Abstract
Aims/Introduction: The effects of 5‐day voluntary exercise on muscle damage and muscle protein degradation were investigated in a streptozotocin‐induced rat model of moderately glycemic, uncontrolled, type 2 diabetes.
Materials and Methods: In the preliminary experiment, an oral glucose tolerance (1.0 g/kg) test was carried out to confirm the development of diabetes 3 days after streptozotocin treatment (30 mg/kg). In the genuine experiment, rats were divided into four groups: (i) non‐diabetic rats without exercise (controls); (ii) non‐diabetic rats with exercise; (iii) diabetic rats without exercise; and (iv) diabetic rats with exercise. After 5 days of voluntary wheel running exercise, blood and 24‐h urine were collected, and levels of serum creatine kinase, a marker of muscle damage, and 24‐h urinary excretion of muscle degradation products were determined.
Results: Type 2 diabetic rats with insulin deficiency that exercised had higher serum creatine kinase and greater urinary excretions of creatinine, urea nitrogen and 3‐methylhistidine compared with both type 2 diabetic rats with insulin deficiency and non‐diabetic rats that did not exercise. However, there were no differences in serum creatine kinase and urinary excretions of creatinine, urea nitrogen and 3‐methylhistidine between non‐diabetic rats that did and did not exercise.
Conclusions: These findings suggest that muscle damage is induced and muscle protein degradation are enhanced by chronic moderate exercise in moderately glycemic uncontrolled type 2 diabetic rats with insulin deficiency at an intensity level of exercise that does not affect muscle damage and muscle protein degradation in non‐diabetic rats. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00130.x, 2011)
Keywords: Type 2 diabetes mellitus, Muscle damage, Exercise
Introduction
The International Diabetes Federation (IDF)1 predicts that the prevalence of people with diabetes mellitus will increase from 5.1% (194 million people worldwide) in 2003 to 6.3% (333 million people worldwide) by 2025. In this context, the United Nations passed a landmark resolution in December 2006 on diabetes mellitus, recognizing the disease as a chronic, debilitating and costly social burden2. Multidimensional efforts to fight diabetes mellitus are taking place worldwide. In clinical practice for type 2 diabetes, exercise is one of three major treatments, along with diet and pharmacological treatment3–6. Skeletal muscle mass plays an important role in glycemic control in type 2 diabetes, especially through exercise. Acute (non‐aerobic) exercise improves the glycemic level by increasing skeletal muscle glucose uptake through muscle contraction, whereas chronic resistance and/or aerobic exercise improves glycemic control by enhancing insulin sensitivity7,8. Regular physical activity also increases cardiorespiratory fitness, maintains muscle mass, reduces the risk of cardiovascular mortality and enhances psychosocial well‐being9.
Skeletal muscle is also important for maintaining normal body composition, because it comprises nearly 40% of bodyweight, constitutes between 50 and 75% of all body proteins10 and is required for movement11. Protein turnover in skeletal muscle normally occurs at a rate of 1–2% of protein synthesis and protein degradation daily12, and skeletal muscle mass is maintained through a regulated balance between muscle protein synthesis (MPS) and muscle protein degradation (MPD)11,12. Regular resistance exercise results in increased MPS in the post‐exercise recovery period13. However, chronic intense unaccustomed exercise or endurance exercise might induce muscle damage by enhancing MPD through excessive muscle contraction, absolute or relative insulin deficiency, and inflammation14–16. Type 2 diabetes is characterized by a deficiency in insulin level or action and by inflammation16–18. Therefore, patients with type 2 diabetes might be susceptible to exercise‐induced muscle damage by enhanced MPD.
The treadmill or wheel exercise is commonly applied to test exercise tolerance in small animals19–22. Wheel exercise involves voluntary running, but there is some difficulty in recording the details of the running behavior (intensity, duration or distance); however, several studies have used voluntary wheel running in rats20,21. Rodnick et al.20 characterized intermittent voluntary wheel running run by rats at fairly high speeds (40–50 m/min) as “sprint training”. Seburn and Gardiner21 estimated that the distance covered in wheel exercise was two to three times longer than that in treadmill exercise. Therefore, we applied voluntary wheel running in the current study as a moderate intensity exercise that might be sufficient enough to discriminate the effects of exercise in diabetic rats from that in non‐diabetic rats.
