Abstract
Aims/Introduction: We compared the safety and efficacy of liraglutide vs glibenclamide in patients with poorly controlled (HbA1c, 7.4–10.4%) type 2 diabetes.
Materials and Methods: Subjects were randomly assigned at a 1:2 ratio to receive 1‐year treatment with glibenclamide 1.25–2.5 mg/day or liraglutide 0.9 mg/day. Other oral anti‐diabetic drugs (OAD) were prohibited during the trial. Adverse events (AE) were monitored.
Results: A total of 400 patients (liraglutide group, n = 268; glibenclamide group, n = 132) were randomized and exposed to trial products. At week 52 vs baseline, HbA1c in the liraglutide and glibenclamide groups was reduced from 9.3 to 7.8% and from 9.2 to 8.2%, respectively. Treatment difference (liraglutide – glibenclamide) at the end of the study was −0.49 (95% CI, −0.71 to −0.27). In the liraglutide and glibenclamide groups, Japan Diabetes Society target HbA1c < 6.9% was achieved by 22.1 and 8.5% of patients, respectively. Fasting plasma glucose fell from 202.8 and 202.1 mg/dL, respectively, to 145.3 and 156.7 mg/dL, respectively. Mean plasma glucose and mean postprandial plasma glucose increment were lower in the liraglutide group. Mean bodyweight was reduced by −0.8 kg in the liraglutide group and increased by 1.0 kg in the glibenclamide group. The proportion of patients reporting at least one treatment‐emergent AE (TEAE) in the liraglutide and glibenclamide groups was 91.4 and 91.7%, respectively. Most TEAE were mild in severity. No major hypoglycemic episode was observed.
Conclusions: Once‐daily administration of liraglutide 0.9 mg for 52 weeks provides more favorable metabolic control and safety profile compared with glibenclamide. Patients on liraglutide lost bodyweight, whereas those on glibenclamide gained weight. This trial was registered with ClinicalTrial.gov (no. NCT00393718). (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00128.x, 2011)
Keywords: GLP‐1 receptor agonist, Liraglutide, Type 2 diabetes mellitus
Introduction
The treatment of type 2 diabetes mellitus aims at preventing the onset and progression of diabetic complications by normalizing glycemic control. The United Kingdom Prospective Diabetes Study (UKPDS)1, as well as the Kumamoto Study2 in Japan, have shown that improvement of blood glucose levels might contribute to delaying the development and progression of diabetic complications in people with type 2 diabetes. However, currently available treatments for diabetes are not satisfactory, as evidenced by the high morbidity and mortality resulting from this condition. In addition, the incidence of diabetes is increasing worldwide. Consequently, there is an incentive to develop new drugs with novel mechanisms of action for the treatment of type 2 diabetes.
Glucagon‐like peptide (GLP)‐1 is an incretin hormone released by enteroendocrine L cells that stimulates endogenous pancreatic insulin secretion and decreases glucagon secretion, both in a glucose‐dependent manner. GLP‐1 also reduces gastric motility and emptying, and decreases appetite. Thus, GLP‐1 is a potent blood glucose‐lowering agent1. However, after intravenous administration, GLP‐1 has a very short half‐life (t½, < 1.5 min) because of rapid cleavage by dipeptidyl peptidase (DPP)‐4. Therefore, liraglutide, a GLP‐1 analog (sequence homology, 97%) with the same mechanism of action as endogenous GLP‐1, but with a longer half‐life in vivo, was rationally designed. After subcutaneous injection, liraglutide has a protracted pharmacokinetic and pharmacodynamic profile based on delayed absorption from the injection site, albumin binding and decreased susceptibility to metabolism by DPP‐4; therefore, liraglutide is suitable for once‐daily administration3,4. Liraglutide binds to GLP‐1 receptors equipotently to endogenous GLP‐1 and is expected to induce the same effects as native GLP‐15–7.
Liraglutide given at doses ≤25 μg/kg showed good tolerability with no gastrointestinal (GI) adverse events (AE) after stepwise dose escalation in a 5‐week study carried out in Japanese healthy volunteers8. In phase II, liraglutide given for 14 weeks to diabetic patients exerted significant reductions of HbA1c vs placebo, and was well tolerated with no report of major hypoglycemia and no increase of calcitonin concentration detected9.
