Abstract
Aims/Introduction: When monotherapy with an oral hypoglycemic agent (OHA) is not sufficiently effective for blood glucose control, combination therapy with OHA having different mechanisms of action might be indicated.
Materials and Methods: In the present study, we compared the efficacy of two options in type 2 diabetes mellitus patients whose blood glucose had not been well controlled with mitiglinide (30 mg/day) alone. A total of 20 patients were included in the study and divided into two groups: group A, in which mitiglinide was given concomitantly with the α‐glucosidase inhibitor voglibose (0.6 mg/day); and group B, in which a double dose of mitiglinide was given (60 mg/day). Twelve weeks after changing the medication, HbA1c, glycoalbumin and 1,5‐anhydroglucitol (1,5‐AG) were measured. In addition, at weeks 0 and 12, a meal tolerance test was carried out, and plasma glucose, insulin, glucagon, active glucagon‐like peptide‐1 (GLP‐1) and total glucose‐dependent insulinotropic polypeptide levels were measured.
Results: The plasma level of 1,5‐AG improved in both groups at week 12. In group A, the plasma insulin level significantly decreased and the plasma active GLP‐1 level significantly increased during the meal tolerance test at week 12; thus, bodyweight significantly decreased only in group A.
Conclusions: Our findings suggested that concomitant administration of mitiglinide with voglibose could achieve better glycemic control, particularly in the postprandial period, without bodyweight gain and might have beneficial effects in type 2 diabetic patients at risk of macrovascular complications. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.0082.x, 2011)
Keywords: α‐Glucosidase inhibitor, Glucagon‐like peptide‐1, Mitiglinide
Introduction
Mitiglinide calcium hydrate (mitiglinide) is a potent, fast‐acting, short‐duration insulin secretagogue that promotes insulin secretion just after meals and thereby inhibits postprandial hyperglycemia. It is an oral hypoglycemic agent (OHA) that rarely induces hypoglycemia because of its short duration of action. α‐Glucosidase inhibitors (α‐GI) inhibit glucose absorption in the upper small intestine, improve postprandial hyperglycemia, and regulate delayed and excessive insulin secretion. These OHA are effective for both postprandial hyperglycemia and hyperinsulinemia. Furthermore, α‐GI might have other beneficial effects on the secretion of incretins, including glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), which have been reported to possess trophic effects on β‐cells. In animal models, native GLP‐1 stimulates β‐cell proliferation and inhibits apoptosis, which might increase β‐cell mass and function1.
When monotherapy with an OHA is not sufficiently effective in blood glucose control, combination therapy with OHA having different mechanisms of action could be indicated. In case monotherapy with mitiglinide is not sufficient to treat postprandial hyperglycemia, one of the risk factors for macroangiopathy, it remains unclear whether it is better to increase the dose of mitiglinide or to use it concomitantly with an α‐GI.
In the present study, we compared these two options in type 2 diabetes mellitus patients, whose plasma glucose levels had not been well controlled by dietary therapy and mitiglinide administration (30 mg/day t.i.d.).
Methods
Subjects
The subjects included 20 outpatients with type 2 diabetes mellitus (age ≥ 20 years) whose plasma glucose levels had not been well controlled with dietary therapy and mitiglinide (10 mg/day t.i.d.) for at least 8 weeks before week 0. The protocol was approved by the Ethics Committee of Hyogo College of Medicine. Each participant gave written informed consent before the start of the study and fulfilled all the following inclusion criteria:
-
1
A HbA1c level of 6.9–8.9% at week 0;
-
2
Not treated with OHA other than mitiglinide for 24 weeks (168 days) before week 0;
-
3
Not treated with insulin for over 8 weeks (56 days) before week 0.
The patient background characteristics (sex, age, duration of diabetes, body mass index [BMI], HbA1c and levels of fasting plasma glucose, insulin, total cholesterol, low‐density lipoprotein cholesterol, triglyceride, and blood pressure) at week 0 are summarized in Table 1.
