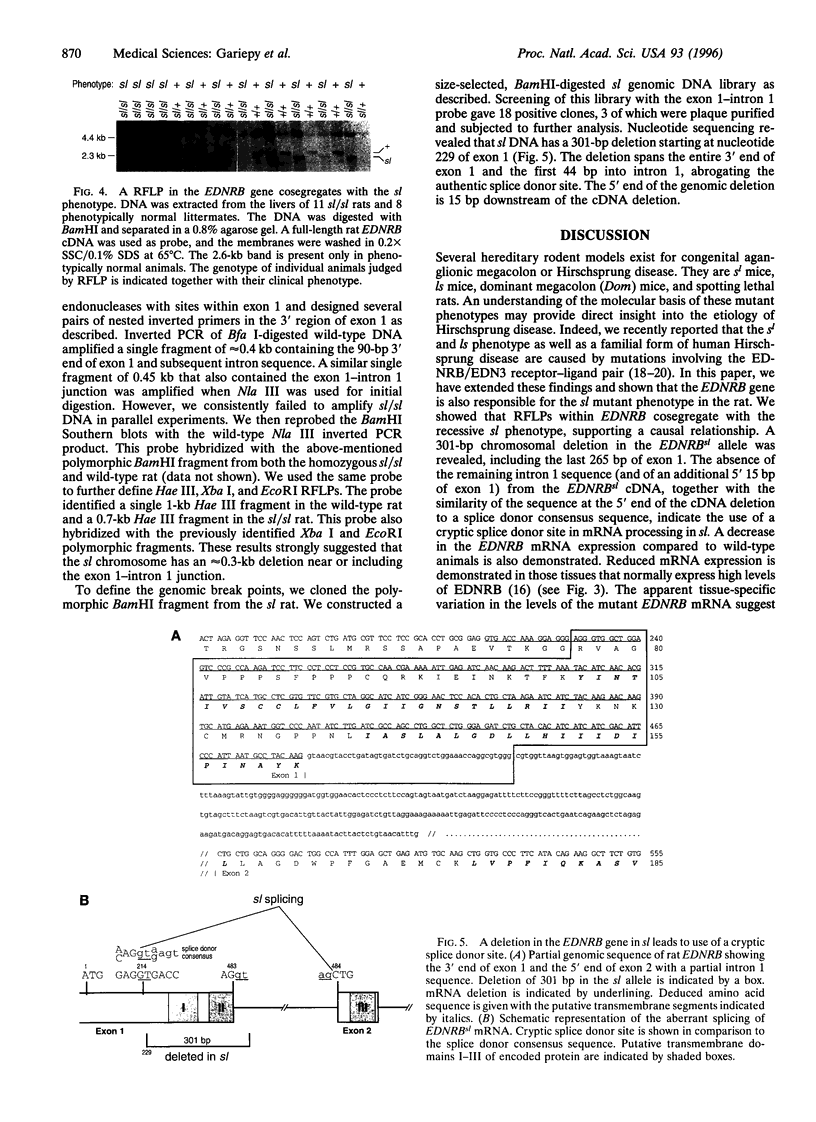
Mutations in the gene encoding the endothelin receptor type B (EDNRB) produce congenital aganglionic megacolon and pigment abnormalities in mice and humans. Here we report a naturally occurring null mutation of the EDNRB gene in spotting lethal (sl) rats, which exhibit aganglionic megacolon associated with white coat color. We found a 301-bp deletion spanning the exon 1-intron 1 junction of the EDNRB gene in sl rats. A restriction fragment length polymorphism caused by this deletion perfectly cosegregates with the sl phenotype. The deletion leads to production of an aberrantly spliced EDNRB mRNA that lacks the coding sequence for the first and second putative transmembrane domains of the G-protein-coupled receptor. Radioligand binding assays revealed undetectable levels of functional EDNRB in tissues from homozygous sl/sl rats. We conclude that EDNRB plays an essential role in the normal development of two neural crest-derived cell lineages, epidermal melanocytes and enteric neurons, in three mammalian species--humans, mice, and rats. The EDNRB-deficient rat may also prove valuable in defining the postnatal physiologic role of this receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J. Stem cells and transcription factors in the development of the mammalian neural crest. FASEB J. 1994 Jul;8(10):707–713. doi: 10.1096/fasebj.8.10.8050669. [DOI] [PubMed] [Google Scholar]
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Baynash A. G., Hosoda K., Giaid A., Richardson J. A., Emoto N., Hammer R. E., Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994 Dec 30;79(7):1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Neural crest cell formation and migration in the developing embryo. FASEB J. 1994 Jul;8(10):699–706. doi: 10.1096/fasebj.8.10.8050668. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A., Duong T. D., Tosney K. W. Descriptive and experimental analysis of the dispersion of neural crest cells along the dorsolateral path and their entry into ectoderm in the chick embryo. Dev Biol. 1992 May;151(1):251–272. doi: 10.1016/0012-1606(92)90231-5. [DOI] [PubMed] [Google Scholar]
- Gershon M. D., Chalazonitis A., Rothman T. P. From neural crest to bowel: development of the enteric nervous system. J Neurobiol. 1993 Feb;24(2):199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- Hori S., Komatsu Y., Shigemoto R., Mizuno N., Nakanishi S. Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology. 1992 Apr;130(4):1885–1895. doi: 10.1210/endo.130.4.1312429. [DOI] [PubMed] [Google Scholar]