In many cases of diabetes that are poorly controlled by diet and/or exercise therapy alone, treatment with an oral anti‐diabetic drug (OAD), such as sulfonylurea (SU), is initiated. Glibenclamide is one of the most potent sulfonylureas and has long been used as either a first‐ or second‐line therapy for type 2 diabetes. However, as SU agents continuously stimulate β‐cells to secrete insulin, over the clinical course of diabetes dose escalation is often necessary because of increasing β‐cell exhaustion, which might become a vicious circle. To delay the necessity of insulin treatment, it is important to keep β‐cells responsive for as long as possible.
The present randomized trial compared the safety and efficacy of liraglutide, which has been shown to correct hyperglycemia with a low frequency of hypoglycemia, as well as to reproduce physiological insulin secretion patterns with once‐daily administration10–15, with those of glibenclamide in patients with type 2 diabetes on diet therapy and/or monotherapy with an OAD. In this 52‐week trial, treatment over the first 24 weeks was given in a double blind fashion, followed by a 28‐week open label treatment period. The results of the initial portion of the trial have been reported elsewhere16. This report focuses on the overall 52‐week findings, including those of the second 28‐week part of the trial, which was carried out following an open label design.
Materials and Methods
Patients
Male and female type 2 diabetic patients aged ≥20 years whose HbA1c was 7.4–10.4%, regardless of whether they were previously taking OAD, were recruited. In addition, patients were required to be able to carry out self‐monitoring of blood glucose (SMBG). Informed consent was obtained from all patients. The present trial was carried out in accordance with the Helsinki Declaration and the Japanese Ministry of Health, Labour and Welfare (MHLW) ordinance on good clinical practice (GCP). The present multicenter study was approved by the Institutional Review Board (IRB) at each of the 75 trial sites.
Study Design
Following an initial screening visit carried out so as to verify patients’ eligibility to enter the trial and a 4–6‐week run‐in period designed to wash out pretrial OAD in patients previously receiving these medications, the subjects were randomly assigned at a 1:2 ratio to receive 1‐year treatment with glibenclamide 1.25–2.5 mg/day or liraglutide given as follows: at first the patients were entered into a 2‐week dose‐escalation period (in 0.3‐mg increments), followed by a 50‐week maintenance period during which they received liraglutide 0.9 mg/day given subcutaneously (in the morning or evening). Glibenclamide was chosen as the comparator, because it was one of the most widely used oral anti‐diabetic agents at the time of planning and initiating the study (2006), and its efficacy and safety are well established.
During this trial, concomitant medication with OAD was prohibited. Patients were, however, instructed to adhere to previous diet or exercise therapy, if any.
The present study looked at HbA1c level after 52‐week treatment, as well as the proportion of patients achieving the Japan Diabetes Society (JDS) target of diabetes therapy, such as having a HbA1c < 6.9%. Considering the relational expression of HbA1c, as measured by the former Japanese standard substance and measurement methods (HbA1c [JDS]), in the present report, HbA1c was estimated as National Glycohemoglobin Standardization Program (NGSP)‐equivalent value calculated by the formula HbA1c (%) = HbA1c (JDS) (%) + 0.4%.
Secondary end‐points, such as fasting plasma glucose (FPG), postprandial plasma glucose (PPG) and 7‐point plasma glucose profile (defined as SMBG measured before and 120 min after start of breakfast, lunch and dinner, and at bedtime), were examined; also assessed were the test agents’ effects on bodyweight, waist circumference, indicators of β‐cell function, cardiovascular biomarkers (BNP, PAI‐1, hsCRP) and lipid profiles (total cholesterol [TC], low‐density lipoprotein cholesterol [LDL‐C], very low‐density lipoprotein cholesterol [VLDL‐C], high‐density lipoprotein cholesterol [HDL‐C], triacylglycerides [TG], free fatty acids [FFA] and apolipoprotein B [apo B]).
HbA1c was measured by the high‐performance liquid chromatography method. SMBG was carried out at home by an automated glucose meter. Other efficacy blood parameters were assessed using samples subjected to centrifugation and cryopreserved at a central laboratory (Mitsubishi Chemical Medience Corporation).
Safety was assessed in terms of AE, including hypoglycemic episodes (defined as major, hypoglycemia requiring third‐party assistance; minor, self‐treated hypoglycemia; and symptoms only, remainder), clinical laboratory parameters, vital signs, electrocardiogram, funduscopy and liraglutide antibodies. Furthermore, as it was reported that dosing with liraglutide might cause thyroid C‐cell tumors in mice and rats17, although the mode of action of this unwanted effect is of uncertain relevance to humans, the levels of calcitonin – a biomarker for C‐cell hyperplasia – were monitored in patients enrolled in the present study.
