Abstract
Monotherapy of α‐glucosidase inhibitor (α‐GI) or dipeptidyl peptidase 4 (DPP4) inhibitor does not sufficiently minimize glucose fluctuations in the diabetic state. In the present study, we evaluated the combined effects of various of α‐GI inhibitors (acarbose, voglibose or miglitol) and sitagliptin, a DPP4 inhibitor, on blood glucose fluctuation, insulin and active glucagon‐like peptide‐1 (GLP‐1) levels after nutriment loading in mice. Miglitol and sitagliptin elicited a 47% reduction (P < 0.05) of the area under the curve of blood glucose levels for up to 2 h after maltose‐loading, a 60% reduction (P < 0.05) in the range of blood glucose fluctuation, and a 32% decrease in plasma insulin compared with the control group. All three of the combinations elicited a 2.5–4.9‐fold synergistic increase in active GLP‐1 (P < 0.05 vs control). Thus, combined treatment with the α‐GI miglitol, which more strongly inhibits the early phase of postprandial hyperglycemia, and DPP4 inhibitor yields both complementary and synergistic effects, and might represent a superior anti‐hyperglycemic therapy. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00081.x, 2011)
Keywords: Postprandial hyperglycemia, Insulin, Glucagon‐like peptide‐1
Introduction
Although active intervention for postprandial hyperglycemia by acarbose, an α‐glucosidase inhibitor (α‐GI), prevents development of cardiovascular events1, it is also important to flatten the postprandial glucose fluctuation to prevent macroangiopathy. α‐GI not only inhibits the rapid elevation of postprandial blood glucose level without excessive insulin secretion, but also enhances active glucagon‐like peptide‐1 (GLP‐1) secretion2. In contrast, the dipeptidyl peptidase 4 (DPP4) inhibitor, sitagliptin, increases insulin secretion and reduces late‐phase elevation of postprandial blood glucose level. We hypothesized that a combination of α‐GI and sitagliptin might yield a greater minimizing effect on blood glucose fluctuation while conserving insulin secretion and enhancing active GLP‐1 secretion to prevent atherosclerosis3–5. Recently, the combined effects of voglibose, an α‐GI, and a DPP4 inhibitor on plasma insulin and active GLP‐1 levels were reported in mice6,7. However, comparison of the combined effect of various α‐GIs and sitagliptin on blood glucose fluctuation has not been reported.
In the present study, we showed that combination therapy of α‐GIs and sitagliptin can yield complementary and synergistic beneficial effects in mice.
Materials and methods
Because α‐GIs are inhibitors of α‐glucosidase, which converts disaccharide to glucose, 7–9‐week‐old male C57BL/6J mice (Charles River Japan, Tokyo, Japan) were subjected to an overnight fast and orally loaded with 2.5 g/kg of maltose. Blood was collected from the end of the tail just before loading until 2 h after loading, and blood glucose level was measured using the glucose dehydrogenase method. The area under the curve of blood glucose levels for up to 2 h after maltose‐loading (ΔAUC0–2 h) was calculated using the trapezoid method. The range of blood glucose fluctuation was determined as the difference between the maximal and minimal blood glucose levels for up to 2 h.
To evaluate initial insulin‐secreting capacity, plasma insulin concentration in blood collected from the end of the tail at 0.25 h after loading was measured using an ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan).
Ten‐week‐old male C57BL/6J mice freely fed a high‐fat diet (D12492 Rodent Diet; Research Diets, New Brunswick, NJ, USA) for 6 weeks were orally loaded with 10 mL/kg of enteral nutrition (Ensure H; Abbot Japan, Tokyo, Japan) to stimulate GLP‐1 secretion. To analyze the delayed effect of GLP‐1 secretion by α‐GI, blood was collected after 0.5 h from the abdominal vein using a syringe containing diprotin A and EDTA at a final concentration of 3 mmol/L and 0.15%, respectively. The concentration of plasma active GLP‐1 was measured using an ELISA kit (Millipore Corporation, Billerica, MA, USA).
α‐Glucosidase inhibitors (acarbose 10 mg/kg, voglibose 0.1 mg/kg or miglitol 3 mg/kg) was given orally at the time of maltose or enteral nutrition‐loading, and sitagliptin (0.3 mg/kg) was given 0.5 h before oral loading. Test doses of α‐GIs were determined from the ED50 doses of the inhibition effect on sucrose or maltose‐loading in normal rats, respectively.
