Abstract
Aims/Introduction: We assessed the long‐term (52 weeks) safety and efficacy of exenatide b.i.d. in Japanese patients with type 2 diabetes and suboptimal glycemic control.
Materials and Methods: Participants completing a 24‐week randomized controlled trial of exenatide (5 μg, 10 μg or placebo b.i.d.) were invited to continue in a 28‐week open‐label extension study (5 μg, n = 64; 10 μg, n = 53). Safety measures included treatment‐emergent adverse events (TEAE). Efficacy measures included change from baseline in glycosylated hemoglobin A1c (HbA1c) levels, proportion of participants achieving HbA1c target levels, fasting and seven‐point, self‐monitored blood glucose concentrations (SMBG), 1,5‐anhydroglucitol concentrations, and bodyweight.
Results: A total of 60 and 49 participants in the exenatide 5 and 10 μg groups completed the study. The 52‐week incidence of TEAE considered by investigators as related to the study drug was 80.6% (58/72) and 88.9% (64/72) in the exenatide 5 and 10 μg groups, respectively. Mild hypoglycemia and nausea were the most common TEAE. Most TEAE occurred during the first 24 weeks. Eight participants experienced serious adverse events. Exenatide treatment was associated with sustained decreases in HbA1c values, with 33.3–47.9% of participants achieving <6.9% HbA1c, sustained decreases in fasting plasma glucose concentrations and SMBG, and sustained increases in 1,5‐anhydroglucitol concentrations. Exenatide 10 μg was associated with sustained weight loss.
Conclusions: Long‐term exenatide treatment had a similar safety profile to that observed previously and was efficacious in improving glycemic control in Japanese patients with suboptimally controlled type 2 diabetes. This trial was registered with ClinicalTrials.gov (no. NCT00577824). (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00137.x, 2011)
Keywords: Exenatide, Japan, Type 2 diabetes mellitus
Introduction
The prevalence of type 2 diabetes mellitus in Japan is increasing1–3. Indeed, findings from a recent survey carried out by the Japanese Health Service Bureau suggested that approximately 8.9 million Japanese have glycosylated hemoglobin A1c (HbA1c) values ≥6.5% or are taking glucose‐lowering medication and are therefore highly likely to have diabetes4. The same survey also suggested that approximately 21.1 million Japanese have HbA1c values between 6.0% and 6.5%, and therefore may have diabetes4. Unfortunately, currently available treatments for type 2 diabetes in Japan, including insulin, sulfonylurea (SU), biguanide (BG) and thiazolidinedione (TZD), do not always provide adequate glycemic control5–7, and can have adverse side‐effects, such as hypoglycemia and weight gain8,9. Given the increasing prevalence of type 2 diabetes in Japan and the risks associated with current treatment, there is a need for new therapies that provide adequate glycemic control.
Exenatide is a glucagon‐like peptide‐1 receptor agonist that has been shown to improve glycemic control, decrease bodyweight and improve β‐cell function in patients with type 2 diabetes from Western countries10–15. Consequently, exenatide b.i.d. has been approved in the USA and Europe for use as adjunct therapy, with diet and exercise, for patients with type 2 diabetes who have not achieved adequate glycemic control with metformin (Met), SU or a combination of Met and SU. Exenatide has also been approved in the USA for use as monotherapy adjunct to diet and exercise, and as adjunct therapy with TZD or combined Met and TZD. We have recently reported the findings of the first phase III, double‐blind, randomized controlled trial of exenatide b.i.d. in Japan16. After 24 weeks of adjunct treatment with exenatide, we found that participants with type 2 diabetes and suboptimal glycemic control had improved glycemic control and, at a 10‐μg b.i.d. dose, decreased bodyweight. Exenatide also had a favorable safety profile and was generally well tolerated. In October 2010, exenatide b.i.d. was approved in Japan as an adjunct therapy for patients with type 2 diabetes who had not achieved adequate glycemic control with SU, alone or in combination with BG or TZD.
The purpose of the present extension study was to determine the long‐term (52 weeks) safety and efficacy of adjunct exenatide treatment in Japanese patients with type 2 diabetes and suboptimal glycemic control.
Materials and Methods
Study Design
The present study was a 28‐week, open‐label extension study carried out at 23 centers in Japan. Participants were enrolled immediately after completing a 24‐week, double‐blind, randomized controlled trial (ClinicalTrials.gov registration number NCT00577824)16. In the 24‐week trial, a total of 181 participants were randomized (1:2:2) to receive placebo, exenatide 5 μg or exenatide 10 μg b.i.d. Participants in the placebo group were further randomized (before starting the 24‐week trial) to receive exenatide 5 μg or exenatide 10 μg during the extension study. Throughout the 52‐week study, participants were instructed to inject exenatide (Eli Lilly and Company, Indianapolis, IN, USA) or placebo 15 min before both the morning and evening meals; however, injections made between 0 and 60 min before meals were allowed.