Table 1. Patient characteristics.
| All subjects | Group A (concomitant voglibose group) | Group B (double mitiglinide group) | P‐value (A vs B) | |
|---|---|---|---|---|
| No. subjects (male, female) | 20 (12, 8) | 10 (7, 3) | 10 (5, 5) | – |
| Age (years) | 59.9 ± 11.4 | 57.8 ± 10.9 | 62.0 ± 12.1 | N.S. |
| Duration of diabetes (years) | 9.8 ± 6.0 | 7.1 ± 3.6 | 12.4 ± 6.8 | 0.0460 |
| BMI (kg/m²) | 24.6 ± 4.1 | 25.9 ± 2.6 | 23.2 ± 5.0 | N.S. |
| HbA1c (%) | 7.8 ± 0.6 | 8.1 ± 0.6 | 7.4 ± 0.3 | 0.0130 |
| Fasting plasma glucose level (mg/dL) | 167.9 ± 27.3 | 179.5 ± 30.8 | 156.3 ± 18.0 | 0.0410 |
| SBP (mmHg) | 126.9 ± 5.6 | 125.8 ± 6.4 | 128.0 ± 4.9 | N.S. |
| DBP (mmHg) | 72.6 ± 2.7 | 73.4 ± 3.4 | 71.8 ± 1.5 | N.S. |
| TC (mg/dL) | 213.0 ± 29.6 | 210.5 ± 23.6 | 215.5 ± 35.8 | N.S. |
| LDL‐C (mg/dL) | 128.7 ± 27.2 | 129.4 ± 16.9 | 128.0 ± 35.7 | N.S. |
| TG (μU/mL) | 123.0 ± 99.8 | 143.1 ± 122.2 | 102.9 ± 72.1 | N.S. |
BMI, body mass index; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; LDL‐C, low‐density lipoprotein cholesterol; N.S., not significant; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Study Design
The study was carried out at the Division of Diabetes and Metabolism, Department of Internal Medicine, Hyogo College of Medicine and Watanabe Medical Clinic in Nishinomiya, Hyogo, Japan. The subjects were randomly allocated to two groups using a lottery system on an open trial basis: group A (concomitant voglibose group), in which mitiglinide was given concomitantly with voglibose; and group B (double mitiglinide group), in which a double dose of mitiglinide was given.
The study design is shown in Figure 1. During the observation period, 10 mg mitiglinide was given orally three times a day before meals (within 5 min before each meal). During the treatment period, 10 mg mitiglinide and 0.2 mg voglibose were given orally to subjects in group A three times a day before meals, whereas 20 mg mitiglinide was given orally to subjects in group B three times a day before meals. During the observation and treatment periods, neither agent was given after a meal or without a meal. The administration period was 24 weeks, including 12 weeks of observation and 12 weeks of treatment. Dietary therapy was started at least 8 weeks (56 days) before the start of the observation period, and continued without change throughout the study period. If exercise therapy had been used before the study, it was continued without change throughout the study period; however, subjects were not allowed to initiate exercise therapy during the study period. The levels of HbA1c, glycoalbumin (GA) and 1,5‐AG were measured at week 12. Considering the relationship between HbA1c value measured by the traditional Japanese standard measurement method (JDS) and the National Glycohemoglobin Standardization Program (NGSP), the HbA1c value (%) in the present study is shown as the NGSP equivalent value (%) calculated on the basis of the following formula2: 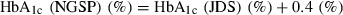 . At weeks 0 and 12, a meal tolerance test was carried out using a test meal (JANEF E460F18: 460 kcal, 56.5 g carbohydrate, 18 g protein and 18 g fat). The levels of plasma glucose, insulin, glucagon, active GLP‐1 and total GIP were measured at 0, 30, 60 and 120 min during the meal tolerance test.
. At weeks 0 and 12, a meal tolerance test was carried out using a test meal (JANEF E460F18: 460 kcal, 56.5 g carbohydrate, 18 g protein and 18 g fat). The levels of plasma glucose, insulin, glucagon, active GLP‐1 and total GIP were measured at 0, 30, 60 and 120 min during the meal tolerance test.
Figure 1.
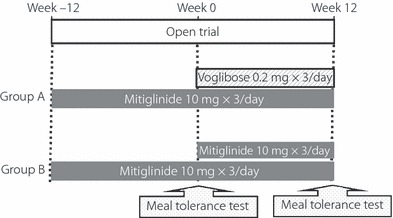
Study design. Test meal (460 kcal, 56.5 g carbohydrate, 18 g protein, 18 g fat).
The plasma level of active GLP‐1 was measured by enzyme‐linked immunosorbent assay (ELISA) using a GLP‐1 Active ELISA Kit (Millipore Corporation, Billerica, MA, USA) according to the manufacturer’s instructions. The plasma level of total GIP was also measured by ELISA using a Human GIP (total) ELISA kit (Millipore Corporation). Blood samples for measurement of GLP‐1 and GIP were collected in EDTA tubes containing aprotinin and a dipeptidyl peptidase 4 inhibitor (10 μL/mL of blood; Millipore Corporation).