The experiment was carried out according to the guidelines of Gifu University. Results are expressed as mean ± SD. Significance of difference among groups was analyzed using Dunnett’s or Tukey’s multiple comparison test based on anova. P‐values of <0.05 were considered to show statistical significance.
Results
In the maltose‐loading test, treatment with each of the three α‐GIs alone significantly suppressed the blood glucose peak level at 0.5 h. Administration of miglitol alone significantly suppressed the blood glucose elevation as early as 0.25 and 0.5 h, and delayed the blood glucose peak until 1 h after loading. With administration of sitagliptin alone, the blood glucose level peaked at 0.25 h and was significantly lower at 0.5 and 1 h. Consequently, a complementary suppression of blood glucose elevation was observed at 0.25, 0.5 and 1 h in the combined miglitol and sitagliptin group (Figure 1c). As a result, the ΔAUC0–2 h of the blood glucose concentration of the group receiving miglitol alone was almost equal to that with acarbose or voglibose alone. The rate of decrease of the ΔAUC0–2 h of the blood glucose concentration in the miglitol and sitagliptin combination group was 47% (P < 0.05) compared with the control group, which was larger than that observed in the combined groups with acarbose or voglibose at the tested doses (Table 1). Similarly, combined treatment of miglitol and sitagliptin showed a 60% reduction (P < 0.05) of the range of blood glucose fluctuation compared with the control group, and also a significant decrease compared with miglitol or sitagliptin alone (Table 1).
Figure 1.
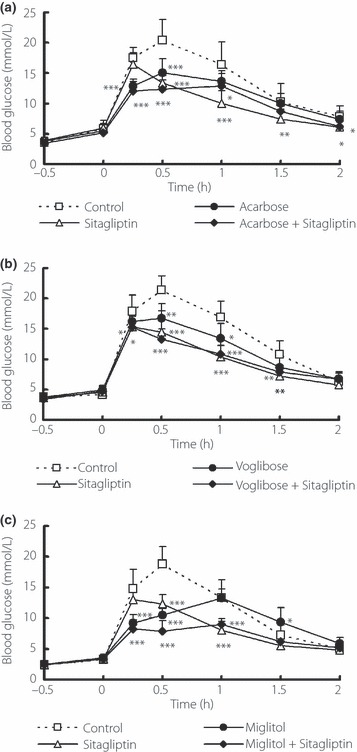
Blood glucose profiles after oral administration of α‐glucosidase inhibitor (α‐GI) and sitagliptin (0.3 mg/kg) in maltose‐loaded normal mice. (a) Acarbose, 10 mg/kg; (b) voglibose, 0.1 mg/kg; (c) miglitol, 3 mg/kg. Each value represents mean ± SD of 8–10 mice. *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group using to Dunnett’s multiple comparison test.
Table 1. Incremental blood glucose, range of glucose fluctuation and plasma insulin in maltose‐loaded mice and plasma active glucagon‐like peptide‐1 in enteral nutrition‐loaded mice.