Efficacy and safety parameters were assessed during every 4‐week visit. In addition, patients were asked to carry out 7‐point SMBG at baseline and every 3 months thereafter through to week 52.
Statistical Analysis
For each of the efficacy end‐points after the 52‐week treatment, the 95% confidence interval (95% CI) for the mean intergroup difference was determined by analysis of variance (anova) with treatment group and pretrial treatment as fixed effects, and corresponding baseline value as covariate using full analysis set (FAS) data on all patients who took at least one dose of the study drug. The last observation carried forward (LOCF) approach was used for patients who had at least one valid post‐baseline measurement. AE and hypoglycemic episodes in each treatment group were summarized and presented in frequency distribution tables.
Results
Of the 464 patients screened, 411 were randomized and 400 were exposed to trial products (liraglutide, n = 268; glibenclamide, n = 132); baseline demographics of enrolled subjects are shown in Table 1. The proportion of subjects completing 52 weeks of study treatment was 84.0% in the liraglutide group and 83.3% in the glibenclamide group. The most common reason for withdrawal from the trial was AE (liraglutide group, 7.5%; glibenclamide group, 6.1%). In contrast, withdrawal because of ineffective therapy accounted for 3.7 and 6.8% of the two groups, respectively.
Table 1. Baseline demographic characteristics of subjects.
| Item | Liraglutide | Glibenclamide | Total |
|---|---|---|---|
| No. patients | 268 | 132 | 400 |
| Sex (male/female), n (%) | 183 (68.3)/85 (31.7) | 86 (65.2)/46 (34.8) | 269 (67.3)/131 (32.8) |
| Age, years | 58.2 (10.4) | 58.5 (10.4) | 58.3 (10.4) |
| Bodyweight, kg | 66.2 (12.6) | 65.4 (12.9) | 65.9 (12.7) |
| BMI, kg/m2 | 24.9 (3.7) | 24.6 (3.8) | 24.8 (3.7) |
| HbA1c, % | 9.32 (1.08) | 9.18 (0.97) | 9.27 (1.04) |
| Duration of diabetes, years | 8.1 (6.7) | 8.5 (6.8) | 8.3 (6.7) |
| Pretrial treatment, n (%) | |||
| Without OAD | 50 (18.7) | 23 (17.4) | 73 (18.3) |
| With OAD | 218 (81.3) | 109 (82.6) | 327 (81.8) |
| Concomitant illness, n (%) | |||
| Yes | 266 (99.3) | 131 (99.2) | 397 (99.3) |
| No | 2 (0.7) | 1 (0.8) | 3 (0.8) |
Mean (SD) except where indicated. BMI, body mass index; OAD, oral anti‐diabetic drugs.
At baseline, HbA1c in the liraglutide and glibenclamide groups was 9.3 and 9.2%, respectively. At week 52, this parameter was 7.8 and 8.2%, respectively. Treatment difference (liraglutide – glibenclamide) at end of study was −0.49 (95% CI, −0.71 to −0.27). HbA1c at each visit is presented in Table 2 and Figure 1. JDS target HbA1c < 6.9% was achieved by 22.1% of patients in the liraglutide group and 8.5% of those in the glibenclamide group.
Table 2. Analysis of variance of HbA1c, fasting plasma glucose, mean plasma glucose, and mean postprandial plasma glucose increment at week 52.
| Parameter | n | Least squares means (SE) | Treatment difference (95% CI) |
|---|---|---|---|
| HbA1c, % | |||
| Liraglutide | 263 | 7.7 (0.1) | −0.49 (−0.71 to −0.27) |
| Glibenclamide | 130 | 8.2 (0.1) | |
| FPG, mg/dL | |||
| Liraglutide | 261 | 145.8 (2.4) | −11.7 (−18.6 to −4.9) |
| Glibenclamide | 130 | 157.5 (3.2) | |
| Mean PG, mg/dL | |||
| Liraglutide | 237 | 167.4 (3.2) | −17.2 (−26.3 to −8.1) |
| Glibenclamide | 119 | 184.6 (4.3) | |
| Mean PPG increment, mg/dL | |||
| Liraglutide | 238 | 63.6 (3.0) | −13.0 (−21.5 to −4.6) |
| Glibenclamide | 119 | 76.6 (3.9) | |
FPG, fasting plasma glucose; PG, plasma glucose; PPG, postprandial plasma glucose.
Figure 1.