The importance of muscle mass in exercise for glycemic control in type 2 diabetes has been widely reported3–6. However, to the best of our knowledge, few studies have examined muscle protein damage and MPD by exercise in animals and humans with type 2 diabetes. In the current study, we investigated exercise‐induced muscle damage and MPD in streptozotocin (STZ)‐induced moderately glycemic uncontrolled type 2 diabetic rats placed in wheel‐equipped cages by measuring serum creatine kinase (CK), a marker of muscle damage23, and urinary excretions of creatinine (Cr), urea nitrogen (UN) and 3‐methylhistidine (3‐MH), which are muscle protein degradation products24–26.
Materials and Methods
Animals
Ten‐week‐old male Wistar rats were housed at 25°C and 50% humidity with a 12‐h light/dark cycle (06.00 hours light on, 18.00 hours light off). They were allowed free access to food (carbohydrate 81.3%, protein 12.7%, fat 6.0%; Oriental East, Tokyo, Japan) and water. In the preliminary experiment, rats were given STZ (30 mg/kg; Sigma, St. Louis, MO, USA) or saline through the tail vein under ethanol anesthesia. On the third day after STZ injection, saline‐treated normal (non‐diabetic) and STZ‐treated rats received an oral glucose tolerance test (OGTT; 1.0 g/kg) without anesthesia after an overnight fast. Diabetes was judged to be present when the blood glucose concentration at 120 min was >200 mg/dL27. Rats found to be diabetic were then used as diabetic rats in the study. All animals were treated in accordance with the Kiryu University Guidelines for the Care and Use of Laboratory Animals, which are based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Procedures
Normal (non‐diabetic) and diabetic rats were divided into four groups: (i) non‐diabetic rats without exercise (designated as controls); (ii) non‐diabetic rats with exercise; (iii) diabetic rats without exercise; and (iv) diabetic rats with exercise (n = 5 in each group). Exercise was carried out throughout for five consecutive days by placing rats in wheel‐equipped cages. On the sixth day, rats were transferred into individual metabolic cages and 24‐h urine samples were collected. Blood samples were taken in fed conditions from the abdominal aorta under pentobarbital anesthesia at the end of the experiment.
Wheel Running Exercise
The rats in the exercise groups were placed in wheel‐equipped cages for five consecutive days. Each cage had an in‐built stainless steel wheel (21 × 46 cm, circumference 1.445 m) mounted on a shaft containing a ball‐bearing collar with a 4‐mm wide running surface of stainless steel mesh20. Rats intermittently played with the wheel voluntarily, arbitrarily and willingly during placement in wheel‐equipped cages.
Measurements
Blood glucose was measured using the oxidase method and serum insulin was measured by ELISA (Rat Insulin ELISA kit; Cosmic Corporation, Tokyo, Japan). Serum CK was measured using a Hitachi 7450 automatic analyzer (Hitachi Medical System, Tokyo, Japan). Urine Cr and UN were measured using commercial kits (Eiken Chemical Co., Tokyo, Japan). 3‐MH was separated from urine by high performance liquid chromatography using the method of Nagasawa et al.28 and measured using a RF‐535 spectrophotometer (Shimadzu, Kyoto, Japan) at 475 nm. Urinary Cr, UN and 3‐MH excretion over 24 h was calculated from the urine concentration × 24‐h urine volume.
Statistical Analysis
All data are presented as the mean ± SE. Significance was determined by one‐way anova with a Tukey’s test or Student’s t‐test, as appropriate. A level of P < 0.05 was considered statistically significant.