Bodyweight was measured every 4 weeks and the subjects were asked about the occurrence of hypoglycemia throughout the study period. The study was carried out from April 2008 to December 2009.
Statistical Analysis
Data are represented as mean ± standard deviation unless otherwise specified. For intergroup comparisons, the Wilcoxon signed‐rank test was used. For multiple comparisons, two‐way analysis of variance (anova) was carried out. The results of anova at each time‐point of measurement were examined using the Wilcoxon signed‐rank test. The area under the curve (AUC) was estimated using the trapezoid method.
Results
Characteristics of Subjects
The characteristics of subjects in groups A and B at week 0 are presented in Table 1. The duration of diabetes was significantly shorter in group A than in group B. HbA1c and fasting plasma glucose levels were significantly higher in group A than in group B (8.1 ± 0.6%vs 7.4 ± 0.3% and 179.5 ± 30.8 mg/dL vs 156.3 ± 18.0 mg/dL, respectively). BMI was also higher in group A than in group B, though not to a significant degree. Blood pressure and lipid profiles did not differ between the groups.
Changes in HbA1c, GA and 1,5‐AG Levels
In group A, 1,5‐AG level had improved significantly at week 12 (3.5 ± 2.9 to 6.9 ± 6.6 μg/mL, P = 0.0039); GA and HbA1c levels had also improved, though not to a significant degree. In group B, HbA1c, GA and 1,5‐AG levels were all improved significantly at week 12 (7.4 ± 0.3 to 7.2 ± 0.4%, P = 0.0469; 22.1 ± 2.7 to 20.5 ± 1.9%, P = 0.0078; and 4.1 ± 2.0 to 5.9 ± 3.6 μg/mL, P = 0.0234, respectively; Table 2).
Table 2. Changes in parameters from week 0 to 12 in groups A and B.
| Group A (concomitant voglibose group) | P‐value (vs week 0) | Group B (double mitiglinide group) | P value (vs week 0) | |||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |||
| HbA1c (%) | 8.1 ± 0.6 | 7.9 ± 0.8 | N.S. | 7.4 ± 0.3 | 7.2 ± 0.4 | 0.0469 |
| GA (%) | 22.3 ± 2.2 | 21.8 ± 3.4 | N.S. | 22.1 ± 2.7 | 20.5 ± 1.9 | 0.0078 |
| 1,5 AG (μg/mL) | 3.5 ± 2.9 | 6.9 ± 6.6 | 0.0039 | 4.1 ± 2.0 | 5.9 ± 3.6 | 0.0234 |
| Fasting plasma glucose (mg/dL) | 179.5 ± 30.8 | 168.7 ± 27.6 | N.S. | 156.3 ± 18.0 | 150.5 ± 16.2 | N.S. |
| Weight (kg) | 71.9 ± 12.7 | 70.8 ± 12.6 | 0.0039 | 60.0 ± 14.0 | 59.8 ± 16.8 | N.S. |
| Glucose AUC0–120 (mg h/dL) | 30196.5 ± 5627.4 | 26044.5 ± 4394.3 | 0.0098 | 25363.5 ± 5443.6 | 24180.0 ± 5366.1 | N.S. |
| Insulin AUC0–120 (μU h/mL) | 3741.8 ± 2184.6 | 3229.8 ± 1551.8 | N.S. | 2878.0 ± 1840.5 | 3221.1 ± 2365.3 | N.S. |
| GLP‐1 AUC0–120 (pmol h/L) | 648.9 ± 91.6 | 843.3 ± 336.9 | 0.0137 | 604.2 ± 58.8 | 664.5 ± 103.7 | N.S. |
| GIP AUC0–120 (pg h/mL) | 24151.1 ± 9506.3 | 22856.1 ± 10277.1 | N.S. | 24481.2 ± 8888.7 | 26751.1 ± 12145.2 | N.S. |
| Glucagon AUC0–120 (pg h/mL) | 10347.6 ± 2029.6 | 11090.4 ± 1948.1 | N.S. | 10373.0 ± 2590.2 | 9820.5 ± 2151.4 | N.S. |
1,5‐AG, 1,5‐anhydroglucitol; AUC, area under the curve; GA, glycoalbumin; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1; HbA1c, hemoglobin A1c; N.S., not significant.