The study was approved by the Institutional Review Board of each participating center and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All study participants provided written informed consent.
Participants
The main study inclusion criteria at the time of enrolment in the initial study16 were as follows: a diagnosis of type 2 diabetes mellitus according to the Japan Diabetes Society (JDS)17 and the World Health Organization18 definitions; currently on SU monotherapy, SU and BG combination therapy, or SU and TZD combination therapy without any change in dose within 90 days of enrolment; suboptimal glycemic control as defined by the JDS17 (i.e. HbA1c value ≥ 7.4% and ≤10.4%); age between 20 and 75 years; and bodyweight ≥ 50 kg. Patients taking α‐glucosidase inhibitors or short‐acting insulin secretion inducers were eligible for inclusion, but stopped taking these drugs before beginning the study.
The main study exclusion criteria at the time of enrolment in the initial study16 were as follows: treatment with exogenous insulin or any drug affecting gastrointestinal motility within 90 days of enrolment; currently on triple therapy (i.e. SU, BG and TZD); any clinically significant gastrointestinal or hepatic disorder; suspected malignant tumor or a history of malignant tumor within 5 years of enrolment; serum creatinine ≥ 1.5 mg/dL in men or ≥1.4 mg/dL in women; systolic blood pressure (sitting) ≥ 160 mmHg or diastolic blood pressure (sitting) ≥ 100 mmHg; fasting plasma glucose ≥ 250 mg/dL or casual blood glucose ≥ 350 mg/dL or at least one episode of severe hypoglycemia within 90 days of enrolment; and women who were breastfeeding, pregnant, intending to become pregnant during the study, not practicing a reliable method of contraception within 90 days of enrolment or unable to practice a reliable method of contraception during the study.
Interventions
From week 24 to week 28 (i.e. the first 4 weeks of the extension study), all participants received exenatide 5 μg, b.i.d. Participants who received exenatide (5 μg or 10 μg) in the 24‐week trial received the same dose of exenatide from week 28 to week 52 (exenatide 5 μg and exenatide 10 μg groups, respectively). Participants who received placebo in the 24‐week trial received exenatide 5 μg (placebo/exenatide 5 μg group) or exenatide 10 μg (placebo/exenatide 10 μg group) from week 28 to week 52.
Baseline clinical characteristics of participants in the exenatide groups were determined at week 0 and included all participants who received at least one dose of exenatide in the 24‐week trial. Baseline clinical characteristics of participants in the placebo/exenatide groups were determined at week 24. Follow‐up assessments were made every 4 weeks.
Efficacy Outcomes
The following efficacy outcomes were recorded: change from baseline in HbA1c values; the proportion of participants who achieved an HbA1c value < 7.4% and <6.9% at week 52; fasting plasma glucose concentrations; seven‐point, self‐monitored blood glucose concentrations (SMBG; measured before breakfast, before lunch, before dinner, 2 h after starting each meal and before bedtime); bodyweight; serum lipid concentrations (total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and triglyceride); and glycemic control, as indicated by 1,5‐anhydroglucitol concentrations. Please note that all HbA1c values in the present study are expressed as National Glycohemoglobin Standardization Program equivalent values (i.e. JDS HbA1c value + 0.4%)17. We used the JDS definition of good glycemic control (i.e. HbA1c value < 6.9%)17.
All laboratory tests were carried out using standard methods at a central laboratory (Bio Medical Laboratories Inc., Tokyo, Japan).
Safety Outcomes
Treatment‐Emergent Adverse Events Treatment‐emergent adverse events (TEAE) were coded and summarized using the Medical Dictionary for Regulatory Activities (version 12.0) and were defined as events that occurred during the treatment period or worsened from baseline. Only common TEAE, defined as TEAE with an incidence >10% in the exenatide 10 μg group during the 52‐week study, and serious adverse events (SAE) are described.
Hypoglycemia Hypoglycemia was defined as the presence of signs or symptoms of hypoglycemia, regardless of blood glucose concentration. Decreased blood glucose was defined as a blood glucose concentration < 70 mg/mL in the absence of signs or symptoms of hypoglycemia.
Exenatide Antibody Status Participants were considered to have treatment‐emergent exenatide antibodies if antibodies were undetectable at baseline and detectable (a titer ≥ 25) at any subsequent visit. Participants were also considered to have treatment‐emergent exenatide antibodies if antibodies were detectable at baseline and the titer increased by three dilution factors at any subsequent visit. Exenatide antibody concentrations were measured by Millipore Corporation (St. Charles, MO, USA) using a solid‐phase enzyme‐linked immunosorbent assay10.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as frequency and percentage.