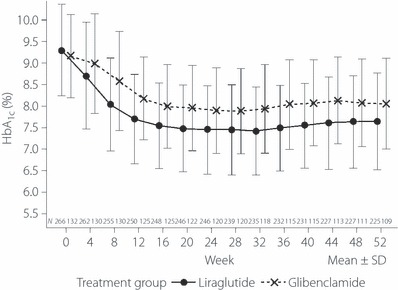
Time‐course of HbA1c.
FPG in the liraglutide and glibenclamide groups fell from 202.8 and 202.1 mg/dL, respectively, to 145.3 and 156.7 mg/dL, respectively, at week 52. Treatment difference at the end of the study was −11.7 (95% CI, −18.6 to −4.9; Table 2). At week 52, mean PG and mean PPG increment were lower in the liraglutide vs glibenclamide groups based on 95% CI (Table 2). Furthermore, at week 52 vs baseline, mean bodyweight was reduced by −0.8 kg in the liraglutide group and increased by 1.0 kg in the glibenclamide group (intergroup difference, −1.7; 95% CI, −2.3 to −1.2). Waist circumference at week 52 was lower in the liraglutide than glibenclamide group based on the 95% CI of treatment difference (−1.1 cm; 95% CI, −1.9 to −0.2). We also analyzed the changes in bodyweight in two subgroups of patients according to baseline BMI (i.e. >25 and <25 kg/m2). In the liraglutide group, the change in bodyweight was −0.51 kg in patients with BMI < 25 kg/m2 and −1.05 kg in patients with BMI ≥ 25 kg/m2, showing a slightly greater decrease in bodyweight in overweight individuals. In contrast, in the glibenclamide group, bodyweight increased similarly in both groups (+1.05 and +0.84 kg, respectively).
anova of lipid profiles at week 52 is presented in Table 3. Mean of FFA was lower in the liraglutide than glibenclamide group based on 95% CI of treatment difference; however, no other intergroup difference of lipid parameters was observed based on 95% CI.
Table 3. Analysis of variance of lipid profiles at week 52.
| Parameter | n | Least squares means (SE) | Treatment difference (95% CI) |
|---|---|---|---|
| TC, mg/dL | |||
| Liraglutide | 262 | 195.5 (1.8) | −4.0 (−9.2 to 1.3) |
| Glibenclamide | 129 | 199.5 (2.4) | |
| LDL‐C, mg/dL | |||
| Liraglutide | 262 | 116.9 (1.6) | −2.1 (−6.6 to 2.3) |
| Glibenclamide | 129 | 119.1 (2.1) | |
| VLDL‐C, mg/dL | |||
| Liraglutide | 262 | 17.9 (0.9) | −2.0 (−4.6 to 0.5) |
| Glibenclamide | 129 | 20.0 (1.2) | |
| HDL‐C, mg/dL | |||
| Liraglutide | 262 | 61.5 (0.7) | 1.0 (−1.0 to 3.0) |
| Glibenclamide | 129 | 60.5 (0.9) | |
| TG, mg/dL | |||
| Liraglutide | 262 | 137.3 (5.5) | −15.3 (−31.0 to 0.3) |
| Glibenclamide | 129 | 152.6 (7.2) | |
| FFA, mEq/L | |||
| Liraglutide | 262 | 0.577 (0.014) | −0.065 (−0.106 to −0.025) |
| Glibenclamide | 129 | 0.643 (0.019) | |
| apo B, mg/dL | |||
| Liraglutide | 262 | 92.6 (1.0) | −1.6 (−4.4 to 1.3) |
| Glibenclamide | 129 | 94.2 (1.3) | |
apo B, Apolipoprotein B; FFA, free fatty acids; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triacylglycerides; VLDL‐C, very low‐density lipoprotein cholesterol.
In terms of markers of β‐cell function at week 52, the proinsulin/insulin ratio (0.98 vs 1.57 [intergroup difference: −0.59; 95% CI: −0.97 to −0.22]) and the proinsulin/C‐peptide ratio (2.03 vs 3.16 [−1.13; −1.46 to −0.80]) were significantly lower in the liraglutide group than in the glibenclamide group based on 95% CI, whereas there was no difference in homeostasis model assessment‐β (34.7 vs 30.8 [3.9; −0.1 to 7.7]) based on 95% CI. In terms of cardiovascular biomarkers, only BNP was significantly different between the two groups based on 95% CI, favoring liraglutide (16.8 vs 25.4 pg/mL [−8.6; −13.6 to −3.6]).