- Hosoda K., Hammer R. E., Richardson J. A., Baynash A. G., Cheung J. C., Giaid A., Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994 Dec 30;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Inagaki H., Bishop A. E., Escrig C., Wharton J., Allen-Mersh T. G., Polak J. M. Localization of endothelinlike immunoreactivity and endothelin binding sites in human colon. Gastroenterology. 1991 Jul;101(1):47–54. doi: 10.1016/0016-5085(91)90458-w. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J. Molecular and developmental genetics of mouse coat color. Annu Rev Genet. 1994;28:189–217. doi: 10.1146/annurev.ge.28.120194.001201. [DOI] [PubMed] [Google Scholar]
- Jacobs-Cohen R. J., Payette R. F., Gershon M. D., Rothman T. P. Inability of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice: requirement for a permissive microenvironment. J Comp Neurol. 1987 Jan 15;255(3):425–438. doi: 10.1002/cne.902550309. [DOI] [PubMed] [Google Scholar]
- Kapur R. P., Sweetser D. A., Doggett B., Siebert J. R., Palmiter R. D. Intercellular signals downstream of endothelin receptor-B mediate colonization of the large intestine by enteric neuroblasts. Development. 1995 Nov;121(11):3787–3795. doi: 10.1242/dev.121.11.3787. [DOI] [PubMed] [Google Scholar]
- Kapur R. P., Yost C., Palmiter R. D. Aggregation chimeras demonstrate that the primary defect responsible for aganglionic megacolon in lethal spotted mice is not neuroblast autonomous. Development. 1993 Mar;117(3):993–999. doi: 10.1242/dev.117.3.993. [DOI] [PubMed] [Google Scholar]
- Kurihara Y., Kurihara H., Suzuki H., Kodama T., Maemura K., Nagai R., Oda H., Kuwaki T., Cao W. H., Kamada N. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994 Apr 21;368(6473):703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Lane P. W. Association of megacolon with two recessive spotting genes in the mouse. J Hered. 1966 Jan-Feb;57(1):29–31. doi: 10.1093/oxfordjournals.jhered.a107457. [DOI] [PubMed] [Google Scholar]
- Lane P. W., Liu H. M. Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J Hered. 1984 Nov-Dec;75(6):435–439. doi: 10.1093/oxfordjournals.jhered.a109980. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Suzuki N., Onda H., Fujino M. Abundance of endothelin-3 in rat intestine, pituitary gland and brain. Biochem Biophys Res Commun. 1989 Oct 16;164(1):74–80. doi: 10.1016/0006-291x(89)91684-7. [DOI] [PubMed] [Google Scholar]
- McCabe L., Griffin L. D., Kinzer A., Chandler M., Beckwith J. B., McCabe E. R. Overo lethal white foal syndrome: equine model of aganglionic megacolon (Hirschsprung disease). Am J Med Genet. 1990 Jul;36(3):336–340. doi: 10.1002/ajmg.1320360319. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Mankoo B., Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993 Dec;119(4):1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Pavan W. J., Tilghman S. M. Piebald lethal (sl) acts early to disrupt the development of neural crest-derived melanocytes. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7159–7163. doi: 10.1073/pnas.91.15.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz H. D., Rothman T. P., Chalazonitis A., Tennyson V. M., Gershon M. D. Neural crest-derived cells isolated from the gut by immunoselection develop neuronal and glial phenotypes when cultured on laminin. Dev Biol. 1993 Apr;156(2):341–361. doi: 10.1006/dbio.1993.1082. [DOI] [PubMed] [Google Scholar]
- Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994 Dec 30;79(7):1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Rothman T. P., Goldowitz D., Gershon M. D. Inhibition of migration of neural crest-derived cells by the abnormal mesenchyme of the presumptive aganglionic bowel of ls/ls mice: analysis with aggregation and interspecies chimeras. Dev Biol. 1993 Oct;159(2):559–573. doi: 10.1006/dbio.1993.1264. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Polokoff M. A. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994 Sep;46(3):325–415. [PubMed] [Google Scholar]
- Ruíz-Ortega M., Gómez-Garre D., Alcázar R., Palacios I., Bustos C., González S., Plaza J. J., González E., Egido J. Involvement of angiotensin II and endothelin in matrix protein production and renal sclerosis. J Hypertens Suppl. 1994 Jul;12(4):S51–S58. [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994 Jan 27;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Shah K. N., Dalal S. J., Desai M. P., Sheth P. N., Joshi N. C., Ambani L. M. White forelock, pigmentary disorder of irides, and long segment Hirschsprung disease: possible variant of Waardenburg syndrome. J Pediatr. 1981 Sep;99(3):432–435. doi: 10.1016/s0022-3476(81)80339-3. [DOI] [PubMed] [Google Scholar]
- Sogabe K., Nirei H., Shoubo M., Nomoto A., Ao S., Notsu Y., Ono T. Pharmacological profile of FR139317, a novel, potent endothelin ETA receptor antagonist. J Pharmacol Exp Ther. 1993 Mar;264(3):1040–1046. [PubMed] [Google Scholar]
- Suzuki N., Matsumoto H., Miyauchi T., Goto K., Masaki T., Tsuda M., Fujino M. Endothelin-3 concentrations in human plasma: the increased concentrations in patients undergoing haemodialysis. Biochem Biophys Res Commun. 1990 Jun 15;169(2):809–815. doi: 10.1016/0006-291x(90)90403-a. [DOI] [PubMed] [Google Scholar]
- Takagi S., Kimura M., Katsuki M. A rapid and efficient protocol of the inverted PCR using two primer pairs. Biotechniques. 1992 Aug;13(2):176–178. [PubMed] [Google Scholar]
- Yada Y., Higuchi K., Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991 Sep 25;266(27):18352–18357. [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M. The endothelin system. A new target for therapeutic intervention. Circulation. 1994 Mar;89(3):1320–1322. doi: 10.1161/01.cir.89.3.1320. [DOI] [PubMed] [Google Scholar]
- Yohn J. J., Smith C., Stevens T., Hoffman T. A., Morelli J. G., Hurt D. L., Yanagisawa M., Kane M. A., Zamora M. R. Human melanoma cells express functional endothelin-1 receptors. Biochem Biophys Res Commun. 1994 May 30;201(1):449–457. doi: 10.1006/bbrc.1994.1722. [DOI] [PubMed] [Google Scholar]