Results
In the preliminary OGTT, diabetic rats showed significantly higher blood glucose levels at 30, 60 and 120 min, and significantly lower serum insulin levels at 30 and 60 min compared with non‐diabetic rats (Figure 1). This profile is similar to that of moderately glycemic uncontrolled type 2 diabetes.
Figure 1.
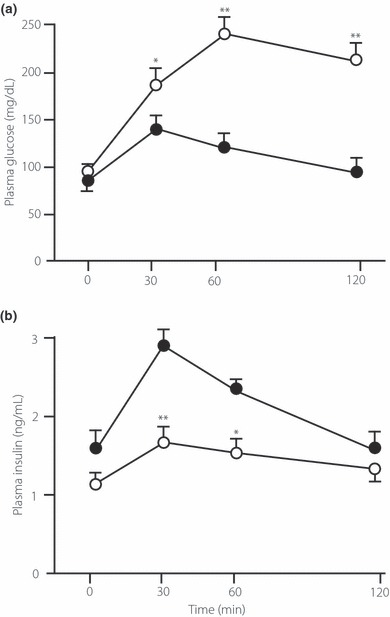
(a) Blood glucose and (b) plasma insulin during an oral glucose tolerance test (1.0 g/kg) in control (non‐diabetic) and streptozotocin‐induced type 2 diabetic rats in the preliminary experiment. *P < 0.05, **P < 0.01 vs non‐diabetic rats (controls). (•) non‐diabetic (control) group; (○) diabetic group.
Comparisons of parameters among the four groups in the genuine experiment at the end of the study are shown in Table 1. Bodyweight did not differ significantly among the four groups. Blood glucose levels in diabetic rats with and without exercise were significantly higher than those in both groups of non‐diabetic rats. Blood glucose levels in diabetic rats with exercise tended to be higher than those in diabetic rats without exercise, but this difference did not reach statistical significance. Serum insulin levels in both groups of diabetic rats were significantly lower than those in both groups of non‐diabetic rats, and serum insulin levels in diabetic rats with exercise were significantly lower than those in diabetic rats without exercise. Diabetic rats with exercise showed significantly higher serum CK levels than diabetic rats without exercise and both groups of non‐diabetic rats. In contrast, serum CK did not differ significantly between non‐diabetic rats with and without exercise.
Table 1. Comparisons of bodyweight, serum glucose, serum insulin and serum creatine kinase.
| Control (non‐diabetic rats without exercise) | Non‐diabetic rats with exercise | Diabetic rats without exercise | Diabetic rats with exercise | |
|---|---|---|---|---|
| Bodyweight (g) | 252 ± 3 | 253 ± 3 | 251 ± 4 | 250 ± 3 |
| Serum glucose (mg/dL) | 122 ± 17 | 144 ± 8 | 238 ± 41*† | 268 ± 39*† |
| Serum insulin (ng/dL) | 1.58 ± 0.7 | 1.08 ± 0.5 | 0.88 ± 0.3*† | 0.33 ± 0.2**††§ |
| Serum CK (IU/L) | 533 ± 182 | 525 ± 315 | 595 ± 225 | 1213 ± 530*†§ |
CK, creatine kinase.
*P < 0.05, **P < 0.01 vs controls, †P < 0.05, ††P < 0.01 vs non‐diabetic rats with exercise, §P < 0.05 vs diabetic rats without exercise.
The 24‐h urinary excretions of Cr (Figure 2) in both groups of diabetic rats were significantly greater than those in both groups of non‐diabetic rats (Figure 2). Cr excretion in diabetic rats with exercise was significantly greater than that in diabetic rats without exercise, but it did not differ significantly between non‐diabetic rats with and without exercise. The 24‐h urinary excretion of UN (Figure 3) in diabetic rats with exercise was significantly greater than that in diabetic rats without exercise and that in non‐diabetic rats without exercise, but it did not differ significantly between non‐diabetic rats with and without exercise. The 24‐h urinary excretions of 3‐MH (Figure 4) in both groups of diabetic rats were significantly greater than those in both groups of non‐diabetic rats. Diabetic rats with exercise had significantly greater 3‐MH excretion than diabetic rats without exercise, but there was no significant difference between non‐diabetic rats with and without exercise.