Treatment‐emergent AE (TEAE) are summarized in Table 4. The proportion of patients reporting at least one TEAE in the liraglutide and glibenclamide groups was 91.4 and 91.7%, respectively. Most TEAE were mild in severity. The most common TEAE in both treatment groups was nasopharyngitis (liraglutide group, 37.3%; glibenclamide group, 43.2%). More patients reported GI‐related AE in the liraglutide vs glibenclamide group. However, this difference was observed principally during the first 4 weeks of the study and tended to even out as the study progressed (Figure 2). No major hypoglycemic episode was reported in the present study. The rate of minor hypoglycemic episodes (events/patient/year) was 0.19 in the liraglutide group and 1.10 in the glibenclamide group; that of symptoms‐only hypoglycemic episodes was 0.51 and 2.74, respectively. Overall, the proportion of subjects who withdrew from the study because of AE in the liraglutide and glibenclamide groups was 7.5 and 6.1%, respectively. One death because of acute gastroenteritis was noted in the liraglutide group; this AE was considered unlikely to be related to taking the study product. Most clinical laboratory parameters remained constant throughout the trial. Five patients (1.9%) in the liraglutide group and three (2.3%) in the glibenclamide group withdrew from the study because of AE related to GI disorders. A causal relationship with the study drug could not be ruled out for the AE related to GI disorders in the liraglutide group, whereas the GI disorders in two of the three patients in the glibenclamide group were unrelated to the study drug.
Table 4. Treatment‐emergent adverse events reported in ≥5% of patients.
| System organ class/preferred term | N (%) E | |
|---|---|---|
| Liraglutide | Glibenclamide | |
| Subjects exposed, n | 268 | 132 |
| All AE | 245 (91.4) 1098 | 121 (91.7) 511 |
| Infections/infestations | 132 (49.3) 267 | 79 (59.8) 137 |
| Nasopharyngitis | 100 (37.3) 183 | 57 (43.2) 94 |
| Bronchitis | 11 (4.1) 13 | 8 (6.1) 11 |
| Gastrointestinal disorders | 121 (45.1) 224 | 48 (36.4) 81 |
| Diarrhea | 26 (9.7) 31 | 9 (6.8) 12 |
| Constipation | 22 (8.2) 24 | 7 (5.3) 8 |
| Stomach discomfort | 14 (5.2) 21 | 3 (2.3) 5 |
| Nausea | 14 (5.2) 24 | 2 (1.5) 3 |
| Musculoskeletal/connective tissue disorders | 65 (24.3) 85 | 33 (25.0) 47 |
| Back pain | 17 (6.3) 17 | 9 (6.8) 9 |
| Arthralgia | 8 (3.0) 9 | 11 (8.3) 11 |
| Respiratory/thoracic disorders | 48 (17.9) 76 | 27 (20.5) 32 |
| Upper respiratory tract infection | 25 (9.3) 48 | 9 (6.8) 10 |
| Pharyngolaryngeal pain | 2 (0.7) 2 | 7 (5.3) 7 |
| Eye disorders | 47 (17.5) 53 | 28 (21.2) 33 |
| Diabetic retinopathy | 16 (6.0) 16 | 9 (6.8) 10 |
| Nervous system disorders | 44 (16.4) 62 | 17 (12.9) 26 |
| Headache | 15 (5.6) 22 | 6 (4.5) 11 |
| Skin/subcutaneous tissue disorders | 38 (14.2) 46 | 23 (17.4) 31 |
| General disorders/administration site conditions | 37 (13.8) 51 | 13 (9.8) 14 |
| Injury/procedural complications | 33 (12.3) 44 | 17 (12.9) 27 |
| Investigations | 33 (12.3) 43 | 6 (4.5) 12 |
| Cardiac disorders | 17 (6.3) 20 | 14 (10.6) 14 |
| Vascular disorders | 17 (6.3) 20 | 10 (7.6) 10 |
| Hypertension | 11 (4.1) 11 | 7 (5.3) 7 |
| Metabolism/nutrition disorders | 14 (5.2) 14 | 3 (2.3) 3 |
| Hepatobiliary disorders | 11 (4.1) 12 | 7 (5.3) 8 |
| Psychiatric disorders | 10 (3.7) 12 | 8 (6.1) 8 |
%, Proportion of exposed subjects with treatment‐emergent adverse events, AE, adverse events; E, number of treatment‐emergent adverse events; N, number of subjects with treatment‐emergent adverse events.
Figure 2.