| Test compound | Blood glucose concentration ΔAUC0–2 h | Range of glucose fluctuation | Plasma insulin at 15 min after maltose loading | Plasma active GLP‐1 at 30 min after EN loading | |
|---|---|---|---|---|---|
| (h*mmol/L) | Inhibition rate (%) | mmol/L | pmol/L | pmol/L | |
| Control | 20.9 ± 4.6a | – | 15.3 ± 2.6a | 111.9 ± 44.8ab | 2.18 ± 0.90a |
| Acarbose, 10 mg/kg | 15.6 ± 2.6b | 25.4 | 10.0 ± 2.5b | 89.5 ± 25.8a | 2.37 ± 0.76a |
| Sitagliptin, 0.3 mg/kg | 12.3 ± 3.3b | 41.1 | 10.9 ± 1.7b | 173.9 ± 53.4b | 3.33 ± 1.44a |
| Combination | 13.8 ± 2.3b | 34.0 | 8.5 ± 2.3b | 118.8 ± 68.9ab | 10.76 ± 6.34b |
| Control | 20.9 ± 3.2a | – | 17.2 ± 2.4a | 113.7 ± 48.2a | 2.23 ± 1.86a |
| Voglibose, 0.1 mg/kg | 16.1 ± 3.2b | 23.0 | 12.3 ± 1.7b | 111.9 ± 17.2a | 2.78 ± 1.23a |
| Sitagliptin, 0.3 mg/kg | 12.7 ± 2.9b | 39.2 | 11.6 ± 2.4b | 229.0 ± 93.0b | 3.54 ± 2.33ac |
| Combination | 13.6 ± 1.3b | 34.9 | 10.7 ± 1.8b | 142.9 ± 43.1a | 5.52 ± 2.34bc |
| Control | 17.9 ± 3.6a | – | 15.5 ± 2.6a | 158.9 ± 44.8a | 2.37 ± 1.33a |
| Miglitol, 3 mg/kg | 14.4 ± 1.7b | 19.6 | 9.7 ± 1.3b | 96.4 ± 18.9a | 3.29 ± 1.13a |
| Sitagliptin, 0.3 mg/kg | 11.5 ± 1.6bc | 35.8 | 10.0 ± 1.8b | 252.5 ± 86.1b | 4.62 ± 3.06a |
| Combination | 9.4 ± 1.3c | 47.5 | 6.2 ± 1.3c | 107.3 ± 20.7a | 11.22 ± 9.72b |
Maltose‐loading tests used for normal mice. Enteral nutirition (EN)‐loading tests used for mice fed high‐fat diet for 6 weeks. Each value represents the mean ± SD of 8–10 mice. Means with different letters (a, b, and c) are significantly different at P < 0.05 by Tukey’s multiple comparison test. AUC0–2 h, the area under the curve of blood glucose levels for up to 2 h after maltose‐loading; GLP‐1, glucagon‐like peptide‐1.
Enhancement of plasma insulin occurred with sitagliptin at 0.25 h after loading (1.6–2.0‐fold). In contrast, miglitol alone or combined treatment with miglitol and sitagliptin decreased the plasma insulin concentration by 39 or 32% compared with the control group, respectively (Table 1).
When enteral nutrition was orally loaded in mice fed a high‐fat diet, the plasma active GLP‐1 concentration was increased by 1.5–1.9‐fold after administration of sitagliptin compared with the control group. In contrast, a synergistic increase in the GLP‐1 concentration was observed after treatment with a combination of α‐GI and sitagliptin (2.5–4.9‐fold, P < 0.05 vs control; Table 1).
Discussion
Recently, suppression of postprandial hyperglycemia has come to be considered important for prevention of atherosclerosis3. Repeated episodes of blood glucose fluctuation accelerate the adhesion of monocytes to vascular endothelial cells and enhance the development/progression of atherosclerosis8. In the present study, the combined administration of α‐GI and sitagliptin complementarily lowered the blood glucose level. Furthermore, the combination of miglitol with sitagliptin significantly minimized blood glucose fluctuations. Thus, such combined treatment should reduce the risk of atherosclerosis.
The suppression of postprandial hyperglycemia by α‐GI alone and combination treatment with sitagliptin enables insulin secretion to be conserved, and therefore can be expected to mitigate dysfunction of pancreatic β‐cells9. In contrast, sitagliptin decreases the blood glucose level by enhancing insulin secretion. Thus, long‐term administration of this agent might create a burden on pancreatic β‐cells. In addition, a correlation between hyperinsulinemia and the level of high‐sensitivity C‐reactive protein (CRP), a marker of inflammation, has been reported10. Because the CRP elevation is related to coronary heart disease, stroke and mortality5, insulin secretion should be reduced as much as possible. In the present study, when α‐GI and sitagliptin were used in combination, insulin secretion was suppressed to a level almost the same as that with α‐GI alone. Furthermore, after chronic combined treatment of miglitol and sitagliptin for 8 weeks in high‐fat diet fed mice, the elevation of fasting plasma insulin level was significantly suppressed compared with that of control mice (normal diet group: 137.8 ± 84.4 pmol/L; high‐fat control group: 253.1 ± 72.3 pmol/L, P < 0.05 vs normal diet group; miglitol alone group: 189.4 ± 110.2 pmol/L; sitagliptin alone group: 359.9 ± 187.7 pmol/L; combination group: 161.9 ± 84.4 pmol/L, P < 0.05 vs high fat control; Y.H. and J.T., unpublished data). Thus, combined treatment might reduce risk of the development/progression of atherosclerosis as well as dysfunction of pancreatic β‐cells.