Figure 2.
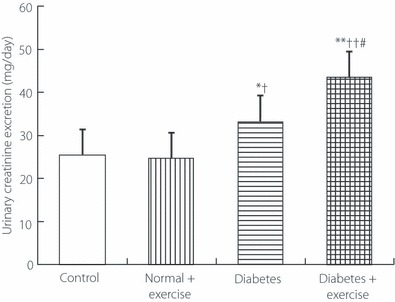
The 24‐h urinary excretion of creatinine in the genuine experiment. *P < 0.05, **P < 0.01 vs non‐diabetic rats without exercise (controls), †P < 0.05, ††P < 0.01 vs non‐diabetic rats with exercise, P < 0.05 vs diabetic rats without exercise.
Figure 3.
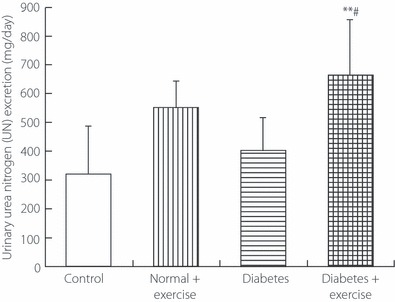
The 24‐h urinary excretion of urea nitrogen in the genuine experiment. **P < 0.01 vs non‐diabetic rats without exercise (controls), #P < 0.05 vs diabetic rats without exercise.
Figure 4.
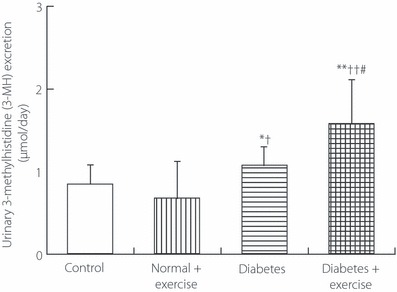
The 24‐h urinary excretion of 3‐methylhistidine in the genuine experiment. *P < 0.05, **P < 0.01 vs non‐diabetic rats without exercise (controls), †P < 0.05, ††P < 0.01 vs non‐diabetic rats with exercise, #P < 0.05 vs diabetic rats without exercise.
Conclusions
Streptozotocin selectively destroys pancreatic β‐cells and is often used to produce models of type 1 diabetes. However, the extent of destruction of pancreatic β‐cells by STZ treatment is dose‐dependent29,30. It is reported that high doses of STZ (40–60 mg/kg) produced type 1 diabetes, whereas low doses of STZ (35 mg/kg) produced type 2 diabetes31,32. In the current study, we applied 30 mg/kg of STZ in male Wistar rats and produced moderately glycemic uncontrolled type 2 diabetes with insulin deficiency, which is a similar profile to that in Goto‐Kakizaki (GK) diabetic rats33 and that in spontaneously diabetic Torii (SDT) rats34, which are animal models of non‐obese type 2 diabetes with insulin deficiency. Using these rats, we found that serum CK, an enzyme marker of muscle damage, was elevated in diabetic rats after 5 days of voluntary exercise, compared with that in diabetic rats without exercise, whereas serum CK in non‐diabetic rats with exercise was not elevated compared with that in non‐diabetic rats without exercise, suggesting that this intensity level of exercise induced muscle damage in diabetic rats, but did not induce muscle damage in non‐diabetic rats.