Results
Participant Disposition
Of the 152 participants who completed the 24‐week trial, 150 were enrolled in the 28‐week extension study, and 137 completed the extension study (Figure 1). A total of 13 participants discontinued the extension study. The most common reason for discontinuation was adverse events (8/13 participants).
Figure 1.
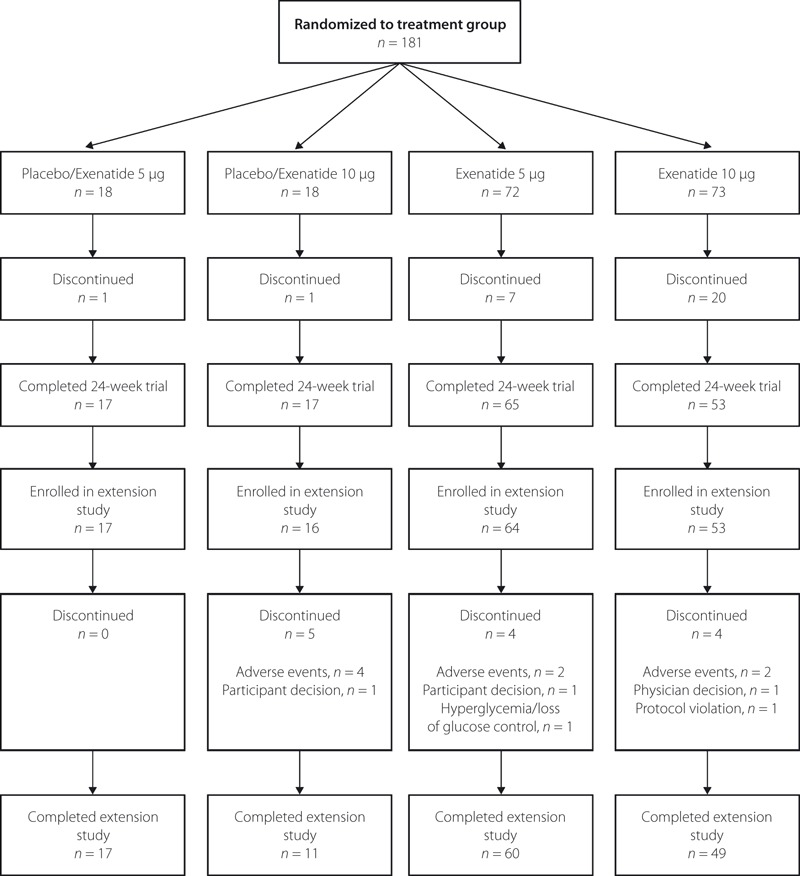
Participant flow diagram.
Baseline Clinical Characteristics
Participants in the different groups had similar baseline clinical characteristics (Table 1). The proportions of participants taking different combinations of oral anti‐diabetic drugs were similar between the groups.
Table 1. Baseline characteristics of Japanese participants with type 2 diabetes mellitus and suboptimal glycemic control.
| Characteristic | Placebo/exenatide 5 μg (n = 17)* | Placebo/exenatide 10 μg (n = 16)* | Exenatide 5 μg (n = 72)† | Exenatide 10 μg (n = 72)† |
|---|---|---|---|---|
| Men, n (%) | 12 (71) | 10 (63) | 49 (68) | 49 (68) |
| Age (years) | 54 ± 12 | 59 ± 11 | 59 ± 9 | 59 ± 10 |
| Weight (kg) | 67 ± 10‡ | 72 ± 17‡ | 67 ± 11 | 69 ± 11 |
| BMI (kg/m2) | 24.6 ± 3.2‡ | 26.4 ± 5.2‡ | 25.0 ± 4.1 | 25.8 ± 3.9 |
| Duration of type 2 diabetes (years) | 11.7 ± 5.5 | 12.7 ± 7.8 | 12.2 ± 6.3 | 11.6 ± 7.0 |
| HbA1c (%) | 8.6 ± 0.9‡ | 8.1 ± 1.2‡ | 8.7 ± 0.8 | 8.6 ± 1.0 |
| Fasting plasma glucose (mg/dL) | 161 ± 35‡ | 147 ± 29‡ | 164 ± 42 | 164 ± 39 |
| Total cholesterol (mg/dL) | 195 ± 27‡ | 192 ± 19‡ | 204 ± 36 | 202 ± 31 |
| HDL cholesterol (mg/dL) | 52 ± 10‡ | 57 ± 13‡ | 57 ± 15 | 55 ± 11 |
| LDL cholesterol (mg/dL) | 120 ± 27‡ | 115 ± 19‡ | 124 ± 28 | 125 ± 27 |
| Triglycerides (mg/dL) | 140 ± 73‡ | 106 ± 49‡ | 133 ± 95 | 131 ± 70 |
| Oral anti‐diabetic agents§ | ||||
| SU alone, n (%) | 1 (5.9) | 1 (6.3) | 4 (5.6) | 8 (11.1) |
| SU + α‐GI, n (%) | 1 (5.9) | 2 (12.5) | 1 (1.4) | 4 (5.6) |
| SU + BG, n (%) | 7 (41.2) | 7 (43.8) | 33 (45.8) | 27 (37.5) |
| SU + BG + α‐GI, n (%) | 4 (23.5) | 5 (31.3) | 22 (30.6) | 13 (18.1) |
| SU + BG + meglitinide derivative, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| SU + TZD, n (%) | 3 (17.6) | 0 (0.0) | 6 (8.3) | 12 (16.7) |
| SU + TZD + α‐GI, n (%) | 1 (5.9) | 1 (6.3) | 6 (8.3) | 7 (9.7) |
Values are mean ± standard deviation, except where indicated.