Changes in Bodyweight and Hypoglycemic Events
In group A, bodyweight significantly decreased (71.9 ± 12.7 to 70.8 ± 12.6 kg, P = 0.0039), whereas in group B, there was no significant change (60.0 ± 14.0 to 59.8 ± 16.8 kg, P = 0.5315; Table 2). No symptoms of hypoglycemia were noted in either group throughout the study period.
Changes in Plasma Glucose and Insulin Levels in Meal Tolerance Tests at Weeks 0 and 12
Changes in plasma glucose levels after a meal are shown in Figure 2. In group A, plasma glucose levels 30 and 60 min after a meal significantly decreased at week 12 (245.7 ± 38.1 to 202.4 ± 37.5 mg/dL, P = 0.0039 and 273.0 ± 51.9 to 229.2 ± 39.2 mg/dL, P = 0.0059, respectively). The plasma glucose level peaked 60 min after a meal at week 0 and 120 min after a meal at week 12. In group B, up to 30 min after a meal, the mean plasma glucose level at week 12 remained almost the same as that at week 0. The mean plasma glucose level 120 min after a meal improved at week 12, though not to a significant degree. The plasma glucose level peaked 120 min after a meal at week 0 and 60 min after a meal at week 12.
Figure 2.
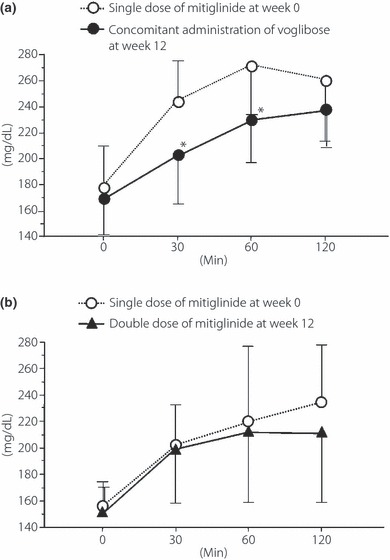
Changes in plasma glucose level after meal tolerance test from week 0 to 12 in (a) group A and (b) group B. A significant decrease 30 and 60 min after a meal was observed at week 12 in group A. *P < 0.05 vs before addition of voglibose (Wilcoxon signed‐rank test).
Changes in plasma insulin level and AUC are presented in Table 2 and Figure 3. In group A, the plasma insulin level 30 min after a meal significantly decreased at week 12 (38.5 ± 27.0 to 27.3 ± 10.4 μU/mL, P = 0.0273). AUC0–30 of the plasma insulin level significantly decreased (698.3 ± 227.1 to 521.8 ± 215.6 μU h/mL, P = 0.0273). AUC0–120 of the plasma insulin level throughout the meal tolerance test at week 12 in group A was less than that at week 0, though not to a significant degree (3741.8 ± 2184.6 to 3229.8 ± 1551.8 μU h/mL). The plasma insulin level peaked 30 min after a meal at week 0 and 60 min after a meal at week 12. In group B, the plasma insulin levels 30 and 60 min after a meal were increased at week 12, though not to a significant degree. The plasma insulin level peaked 30 min after a meal at both week 0 and 12. AUC0–120 of the plasma insulin level at week 12 in group B was higher than that at week 0 (2878.0 ± 1840.5 to 3221.1 ± 2365.3 μU h/mL).
Figure 3.
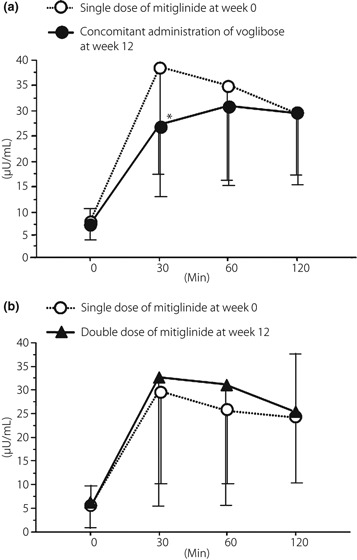
Change in plasma insulin level after meal tolerance test from week 0 to 12 in (a) group A and (b) group B. A significant decrease 30 min after a meal was observed at week 12 in group A. *P < 0.05 vs before addition of voglibose (Wilcoxon signed‐rank test).