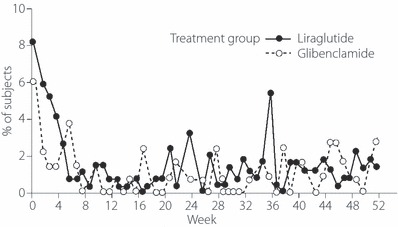
Proportion of patients reporting treatment‐emergent adverse events (TEAE) of the gastrointestinal (GI) system by period. Whereas more subjects reported TEAE of the GI system in the liraglutide group compared with the glibenclamide group during the first 4 weeks, the proportion of subjects with GI disorders was comparable between the groups thereafter.
After 52 weeks, no significant shift of calcitonin category was seen in either of the treatment groups.
At baseline, all samples in the two treatment groups had negative findings to liraglutide antibodies or cross‐reactivity to native GLP‐1. At the end of the study, although 33 patients (14.7%) had detectable liraglutide antibodies, mean HbA1c was not influenced by the presence of antibodies in these individuals; indeed, at week 52 their HbA1c showed a numerically greater decrease than that observed in the liraglutide group as a whole. We found no association between the incidence of TEAE and the presence of liraglutide antibodies.
Discussion
The present trial was designed to compare the long‐term efficacy and safety of liraglutide 0.9 mg once daily vs glibenclamide given as monotherapy in Japanese patients with type 2 diabetes mellitus who were previously receiving diet therapy with or without an OAD.
The results from the present trial show that liraglutide provides more favorable glycemic control compared with glibenclamide monotherapy. Although no formal test was carried out for superiority or non‐inferiority, based on the 95% CI for the treatment difference, HbA1c at 52 weeks was lower in the liraglutide group than in the glibenclamide group (estimated mean treatment difference, −0.49%). This intergroup difference was comparable to that observed at the end of the earlier, double blind part of the study at 24 weeks (−0.50%). Therefore, liraglutide exerts sustained reduction of HbA1c for a period of ≤1 year. Furthermore, a higher proportion of patients in the liraglutide vs glibenclamide group achieved target HbA1c levels as defined by JDS, and durable improvements of FPG, PPG and 7‐point PG profile were seen in the liraglutide group.
Liraglutide given to patients with type 2 diabetes was generally safe and well tolerated; the safety data obtained over 52 weeks did not raise any safety concerns. No major hypoglycemic episodes were reported. As expected in a long‐term trial in diabetic patients, most of the subjects experienced one or more TEAE, although the majority of TEAE were mild in severity. Hypoglycemia is a common AE associated with SU agents and insulin therapy18. Because GLP‐1 and GLP‐1 derivatives’ insulinotropic effects are glucose dependent and attenuated as endogenous glucose levels fall, treatment with liraglutide potentially conveys a low risk of hypoglycemia12, as was observed in the present study. In contrast, liraglutide is known to sometimes cause TEAE, such as nausea and vomiting, particularly at higher dosages. These unwanted effects seem attributable to GLP‐1’s mode of action on delayed gastric emptying10. To mitigate the occurrence of GI‐related AE when initiating GLP‐1 analog therapy, it has been previously shown that stepwise weekly dose increases might be effective; in volunteer subjects, liraglutide incrementally uptitrated in this fashion to ≤10 μg/kg showed good tolerability with no reports of nausea and vomiting during the trial19. Such stepwise dose‐titration strategy was used in the present study. Although there were overall more TEAE from the GI system noted in the liraglutide vs glibenclamide group (45.1 vs 36.4%), these were mainly observed during the first 4 weeks of the study and their incidence rate was comparable between the two groups thereafter.
In the Liraglutide Effect and Action in Diabetes (LEAD)‐3 trial, liraglutide monotherapy significantly reduced HbA1c compared with the long‐acting SU glimepiride; the differences between reductions of HbA1c exerted by glimepiride and liraglutide 1.2 and 1.8 mg/day were −0.33% (95% CI, −0.53 to −0.13; P = 0.0014) and −0.62% (−0.83 to −0.42; P < 0.0001), respectively, after treatment for 52 weeks20. Although no major hypoglycemia occurred in the three treatment arms, there was a slightly higher incidence of GI‐related AE in the liraglutide groups than in the glimepiride group. As in the present study, such AE mostly occurred during the first 4 weeks of the study and then gradually subsided. Furthermore, whereas participants in the liraglutide treatment groups lost weight, those in the glimepiride group gained weight – regardless of the presence of persistent nausea20. Therefore, in Western diabetic patients as in their Japanese counterparts, liraglutide was considered safe and effective and conferred additional advantages over SU, such as less hypoglycemia and greater reductions in weight.