*Includes participants who were enrolled in the 28‐week extension study.
†Includes participants who were enrolled in the initial 24‐week trial and received at least one dose of exenatide.
‡These values were determined at the beginning of the 28‐week extension study.
§Taken at the time of providing informed consent.
α‐GI, α‐glucosidase inhibitors; BMI, body mass index; BG, biguanide; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SU, sulfonylurea; TZD, thiazolidinedione.
Efficacy Outcomes
Exenatide treatment was associated with sustained decreases in HbA1c values (Figure 2a). In the exenatide 5 μg and exenatide 10 μg groups, the decreases in HbA1c values that occurred during the first 24 weeks of treatment were generally maintained from week 24 to week 52. In the placebo/exenatide 5 μg and placebo/exenatide 10 μg groups, HbA1c values were decreased from baseline (week 24) to week 52. Decreases from baseline in HbA1c values were greater in the exenatide 10 μg and placebo/exenatide 10 μg groups than in the exenatide 5 μg and placebo/exenatide 5 μg groups.
Figure 2.
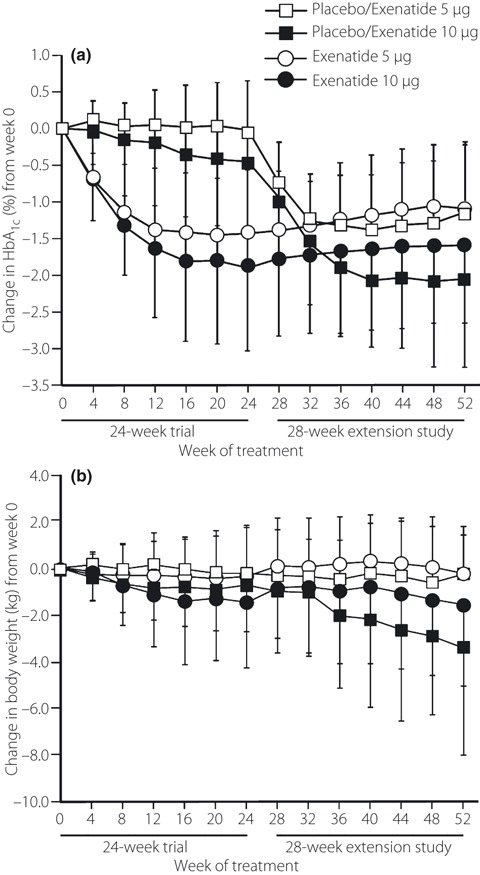
Change from week 0 in (a) glycosylated hemoglobin A1c (HbA1c) values and (b) bodyweight after treatment with exenatide (5 or 10 μg) b.i.d. Participants in the placebo/exenatide groups received placebo for the first 24 weeks and exenatide for the following 28 weeks. Participants in the exenatide groups received exenatide for the entire 52 weeks. Values are means, with standard deviations shown as upward (placebo/exenatide 5 μg [n = 16–18] and exenatide 5 μg [n = 60–72] groups) or downward (placebo/exenatide 10 μg [n = 11–17] and exenatide 10 μg [n = 48–72] groups) error bars. Note: data were not available for all participants.
A greater proportion of participants in the exenatide 10 μg and placebo/exenatide 10 μg groups achieved HbA1c target levels than participants in the exenatide 5 μg and placebo/exenatide 5 μg groups. Among participants who received exenatide for 52 weeks, 32 of 45 (71.1%) and 30 of 59 (50.8%) achieved an HbA1c level of <7.4% in the exenatide 10 μg and exenatide 5 μg groups, respectively. Similarly, 8 of 11 (72.7%) participants in the placebo/exenatide 10 μg group achieved an HbA1c level of <7.4% compared with 8 of 17 (47.1%) participants in the placebo/exenatide 5 μg group. Among participants who received exenatide for 52 weeks, 23 of 48 (47.9%) and 20 of 60 (33.3%) participants achieved an HbA1c level of <6.9% in the exenatide 10 μg and exenatide 5 μg groups, respectively. Similarly, 7 of 11 (63.6%) participants in the placebo/exenatide 10 μg group achieved an HbA1c level of <6.9% compared with 3 of 17 (17.6%) participants in the placebo/exenatide 5 μg group.