Changes in Plasma Glucagon Level in Meal Tolerance Test at Weeks 0 and 12
There was no significant change in plasma glucagon level or plasma glucagon AUC between week 0 and 12 in either group (Table 2).
Changes in Active GLP‐1 and Total GIP Levels in Meal Tolerance Tests at Weeks 0 and 12
Changes in active GLP‐1 levels are shown in Figure 4. In group A, the active GLP‐1 levels were elevated throughout the experiment at week 12. Among them, active GLP‐1 levels 60 and 120 min, and AUC0–120 after a meal significantly increased (5.3 ± 0.7 to 7.5 ± 2.7 pmol/L, P = 0.0039 and 5.3 ± 0.9 to 6.7 ± 2.7 pmol/L, P = 0.0332, and 648.9 ± 91.6 to 843.3 ± 336.9 pmol h/L, P = 0.0137, respectively). Active GLP‐1 levels peaked 30 min after a meal at week 0 and 60 min after a meal at week 12. In group B, there was no significant difference in active GLP levels between week 0 and 12. Active GLP‐1 levels peaked 30 min after a meal at weeks 0 and 12.
Figure 4.
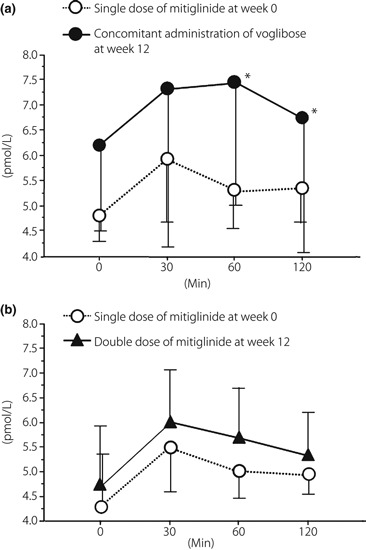
Change in active glucagon‐like peptide‐1 level after meal tolerance tests from week 0 to 12 in (a) group A and (b) group B. A significant increase at 60 and 120 min after a meal was observed at week 12 in group A. *P < 0.05 vs before addition of voglibose (Wilcoxon signed‐rank test).
There was no significant change in total GIP levels between week 0 and 12 in either group (Table 2, Figure 4).
Discussion
In the present study, when mitiglinide was given concomitantly with voglibose for 12 weeks, the peak plasma glucose level after a meal decreased significantly and the time required for plasma glucose level to reach the peak value was prolonged (Figure 2a). Although there was no significant difference in AUC0–120 of plasma insulin levels, a significant decrease was observed in AUC0–30 (P = 0.0273), namely during the early phase of insulin secretion (Figure 3). It has been shown that voglibose inhibits the postprandial increase in plasma glucose level and thereby decreases plasma insulin levels.
It was recently reported that the acute postprandial increase in plasma glucose level (postprandial glucose spike) promotes arteriosclerosis and increases the risk of cardiovascular disease, including myocardial infarction3. It has also been reported that hyperinsulinemia impairs vascular endothelial function and increases the risk of ischemic heart disease4. In the present study, concomitant administration of mitiglinide and voglibose (group A) more markedly inhibited the postprandial glucose spike and decreased insulin secretion than did a single dose of mitiglinide. This suggests that concomitant use of mitiglinide with voglibose might have a beneficial effect in preventing arteriosclerosis.
In group A, the active GLP‐1 levels at 60 and 120 min after a meal were significantly increased at week 12 (Figure 4a). This finding is of particular interest, because there has been no previous report of voglibose significantly increasing the active GLP‐1 level in patients with type 2 diabetes mellitus.
Active GLP‐1 level was reported to increase when voglibose was given to ob/ob mice for 3–4 weeks5. It appears that continuous administration of voglibose evoked chronic glucose absorption from the small intestine and increased the amount of undigested carbohydrates, which results in constant stimulation of the lower small intestine and the large intestine, thus promoting differentiation and proliferation of GLP‐secreting cells (L‐cells)6. This mechanism of action appears to explain why the GLP‐1 levels at 60 and 120 min after a meal were significantly increased at week 12 in group A. These findings suggest that concomitant use of mitiglinide and voglibose could spare excessive insulin secretion, and that the increase in GLP‐1 level might protect the function of pancreatic β‐cells and regulate postprandial plasma glucose levels.