Muscle tissues are damaged after intense prolonged exercise as a consequence of both mechanical (direct) and metabolic (indirect) factors23. Exercise intensity might increase the membrane permeability of muscle cells, and CK and other enzymes in the muscle leak into the circulation, which then leads to leakage of intracellular muscle components by rhabdomyolysis23,35,36. These components include muscle protein degradation (MPD) products, such as Cr, UN and 3‐MH24–26. It is likely that exercise‐induced muscle damage in diabetic rats in the current study mainly occurs through MPD or muscle protein breakdown. Thus, we determined 24‐h urinary excretions of Cr, UN and 3‐MH, products of MPD, and found that these products were elevated in diabetic rats with exercise compared with those in diabetic rats without exercise. In contrast, changes in 24‐h urinary Cr, UN and 3‐MH excretion were not observed in non‐diabetic rats with exercise compared with those in non‐diabetic rats without exercise (controls). Serum Cr arises from damaged muscle24, but is not always directly released into urine, as a creatine pool is present in the body37. Serum UN formed from degradation of endogenous muscle proteins does not differ from serum UN derived from other organs and from ingested protein. Therefore, urinary excretion of UN transferred from serum only partly reflects MPD25. Urinary 3‐MH excretion is considered to be a reliable marker of muscle fiber protein degradation26, but cannot be utilized as a marker of total MPD. Consequently, each marker has a limited capacity for identifying MPD; however, the consistent elevation of all three markers suggests that MPD was enhanced in moderately glycemic uncontrolled type 2 diabetic rats in the current study by 5‐day voluntary moderate exercise at an intensity level that did not enhance MPD in non‐diabetic rats.
Taken together, it might be reasonable to consider that muscle damage was induced mainly through enhanced MPD in moderately glycemic uncontrolled type 2 diabetic rats with insulin deficiency by moderate exercise at an intensity level that did not affect muscle integrity in non‐diabetic rats.
The current study did not deal with the effect of exercise or muscle damage and MPD in uncontrolled type 2 diabetic rats with insulin resistance, and cannot explain the mechanisms of muscle damage and muscle protein degradation by exercise in uncontrolled type 2 diabetic rats with insulin resistance. In addition, we wonder whether a similar exercise effect on muscle damage and muscle protein degradation in diabetic rats in the current study might occur in type 2 diabetic patients with relative insulin secretary deficiency, whose profile consists of a considerably large number of Japanese, as well as Asian, type 2 diabetic patients38. Further studies are needed to elucidate the effects on muscle damage and MPD in uncontrolled type 2 diabetic rats with insulin resistance or with relative insulin secretary deficiency.
In the current study, diabetic rats with exercise showed significant higher serum glucose and lower serum insulin than diabetic rats without exercise. Because the diabetic rats in the current study were type 2 diabetic rats with insulin deficiency, we presume that these phenomena were caused by a decrease in serum insulin by exercise through increased sympathetic nerve activity39.
Many studies have investigated the effects of exercise on type 2 diabetes, but the majority of reports have focused on the effects of exercise on blood glucose control3–6. However, to date, there have been no reports that have addressed the effects of exercise on muscle damage and MPD in type 2 diabetic animals. In the current study, we unexpectedly found that uncontrolled type 2 diabetes was susceptible to exercise‐induced muscle damage that probably occurred through enhanced MPD. The results suggest that moderate exercise might cause muscle damage and enhance MPD in moderately glycemic, uncontrolled type 2 diabetic patients. The results also suggest that the status of glycemic control should be considered carefully when implementing an exercise regimen in patients with type 2 diabetes in clinical practice.
This raises the question of the cause of induced muscle damage and enhanced MPD by exercise in uncontrolled type 2 diabetic rats with insulin deficiency. Insulin is an anabolic hormone40,41. In an early study, Smith et al.42 reported that the total protein degradation rate was increased by 30% and myofibrillar catabolism was accelerated by 60% in STZ‐induced diabetic rats, and that treatment with insulin within a physiological range completely reversed these changes. We suggest that reversal of induced muscle damage and enhanced MPD by insulin is mediated through the anabolic actions of insulin40,41, directly, and through inhibition of increased hepatic gluconeogenesis, indirectly, because amino acids as substrates for gluconeogenesis are derived from whole body proteins, including muscle protein, in uncontrolled type 2 diabetes43. This might make muscle structure and function susceptible to muscle damage and MPD enhancement by exercise. Subinflammation in type 2 diabetes might also contribute to these phenomena14. The results in the current study thus suggest that glycemic control is important to maintain muscle mass in diabetes. Further studies are also needed to investigate which molecular mechanisms underlie muscle damage and muscle protein degradation in type 2 diabetes.