Fasting plasma glucose concentrations decreased from baseline in all groups after the start of exenatide treatment. The decrease in fasting plasma glucose concentration from baseline in participants in the exenatide 5 μg group and the exenatide 10 μg group after 24 weeks of treatment was maintained during the extension study. The mean (±SD) change from baseline in fasting plasma glucose concentration in the exenatide 5 μg group was −29.6 ± 37.1 mg/dL at week 24 and −16.4 ± 41.4 mg/dL at week 52. The mean (±SD) change from baseline in fasting plasma glucose concentration in the exenatide 10 μg group was −36.0 ± 36.3 mg/dL at week 24 and −29.8 ± 39.0 mg/dL at week 52. The mean (±SD) change from week 0 in fasting plasma glucose concentration in the placebo/exenatide 5 μg group was −8.2 ± 23.3 mg/dL at week 24 (baseline for this group) and −19.1 ± 36.9 mg/dL at week 52. The mean (±SD) change from week 0 in fasting plasma glucose concentration in the placebo/exenatide 10 μg group was −3.2 ± 27.1 mg/dL at week 24 (baseline for this group) and −31.6 ± 27.0 mg/dL at week 52.
Seven‐point, SMBG were decreased in all treatment groups at week 52 compared with baseline (Figure 3). Decreases from baseline were most pronounced at the after breakfast and after dinner time‐points.
Figure 3.

Changes in seven‐point, self‐monitored blood glucose concentrations after treatment with exenatide (5 or 10 μg) b.i.d. Plasma glucose concentrations were measured before breakfast, before lunch, before dinner, 2 h after starting each meal and before bedtime. Participants in the placebo/exenatide groups received placebo for the first 24 weeks and exenatide for the following 28 weeks. Participants in the exenatide groups received exenatide for the entire 52 weeks. Values are mean ± standard deviation (n = 16–18 in the placebo/exenatide 5 μg group; n = 11–17 in the placebo/exenatide 10 μg group; n = 59–72 in the exenatide 5 μg group; n = 46–72 in the exenatide 10 μg group). Note: data were not available for all participants.
Bodyweight decreased from baseline in the exenatide 10 μg group and in the placebo/exenatide 10 μg group, but did not change in either the exenatide 5 μg group or the placebo/exenatide 5 μg group (Figure 2b). The bodyweight decrease from baseline in participants in the exenatide 10 μg group after 24 weeks of treatment was maintained during the extension study.
Plasma total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and triglyceride concentrations were generally decreased from baseline or similar to baseline in the exenatide 5 μg and exenatide 10 μg groups at weeks 24 and 52, and in the placebo/exenatide 5 μg and placebo/exenatide 10 μg groups at week 52 (data not shown). Plasma concentrations of all lipids were within the normal range in all groups during the entire study.
Exenatide treatment was associated with sustained increases from baseline in 1,5‐anhydroglucitol concentrations. The mean (±SD) increases from baseline (week 0) in 1,5‐anhydroglucitol concentrations were 5.7 ± 4.4 μg/mL and 5.5 ± 4.4 μg/mL at week 24, and 3.3 ± 3.7 μg/mL and 4.5 ± 4.1 μg/mL at week 52 in the exenatide 5 μg group and the exenatide 10 μg group, respectively. The mean (±SD) increases from baseline (week 24) in 1,5‐anhydroglucitol concentrations were 2.7 ± 3.5 μg/mL and 6.7 ± 4.9 μg/mL at week 52 in the placebo/exenatide 5 μg group and the placebo/exenatide 10 μg group, respectively.
Safety Outcomes
The majority of common TEAE in the exenatide 5 μg and exenatide 10 μg groups occurred during the first 24 weeks, as shown by similar incidence rates between the first 24 weeks and the entire 52 weeks (Table 2). Mild hypoglycemia and nausea were the most common TEAE. With the exception of six episodes of moderate hypoglycemia in two patients in the exenatide 10 μg group, all episodes of hypoglycemia were mild in severity. In all groups, the incidences of hypoglycemia and nausea were highest within the first 4–8 weeks after starting exenatide treatment. Few participants who were randomized to receive exenatide for 52 weeks experienced hypoglycemia (5/144 participants) or nausea (1/144 participants) for the first time during the extension study. No TEAE specifically occurred during the extension study only (data not shown).