It has been reported that GLP‐1 improved abnormal glucagon secretion, particularly the paradoxical rise in glucagon secretion7. However, in the present study, no relationship between GLP‐1 secretion and pancreatic glucagon secretion was observed in either group (Table 2). Further investigation is necessary to elucidate whether the beneficial effects of the concomitant use of α‐GI and mitiglinide treatment, on better long‐term glucose control, would depend on the suppression of glucagon secretion.
In contrast, in group B, HbA1c, GA and 1,5‐AG levels significantly improved at week 12 (Table 2). In a double‐blind comparative phase III clinical study of mitiglinide in China8, HbA1c levels improved when the mitiglinide dose was increased from 10 to 20 mg, which is similar to the results of the present study. However, meal tolerance tests at week 12 showed no significant change in plasma glucose level in group B (Figure 2). It is quite difficult to explain the discrepancy; the plasma glucose level 120 min after a meal in group B showed no significant decrease at week 12, but did tend to decrease compared with that of week 0. In the present study, we investigated the plasma glucose levels only until 120 min after a meal. However, there was a great difference in plasma glucose levels at 120 min or later (Figure 2). Therefore, the HbA1c level might have been significantly improved at 120 min or later after a meal in group B.
In the present study, we randomly allocated the subjects to two groups; incidentally, the background characteristics were significantly different between the groups (Table 1). The duration of diabetes was shorter and the blood glucose control was worse in group A participants on entry to the study.
Mean BMI was 26.0 in participants of group A, which shows that they were slightly more obese than the Japanese patients with type 2 diabetes. Because impairment of early insulin secretion is closely related to the pathogenesis of type 2 diabetes in Japanese patients and the secretory capacity of pancreatic β‐cells is weaker in Japanese patients than those in the USA and Europe9–11, concomitant use of mitiglinide with voglibose could be more useful even in fairly well‐controlled obese Japanese patients with type 2 diabetes mellitus as long as they were switched to concomitant treatment at an early stage.
In contrast, in group B in the present study, mean BMI was 23.2; that is, they were non‐obese, plus their mean duration of diabetes was much longer (12.4 years).
In non‐obese Japanese type 2 patients whose blood glucose levels are fairly well controlled with mitiglinide (30 mg/day) alone, a double dose of mitiglinide treatment could be expected to improve the plasma glucose level without causing bodyweight gain and/or hypoglycemia. This might support the potential for a double dose of mitiglinide to still be effective in non‐obese, long‐standing type 2 diabetes patients with low insulin secretory capacity.
Because the sample size of the present study was limited, a large‐scale study should be carried out to confirm the patient benefits by treatment regimen.
Acknowledgement
The authors declare no financial support or relationships that may pose a conflict of interest.
References
- 1.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008; 60: 470–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 2010; 53: 450–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complication Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 4.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996; 334: 952–957 [DOI] [PubMed] [Google Scholar]
- 5.Moritoh Y, Takeuchi K, Hazama M. Chronic administration of voglibose, an α‐glucosidase inhibitor, increases active glucagon‐like peptide‐1 levels by increasing its secretion and decreasing dipeptidyl peptidase‐4 active in ob/ob mice. JPET 2009; 329: 669–676 [DOI] [PubMed] [Google Scholar]
- 6.Cani PD, Hoste S, Guiot Y, et al. Dietary non‐digestible carbohydrates promote L‐cell differentiation in the proximal colon of rats. Br J Nutr 2007; 98: 32–37 [DOI] [PubMed] [Google Scholar]
- 7.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687 [DOI] [PubMed] [Google Scholar]
- 8.Gao X; Mitiglinide versus nateglinide comparison study group . Multicentre, double‐blind, randomized study of mitiglinide compared with nateglinide in type 2 diabetes mellitus patients in China. J Int Med Res 2009; 37: 812–821 [DOI] [PubMed] [Google Scholar]
- 9.Kosaka K, Hagura R, Kuzuya T. Insulin responses in equivocal and definite diabetes, with special reference to subjects who had mild glucose intolerance but later developed definite diabetes. Diabetes 1977; 26: 944–952 [DOI] [PubMed] [Google Scholar]
- 10.Mitsui R, Fukushima M, Nishi Y, et al. Factors responsible for deteriorating glucose tolerance in newly diagnosed type 2 diabetes in Japanese men. Metabolism 2006; 55: 53–58 [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki T, Miyake Y, Hagura R, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia 1984; 26: 44–49 [DOI] [PubMed] [Google Scholar]