In conclusion, exercise induced muscle damage and enhanced MPD in STZ‐induced moderately glycemic, uncontrolled type 2 diabetic rats with insulin deficiency. These effects were caused by moderate exercise at an intensity level that did not induce muscle damage and did not enhance MPD in non‐diabetic rats. These results suggest that in clinical practice an exercise regimen should be carefully prescribed in patients with uncontrolled type 2 diabetes, as well as in patients with type 1 diabetes, for maintaining muscle mass, as well as regulating glycemic control.
Acknowledgment
The present study was supported by research grants of Kyoritsu Women’s University and Kiryu University. The authors have no conflicts of interest.
References
- 1.International Diabetes Federation . Diabetes Atlas 2th. 2003. Available at http://www.eatlas.idf.org/Diabetes_Atlas_Executive_Summary_download
- 2.The United Nations . The UN Resolution on Diabetes. 2006. Available at http://www.unitefordiabetes.org/campaign/resolution.html
- 3.Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinman B, Ruderman N, Campaigne BN, et al. Physical activity/exercise and diabetes. Diabetes Care 2004; 27(Suppl 1): S58–S62 14693927 [Google Scholar]
- 5.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007; 147: 357–369 [DOI] [PubMed] [Google Scholar]
- 6.Boule NG, Haddad E, Kenny GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA 2001; 286: 1218–1227 [DOI] [PubMed] [Google Scholar]
- 7.Wright DC, Hucker KA, Holloszy JO, et al. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004; 53: 330–335 [DOI] [PubMed] [Google Scholar]
- 8.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther 2008; 88: 1279–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006; 29: 1433–1438 [DOI] [PubMed] [Google Scholar]
- 10.Matthews DE. Proteins and amino acids In: Shils ME, Olson JA, Shike M, Ross AC (eds). Modern Nutrition and Health and Disease, 9th edn Williams & Wilkins, Baltimore, MD, 1999: 11–48 [Google Scholar]
- 11.Drummond MJ, Dreyer HC, Fry CS, et al. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009; 106: 1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welle S, Thornton C, Statt M, et al. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol Endocrinol Metab 1994; 267: E599–E604 [DOI] [PubMed] [Google Scholar]
- 13.MacDougall JD, Gibala MJ, Tarnopolsky MA, et al. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 1995; 20: 480–486 [DOI] [PubMed] [Google Scholar]
- 14.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF‐kappaB signaling in skeletal muscle. J Appl Physiol 2007; 103: 388–395 [DOI] [PubMed] [Google Scholar]
- 15.Riddell M, Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol 2009; 3: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burd NA, Tang JE, Moore DR, et al. Exercise training and protein metabolism: influences of contraction, protein intake, and sex‐based differences. J Appl Physiol 2009; 106: 1692–1701 [DOI] [PubMed] [Google Scholar]
- 17.Steven EK, Daniel P. The pathophysiology of Type II (Noninsulin‐Dependent) Diabetes Mellitus: Implications for Treatment (edited by Daniel P Jr, Robert SS). Ellenbery & Rifkin’s Diabetes Mellitus, Appleton & Lange Stamford, Connecticut, 1997: 487–512 [Google Scholar]
- 18.Antuna‐Puente B, Feve B, Fellahi S, et al. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab 2008; 34: 2–11 [DOI] [PubMed] [Google Scholar]
- 19.Fritsche L, Hoene M, Lehmann R, et al. IL‐6 deficiency in mice neither impairs induction of metabolic genes in the liver nor affects blood glucose levels during fasting and moderately intense exercise. Diabetologia 2010; 53: 1732–1742 [DOI] [PubMed] [Google Scholar]
- 20.Rodnick KJ, Reaven GM, Haskell WL, et al. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol 1989; 66: 1250–1257 [DOI] [PubMed] [Google Scholar]