During liraglutide’s preclinical development program, thyroid C‐cell tumors were observed in 104‐week carcinogenicity studies in mice and rats, heralded by increases of plasma calcitonin concentrations17. It is possible that chronic activation of the GLP‐1 receptor present on thyroid C‐cells by liraglutide can lead to hyperplastic and neoplastic changes of the thyroid in rodent models – although the thyroid tumors and C‐cell proliferative changes induced by liraglutide in rodents are thought to be caused by a non‐genotoxic specific receptor‐mediated mechanism that might not be relevant to humans. In the present trial, the majority of subjects had calcitonin levels below the lower limit of quantification (LLOQ) at baseline, as well as at week 52. No significant shift of this parameter was seen in the two treatment groups, suggesting that treatment with liraglutide for ≤1 year does not pose any risk of thyroid malignancy in patients. This finding is in line with that concluded by a long‐term safety study carried out in North America (data from the LEAD series of studies), in which no difference was observed between mean calcitonin levels in the liraglutide and control groups. In consideration of these results, weighed alongside the drug’s benefits, the European Medicines Agency (EMA) granted approval of liraglutide for the treatment of type 2 diabetes mellitus in 2009, followed by the US Food and Drug Administration in January 201021.
The finding in the present study that subjects treated with liraglutide had a mean bodyweight loss of 0.8 kg from baseline, whereas treatment with glibenclamide resulted in a mean increase of 1 kg, is important and could be a clear benefit of this treatment. It is notable that liraglutide reduces weight while improving glycemic control whereas SU and insulin therapy frequently causes weight gain while providing good glycemic control. It has been reported that GLP‐1 plays a physiological regulatory role in controlling appetite by enhancing satiety and suppressing food intake in humans22. Therefore, the GLP‐1 analog liraglutide could be useful for obese and overweight diabetic individuals who need to reduce fat mass12. Indeed, liraglutide has been shown to induce significant weight reduction in diabetic, as well as non‐diabetic, obese individuals in a randomized trial23. The effect on bodyweight in the present study was not very large compared with that noted in previous trials carried out in non‐Japanese individuals, in which weight reductions ranging at >2 kg have been observed20,24. This might be a result of our patients’ lower baseline BMI (25 vs 30–35 kg/m2 in non‐Japanese subjects enrolled in overseas trials), as well as a possible influence of genetic differences. Further research is required to elucidate the beneficial effects of liraglutide on reducing bodyweight in Japanese patients with diabetes.
In conclusion, once‐daily administration of liraglutide 0.9 mg for 52 weeks provides more favorable metabolic control and safety profile compared with glibenclamide. HbA1c at 52 weeks was lower in the liraglutide group than the glibenclamide group. Furthermore, sustained effects on lowering FPG, PPG and mean daily PG were observed in the liraglutide group. Whereas patients in the glibenclamide group experienced weight gain of 1 kg, those on liraglutide lost 0.8 kg in bodyweight. Liraglutide was generally safe and well tolerated; 1‐year treatment with this agent did not raise any safety concerns. Although AE related to the GI system were higher in the liraglutide group than in the glibenclamide group, especially during the first 4 weeks, the incidence of GI‐related AE was comparable between the two groups thereafter. No major hypoglycemic episodes were reported. Antibodies against liraglutide developing during this trial did not have any clinically significant impact on overall metabolic control.
Acknowledgments
The authors thank all persons who contributed to the present study. This trial was sponsored by Novo Nordisk Pharma Ltd. Kohei Kaku was the sponsor’s consultant medical expert in this trial. Yutaka Seino was the lead investigator in this trial. Mads Frederik Rasmussen is an employee of Novo Nordisk A/S in Denmark. Tomoyuki Nishida is an employee of Novo Nordisk Pharma Ltd in Japan.