Table 2. Common treatment‐emergent adverse events during the 24‐week, double‐blind study (24 weeks: placebo and exenatide groups), the extension study (28 weeks: placebo/exenatide groups only) and the entire study (52 weeks cumulative: exenatide groups only).
| Treatment‐emergent adverse event* | 24 Weeks | 28 Weeks | 24 Weeks | 52 Weeks | |||
|---|---|---|---|---|---|---|---|
| Placebo (n = 35) | Placebo/exenatide 5 μg† (n = 17) | Placebo/exenatide 10 μg† (n = 16) | Exenatide 5 μg (n = 72) | Exenatide 10 μg (n = 72) | Exenatide 5 μg (n = 72) | Exenatide 10 μg (n = 72) | |
| Hypoglycemia, n (%) | 8 (22.9) | 5 (29.4) | 12 (75.0) | 37 (51.4) | 42 (58.3) | 40 (55.6) | 44 (61.1) |
| Nausea, n (%) | 3 (8.6) | 6 (35.3) | 8 (50.0) | 18 (25.0) | 26 (36.1) | 18 (25.0) | 27 (37.5) |
| Blood glucose decreased, n (%) | 4 (11.4) | 4 (23.5) | 4 (25.0) | 10 (13.9) | 18 (25.0) | 14 (19.4) | 24 (33.3) |
| Nasopharyngitis, n (%) | 8 (22.9) | 4 (23.5) | 3 (18.8) | 8 (11.1) | 9 (12.5) | 24 (33.3) | 22 (30.6) |
| Vomiting, n (%) | 1 (2.9) | 1 (5.9) | 4 (25.0) | 3 (4.2) | 12 (16.7) | 4 (5.6) | 15 (20.8) |
| Constipation, n (%) | 1 (2.9) | 1 (5.9) | 1 (6.3) | 10 (13.9) | 11 (15.3) | 12 (16.7) | 13 (18.1) |
| Abdominal discomfort, n (%) | 1 (2.9) | 1 (5.9) | 2 (12.5) | 7 (9.7) | 9 (12.5) | 10 (13.9) | 12 (16.7) |
| Decreased appetite, n (%) | 1 (2.9) | 0 (0) | 1 (6.3) | 7 (9.7) | 9 (12.5) | 7 (9.7) | 9 (12.5) |
| Anorexia, n (%) | 1 (2.9) | 0 (0) | 3 (18.0) | 2 (2.8) | 8 (11.1) | 2 (2.8) | 8 (11.1) |
*Treatment‐emergent adverse events with an incidence >10% in the exenatide 10 μg group during the first 24 weeks are shown.
†These participants received placebo for the first 24 weeks and exenatide for the following 28 weeks.
For the entire 52 weeks, the incidence of TEAE considered by the investigators to be related to the study drug was 80.6% (58/72 participants) in the exenatide 5 μg group and 88.9% (64/72 participants) in the exenatide 10 μg group.
A total of eight participants experienced SAE during the extension study.
One participant in the placebo/exenatide 10 μg group and one participant in the exenatide 5 μg group experienced pancreatitis. Both participants discontinued the study and recovered uneventfully. Both participants had gallstones, a known cause of pancreatitis; however, the investigators could not rule out a relationship between exenatide treatment and pancreatitis.
One participant in the exenatide 5 μg group experienced pneumonia, which was considered to be unrelated to the study procedures. However, the investigators could not rule out a relationship between pneumonia and exenatide treatment.
Three participants experienced two different SAE, including cerebral infarction and hemiplegia (placebo/exenatide 5 μg group, n = 1), cerebral circulatory failure and calculus ureteric (exenatide 5 μg group, n = 1), and diabetic retinopathy and retinal vein occlusion (exenatide 10 μg group, n = 1). Other SAE during the extension study included brain stem infarction (exenatide 10 μg group, n = 1) and large intestine carcinoma (exenatide 10 μg group, n = 1). All of these SAE were considered by the study investigators to be unrelated to exenatide treatment and the study procedures.
There were no increases between week 24 and week 52 in the proportion of participants in the exenatide 5 μg and exenatide 10 μg groups who tested positive for the exenatide antibody. The proportion of participants who tested positive for the exenatide antibody at week 24 was 63.1% (41/65) and 60.4% (32/53) in the exenatide 5 μg and exenatide 10 μg groups, respectively. Corresponding proportions at week 52 were 63.3% (38/60) and 61.2% (30/49).
Exenatide antibody status did not appear to influence changes in HbA1c values or the incidence of TEAE, including hypoglycemia and nausea (data not shown).
Discussion
This is the first study to examine the long‐term (52 weeks) safety and efficacy of exenatide b.i.d. in Japanese participants with type 2 diabetes and suboptimal glycemic control. Importantly, we observed a very low incidence of common TEAE during the second 28 weeks of treatment. We also found that the efficacy of exenatide (as shown by improved glycemic control) previously observed after 24 weeks of treatment16 was maintained after 52 weeks of treatment. Our findings suggest that long‐term exenatide has a similar safety profile to that observed in previous studies and is efficacious for improving glycemic control in Japanese patients with suboptimally controlled type 2 diabetes.