- 21.Seburn KL, Gardiner P. Adaptations of rat lateral gastrocnemius motor units in response to voluntary running. J Appl Physiol 1995; 78: 1673–1678 [DOI] [PubMed] [Google Scholar]
- 22.Laye MJ, Rector RS, Warner SO, et al. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 2009; 587: 3729–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med 2010; 48: 757–767 [DOI] [PubMed] [Google Scholar]
- 24.Ix JH, de Boer IH, Wassel CL, et al. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation 2010; 121: 1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy BD, Tarnopolsky MA, MacDougall JD, et al. Effect of glucose supplement timing on protein metabolism after resistance training. J Appl Physiol 1997; 82: 1882–1888 [DOI] [PubMed] [Google Scholar]
- 26.Sadiq F, Crompton LA, Scaife JR, et al. Effect of prolonged intravenous glucose and essential amino acid infusion on nitrogen balance, muscle protein degradation and ubiquitin‐conjugating enzyme gene expression in calves. Nutr Metab 2008; 12: 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junod A, Lambert AE, Stauffacher W, et al. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest 1969; 48: 2129–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasawa T, Yoshisawa F. Plasma N tau‐methylhistidine concentration is a sensitive index of myofibrillar protein degradation during starvation in rats. Biosci Biotech Biochem 1996; 60: 501–502 [DOI] [PubMed] [Google Scholar]
- 29.Okamoto T, Kanemoto N, Ohbuchi Y, et al. Characterization of STZ‐induced type 2 diabetes in Zucker fatty rats. Exp Anim 2008; 57: 335–345 [DOI] [PubMed] [Google Scholar]
- 30.Jackerott M, Møldrup A, Thams P, et al. STAT5 activity in pancreatic beta‐cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes 2006; 55: 2705–2712 [DOI] [PubMed] [Google Scholar]
- 31.Vasavada RC, Garcia‐Ocaña A, Zawalich WS, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 2000; 275: 15399–15406 [DOI] [PubMed] [Google Scholar]
- 32.Shimabukuro M, Wang MY, Zhou YT, et al. Protection against lipoapoptosis of beta cells through leptin‐dependent maintenance of Bcl‐2 expression. Proc Natl Acad Sci USA 1998; 95: 9558–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohta T, Furukawa N, Komuro G, et al. JTT‐608 restores impaired early insulin secretion in diabetic Goto‐Kakizaki rats. Br J Pharmacol 1999; 126: 1674–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuyama T, Komeda K, Hara A, et al. Chronological characterization of diabetes development in male Spontaneously Diabetic Torii rats. Biochem Biophys Res Commun 2004; 314: 870–874 [DOI] [PubMed] [Google Scholar]
- 35.Bijsterbosch MK, Duursma AM, Smit MJ, et al. Several dehydrogenases and kinases compete for endocytosis from plasma by rat tissues. Biochem J 1985; 229: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 2010; 48: 749–756 [DOI] [PubMed] [Google Scholar]
- 37.Benzi G, Ceci A. Creatine as nutritional supplementation and medicinal product. J Sports Med Phys Fitness 2001; 41: 1–10 [PubMed] [Google Scholar]
- 38.Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835 [DOI] [PubMed] [Google Scholar]
- 39.Ahren B. Autonomic regulation of islet hormone secretion – implications for health and disease. Diabetologia 2000; 43: 393–410 [DOI] [PubMed] [Google Scholar]
- 40.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995; 95: 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow LS, Albright RC, Bigelow ML, et al. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl‐tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 2006; 291: E729–E736 [DOI] [PubMed] [Google Scholar]
- 42.Smith OL, Wong CY, Gelfand RA. Skeletal muscle proteolysis in rats with acute streptozocin‐induced diabetes. Diabetes 1989; 38: 1117–1122 [DOI] [PubMed] [Google Scholar]
- 43.Samuel VT, Beddow SA, Iwasaki T, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. PNAS 2009; 106: 12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]