References
- 1.UK Progressive Diabetes Study Group . Intensive blood‐glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in subjects with non‐insulin‐dependent diabetes mellitus: a randomised prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [DOI] [PubMed] [Google Scholar]
- 3.Elbrønd B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single‐dose of NN2211, a long‐acting glucagon‐like peptide 1 derivative, in healthy male subjects. Diabetes Care 2002; 25: 1398–1404 [DOI] [PubMed] [Google Scholar]
- 4.Agersø H, Jensen LB, Elbrønd B, et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long‐acting GLP‐1 derivative, in healthy men. Diabetologia 2002; 45: 195–202 [DOI] [PubMed] [Google Scholar]
- 5.Juhl CB, Hollingdal M, Sturis J, et al. Bedtime administration of NN2211, a long‐acting GLP‐1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 2002; 51: 424–429 [DOI] [PubMed] [Google Scholar]
- 6.Nauck M, El‐Ouaghlidi A, Hompesch M, et al. No impairment of hypoglycaemia counterregulation via glucagon with the long‐acting GLP‐1 derivative, NN2211, in subjects with type 2‐diabetes. Diabetologia 2003; 46(Suppl 2): A285 [Google Scholar]
- 7.Chang AM, Jakobsen G, Sturis J, et al. The GLP‐1 derivative NN2211 restores β‐cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 2003; 52: 1786–1791 [DOI] [PubMed] [Google Scholar]
- 8.Irie S, Matsumura Y, Zdravkovic M, et al. Tolerability, pharmacokinetics and pharmacodynamics of the once‐daily human GLP‐1 analog liraglutide in Japanese healthy subjects: a randomized, double‐blind, placebo‐controlled dose‐escalation study. Int J Clin Pharmacol Ther 2008; 46: 273–279 [DOI] [PubMed] [Google Scholar]
- 9.Seino Y, Rasmussen MF, Zdravkovic M, et al. Dose‐dependent improvement in glycemia with once‐daily liraglutide without hypoglycemia or weight gain: a double‐blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81: 161–168 [DOI] [PubMed] [Google Scholar]
- 10.Madsbad S, Schmitz O, Ranstam J, et al. Improved glycemic control with no weight increase in patients with type 2 diabetes after once‐daily treatment with the long‐acting glucagon‐like peptide 1 analog liraglutide (NN2211): a 12‐week, double‐blind, randomized, controlled trial. Diabetes Care 2004; 27: 1335–1342 [DOI] [PubMed] [Google Scholar]
- 11.Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the long‐acting glucagon‐like peptide 1 derivative liraglutide (NN2211) markedly improves 24‐h glycemia and α‐ and β‐cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004; 53: 1187–1194 [DOI] [PubMed] [Google Scholar]
- 12.Harder H, Nielsen L, Thi TDT, et al. The effect of liraglutide, a long‐acting glucagon‐like peptide 1 derivative, on glycemic control, body composition, and 24‐h energy expenditure in patients with type 2 diabetes. Diabetes Care 2004; 27: 1915–1921 [DOI] [PubMed] [Google Scholar]
- 13.Saad MF, An B, Santiago O; on behalf of the 2072 Study Group The effect of NN2211, a long‐acting GLP‐1 derivative, on glycemic control and body weight in obese patients with type 2 diabetes. Diabetologia 2002; 45(Suppl 2): A44 [Google Scholar]
- 14.Nauck M, Hompesch M, Filipczak R, et al. Liraglutide significantly improves glycemic control and reduces body weight compared with glimepiride as add‐on to metformin in type 2 diabetes. Diabetes 2004; 53(Suppl 2): A3 [Google Scholar]
- 15.Vilsbøll T, Zdravkovic M, Thi TDL, et al. Liraglutide significantly improves glycaemic control, and lowers body weight without risk of either major or minor hypoglycaemic episodes in subjects with type 2 diabetes. Diabetes 2006; 55(Suppl 1): A27 [Google Scholar]
- 16.Seino Y, Rasmussen MF, Nishida T, et al. Efficacy and safety of the once‐daily human GLP‐1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin 2010; 26: 1013–1022 [DOI] [PubMed] [Google Scholar]
- 17.Knudsen LB, Madsen LW, Andersen S, et al. Glucagon‐like peptide‐1 receptor agonists activate rodent thyroid C‐cells causing calcitonin release and C‐cell proliferation. Endocrinology 2010; 151: 1473–1486 [DOI] [PubMed] [Google Scholar]
- 18.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003; 26: 1902–1912 [DOI] [PubMed] [Google Scholar]
- 19.Kageyama S, Hirao K, Shimizu A, et al. Tolerability, pharmacokinetics, and pharmacodynamics of liraglutide, long acting human GLP‐1 analogue. Endocrinol Diabetol 2007; 24: 95–104 [Google Scholar]
- 20.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481 [DOI] [PubMed] [Google Scholar]
- 21.Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010; 362: 774–777 [DOI] [PubMed] [Google Scholar]
- 22.Flint A, Raben A, Astrup A, et al. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet 2009; 374: 1606–1616 [DOI] [PubMed] [Google Scholar]
- 24.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes. Diabetes Care 2009; 32: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]