We found that long‐term treatment with exenatide was associated with generally mild severity TEAE, a low incidence of TEAE in the extension study and a low proportion of participants who discontinued in the extension study because of TEAE. In keeping with the findings of previous studies of Caucasian participants with type 2 diabetes11–14,19,20, we found that hypoglycemia and nausea were the most common TEAE associated with exenatide treatment and that the severity of these TEAE was generally mild to moderate. Also consistent with previous findings was our finding that TEAE were generally more common during the earlier stages of treatment than during the later stages of treatment11–13,19. Further, more than 75% of participants who received exenatide completed the 52‐week study. Similar completion rates have been reported in long‐term studies of exenatide involving Caucasian participants with type 2 diabetes14,21,22.
Two participants in the present study experienced pancreatitis during the treatment period. Although a relationship between exenatide treatment and pancreatitis could not be ruled out by the study investigators in either case, both participants had gallstones, a known cause of pancreatitis. Furthermore, type 2 diabetes is also a known risk factor for acute pancreatitis23–25. Several recent studies found that exenatide treatment was not associated with an increased risk of pancreatitis compared with other anti‐diabetic treatments24,26,27. Nevertheless, the prescribing information for exenatide acknowledges that postmarketing cases of acute pancreatitis have been reported28.
We found that long‐term exenatide treatment was associated with sustained improvement in glycemic control, as shown by decreased HbA1c values from baseline, an increased proportion of participants achieving target HbA1c values, decreased fasting plasma glucose concentrations and SMBG, and increased 1,5‐anhydroglucitol concentrations. Other researchers have also consistently reported that long‐term (1–3 years) exenatide treatment was associated with sustained improvement in glycemic control in Caucasian participants with type 2 diabetes14,19–22. The present findings suggest that exenatide treatment is effective for long‐term glycemic control in Japanese patients with type 2 diabetes.
Similar to previous long‐term studies of Caucasian patients with type 2 diabetes and suboptimal glycemic control14,19–22,29, we found that exenatide 10 μg treatment was associated with sustained weight loss in Japanese participants with type 2 diabetes and suboptimal glycemic control. Our finding that exenatide 10 μg was associated with sustained weight loss is important given that bodyweight is known to influence glycemic control30. Also worth noting is our finding that participants receiving exenatide 10 μg lost weight despite continuing to take oral anti‐diabetics, which are known to cause weight gain8,9. Interestingly, we found that exenatide 5 μg treatment was not associated with weight loss. Variable effects of exenatide 5 μg on weight loss have been reported in previous, shorter duration studies involving Caucasian participants with type 2 diabetes11,13. These different findings may reflect differences in the mean baseline body mass index of the study participants. Indeed, DeFronzo et al.12 reported that only participants with an initial body mass index ≥ 30 kg/m2 had significant weight loss after 30 weeks of exenatide 5 μg treatment, whereas all participant groups in the present study had a mean baseline body mass index < 30 kg/m2.
The strengths of the present study include the multicenter design, the duration of exenatide treatment, the assessment of multiple indicators of glycemic control and the high participant retention rate during the extension study. The present study, however, does have several limitations that must be acknowledged, including the absence of a group who received placebo for 52 weeks and the lack of control of participant dietary and exercise habits. We did, however, include a placebo group for the initial 24‐week trial in which pronounced differences were observed between the placebo and exenatide treatment groups16. Although different dietary and exercise habits might have confounded our results, we have no reason to believe that these habits would have been markedly different between groups. Furthermore, dietary and exercise habits are rarely strictly controlled in ‘real world’ clinical settings; hence, our demonstration of efficacy and weight loss (in the exenatide 10 μg group) despite this lack of control is important. Finally, we acknowledge that monitoring beyond 52 weeks is necessary to evaluate the continued efficacy of exenatide in Japanese patients with type 2 diabetes.
In conclusion, our findings from this open‐label extension study show that long‐term (52 weeks) treatment with exenatide b.i.d. has a safety profile similar to that observed previously and is associated with sustained improvement in glycemic control in Japanese patients with type 2 diabetes. Taken together, our findings suggest that exenatide may be a viable treatment option for Japanese patients with type 2 diabetes and suboptimal glycemic control.
Acknowledgements
The present study was sponsored by Eli Lilly Japan K.K. with support from Amylin Pharmaceuticals, Inc. and Eli Lilly and Co. The sponsor of this study did not impose any impediment, directly or indirectly, on the publication of the study’s results. The authors acknowledge the independent medical writing assistance provided by Luke Carey and Rebecca Lew of ProScribe Medical Communications (http://www.proscribe.com.au), funded by an unrestricted financial grant from Eli Lilly Japan K.K. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2). In collaboration with the authors, Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis and preparation of the manuscript. AY, HS and TI are employees of, and own shares in, Eli Lilly Japan K.K. NI has been a member of advisory boards and has received consulting fees and financial support/grants from Novo Nordisk, Novartis, Banyu Pharmaceutical Company, Ltd and Takeda Pharmaceutical Co., Ltd. NI has received financial support/grants from Eli Lilly and Company. KU has no conflicts of interest to declare.