In patients with type 2 diabetes, miglitol has been reported to increase plasma active GLP‐1 levels2,11. GLP‐1 has several physiological activities, including a trophic effect on the pancreatic islets and suppression of gastric emptying and appetite12, in addition to enhancement of insulin secretion and inhibition of glucagon secretion. When combined with sitagliptin, each α‐GI tested increased the active GLP‐1 concentration synergistically. The GLP‐1 secretion induced by α‐GI might result from delayed absorption of carbohydrate into the lower parts of the digestive tract13, although the details of this mechanism remain unclear.
In conclusion, combined treatment with α‐GI miglitol, which more strongly inhibits the early phase of postprandial hyperglycemia, and sitagliptin can yield complementary and synergistic effects and therefore might represent a better antihyperglycemic therapy.
Acknowledgements
We thank H Tsuchida and K Yokoyama for technical assistance. This work was supported by a Health and Labor Science Research Grant from the Japanese Ministry of Health, Labor and Welfare, a KAKENHI, Grant‐in‐Aid for Scientific Research from the Japanese Ministry of Science, Education, Sports, Culture and Technology, and a New Energy and Industrial Technology Development Organization Grant. The authors report no conflicts of interest.
References
- 1.Chiasson JL, Josse RG, Gomis R, et al. Stop‐NIDDM Trail Research Group Acarbose prevention of type 2 diabetes mellitus: the STOP‐NIDDM randomised trial. Lancet 2002; 359: 2072–2077 [DOI] [PubMed] [Google Scholar]
- 2.Narita T, Katsuura Y, Sato T, et al. Miglitol induces prolonged and enhanced glucagons‐like peptide‐1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese Type 2 diabetic patients. Diabet Med 2009; 26: 187–188 [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Ciotola M, Carleo D, et al. Post‐meal glucose peaks at home associate with carotid intima‐media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 1345–1350 [DOI] [PubMed] [Google Scholar]
- 4.Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon‐like peptide‐1 receptor agonist, exendin‐4. Diabetes 2010; 59: 1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaptoge S, Di Angelantonio E, Lowe G, et al. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet 2010; 375: 132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki K, Inoue T, Yasuda N, et al. Comparison of efficacies of a dipeptidyl peptidase IV inhibitor and alpha‐glucosidase inhibitors in oral carbohydrate and meal tolerance tests and the effects of their combination in mice. J Pharmacol Sci 2007; 104: 29–38 [DOI] [PubMed] [Google Scholar]
- 7.Moritoh Y, Takeuchi K, Hazama M. Combination treatment with alogliptin and voglibose increases active GLP‐1 circulation, prevents the development of diabetes and preserves pancreatic beta‐cells in prediabetic db/db mice. Diabetes Obes Metab 2010; 12: 224–233 [DOI] [PubMed] [Google Scholar]
- 8.Mita T, Otsuka A, Azuma K, et al. Swings in blood glucose levels accelerate atherogenesis in apolipoprotein E‐deficient mice. Biochem Biophys Res Commun 2007; 358: 679–685 [DOI] [PubMed] [Google Scholar]
- 9.Fukaya N, Mochizuki K, Tanaka Y, et al. The alpha‐glucosidase inhibitor miglitol delays the development of diabetes and dysfunctional insulin secretion in pancreatic beta‐cells in OLETF rats. Eur J Pharmacol 2009; 624: 51–57 [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Abbasi F, Lamendola C, et al. Differentiation between obesity and insulin resistance in the association with C‐reactive protein. Circulation 2002; 106: 2908–2912 [DOI] [PubMed] [Google Scholar]
- 11.Lee A, Patrick P, Wishart J, et al. The effects of miglitol on glucagons‐like peptide‐1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab 2002; 4: 329–335 [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–165 [DOI] [PubMed] [Google Scholar]
- 13.Qualmann C, Nauck MA, Holst JJ, et al. Glucagon‐like peptide 1 (7‐36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha‐glucosidase inhibition (acarbose). Scand J Gastroenterol 1995; 30: 892–896 [DOI] [PubMed] [Google Scholar]