References
- 1.Ministry of Health, Labor, and Welfare . National Survey of Diabetes 1997. Ministry of Health, Labor, and Welfare, Tokyo, Japan, 1998. Available at: http://www.mhlw.go.jp/toukei/kouhyo/indexkk_4_1.html(Last accessed on 2010, October 15). (Japanese). [Google Scholar]
- 2.Kawamori R. Diabetes trends in Japan. Diabetes Metab Res Rev 2002; 18(Suppl. 3): S9–S13 [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health, Labor, and Welfare . National Survey of Diabetes 2002. Ministry of Health, Labor, and Welfare, Tokyo, Japan, 2003. Available at: http://www.mhlw.go.jp/toukei/kouhyo/indexkk_4_2.html(last accessed on 2010, October 15). (Japanese). [Google Scholar]
- 4.Ministry of Health, Labor, and Welfare . Outline of the National Health and Nutrition Survey. Ministry of Health, Labor, and Welfare, Tokyo, Japan, 2007. Available at http://www.mhlw.go.jp/houdou/2008/12/h1225‐5.html(last accessed on 2010, October 15). (Japanese). [Google Scholar]
- 5.Harris MI, Eastman RC, Cowie CC, et al. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care 1999; 22: 403–408 [DOI] [PubMed] [Google Scholar]
- 6.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 291: 335–342 [DOI] [PubMed] [Google Scholar]
- 7.Neville SE, Boye KS, Montgomery WS, et al. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25: 705–716 [DOI] [PubMed] [Google Scholar]
- 8.Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ 2005; 172: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergenstal RM, Bailey CJ, Kendall DM. Type 2 diabetes: assessing the relative risks and benefits of glucose‐lowering medications. Am J Med 2010; 123: 374 e9–18. [DOI] [PubMed] [Google Scholar]
- 10.Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin‐4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 2003; 26: 2370–2377 [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in sulfonylurea‐treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628–2635 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin‐4) on glycemic control and weight over 30 weeks in metformin‐treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092–1100 [DOI] [PubMed] [Google Scholar]
- 13.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 14.Nauck MA, Duran S, Kim D, et al. A comparison of twice‐daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non‐inferiority study. Diabetologia 2007; 50: 259–267 [DOI] [PubMed] [Google Scholar]
- 15.Zinman B, Hoogwerf BJ, Duran Garcia S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146: 477–485 [DOI] [PubMed] [Google Scholar]
- 16.Kadowaki T, Namba M, Imaoka T, et al. Improved glycemic control and reduced body weight with exenatide: a double‐blind, randomized, phase 3 study in Japanese patients with suboptimally controlled type 2 diabetes over 24 weeks. J Diabetes Invest 2011; 2: 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: A Report of the World Health Organization and International Diabetes Federation. WHO Press, Geneva, Switzerland, 2006. [Google Scholar]
- 19.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8: 436–447 [DOI] [PubMed] [Google Scholar]
- 20.Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open‐label, uncontrolled extension of three double‐blind, placebo‐controlled trials. Clin Ther 2007; 29: 139–153 [DOI] [PubMed] [Google Scholar]
- 21.Bunck MC, Diamant M, Corner A, et al. One‐year treatment with exenatide improves beta‐cell function, compared with insulin glargine, in metformin‐treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32: 762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysschaert M, Preumont V, Oriot PR, et al. One‐year metabolic outcomes in patients with type 2 diabetes treated with exenatide in routine practice. Diabetes Metab 2010; 36: 381–388 [DOI] [PubMed] [Google Scholar]
- 23.Noel RA, Braun DK, Patterson RE. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009; 32: 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 2010; 33: 2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 2010; 12: 766–771 [DOI] [PubMed] [Google Scholar]
- 26.Dore DD, Seegar JD, Chan KA. Use of a claims‐based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009; 25: 1019–1027 [DOI] [PubMed] [Google Scholar]
- 27.Dore DD, Bloomgren GL, Wenten M, et al. A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab 2011; 13: 559–566 [DOI] [PubMed] [Google Scholar]
- 28.Eli Lilly Japan K.K. Byetta [package insert]. Eli Lilly Japan K.K., Kobe, Japan, 2010. [Google Scholar]
- 29.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24: 275–286 [DOI] [PubMed] [Google Scholar]
- 30.Maggio CA, Pi‐Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am 2003; 32: 805–822 [DOI] [PubMed] [Google Scholar]


