Abstract
Aims/Introduction: The aim of the present study was to determine the predictors of deterioration of glucose tolerance in individuals with normal glucose tolerance (NGT) and abdominal obesity, and whether a lifestyle intervention to reduce visceral fat is effective in these individuals.
Materials and Methods: The study subjects were 251 individuals who had abdominal obesity with certain risk factors (hypertension, high fasting plasma glucose (FPG), elevated hemoglobin A1c (HbA1c), dyslipidemia and hyperuricemia) and underwent oral glucose tolerance test (OGTT) in 2004 and 2005.
Results: Using the area under the receiver operating characteristic curve, we found that PG at 0 min, 60 min, and area under the curve (AUC) of glucose from 0 to 120 min (AUC [glucose0–120]) in OGTT were significant predictors of deterioration of glucose tolerance, with optimal cut‐off values of 95 mg/dL, 158 mg/dL and 271 mg h/dL, respectively. Although the rate of deterioration of glucose tolerance didn’t decrease with reductions in visceral fat area (VFA) over the 1‐year period in subjects with NGT, the rate tended to decrease with reductions in VFA in high‐risk NGT subjects (PG at 0 min > 95 or at 60 min > 158, or AUC [glucose0–120] > 271).
Conclusions: These results suggest that reduction of visceral fat over 1 year might not be beneficial in all subjects with NGT, but is beneficial in high‐risk NGT. We propose that individuals with values of the aforementioned predictors higher than the cut‐off levels, even those with NGT, should receive a lifestyle intervention program aimed at reducing visceral fat to prevent deterioration of glucose tolerance. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00080.x, 2011)
Keywords: Normal glucose tolerance, Abdominal obesity, Deterioration of glucose tolerance
Introduction
Visceral fat accumulation is associated with the development of metabolic disorders, including glucose intolerance. However, not all subjects with abdominal obesity develop hyperglycemia. Individuals with pre‐diabetic conditions, including impaired glucose tolerance (IGT), are already at high risk of the development of atherosclerosis1–3, as well as microvascular complications4. Thus, to prevent these complications, it is important to identify individuals with normal glucose tolerance (NGT), especially those with abdominal obesity, who could progress to glucose intolerance and design interventions to delay such deterioration. The aim of the present study was to determine predictors that can identify subjects with abdominal obesity who might develop glucose intolerance and to investigate whether a lifestyle intervention aimed at reducing visceral fat is effective in these individuals.
Materials and methods
Subjects
The study group comprised 3827 Japanese subjects (2854 men and 973 women) who were employees of the Amagasaki City Office, Hyogo, Japan (an urban area) and had completed the government‐funded annual health checkup in 2004. Among the tests carried out during the annual examination, oral glucose tolerance test (OGTT) was carried out in 783 subjects who had abdominal obesity and one or more risk factor(s). Abdominal obesity represented a waist circumference ≥85 cm in men and 90 cm in women5. Risk factors included the following: (i) hypertension: systolic blood pressure (sBP) of ≥130 mmHg and/or diastolic blood pressure (dBP) of ≥85 mmHg; (ii) high glucose: fasting plasma glucose (FPG) level of ≥110 mg/dL and/or Hemoglobin A1c (HbA1c) level of ≥5.5%; (iii) dyslipidemia: a serum triglyceride level of ≥150 mg/dL and/or a serum high‐density lipoprotein (HDL) cholesterol level of <40 mg/dL, and/or a serum low‐density lipoprotein (LDL) cholesterol level of ≥140 mg/dL; and (iv) hyperuricemia: a serum uric acid level of ≥7.0 mg/dL. These subjects were provided with health guidance to improve their lifestyle and to reduce visceral fat6. In 2004, there were 373 subjects who were identified as NGT and, finally, 251 subjects (240 men and 11 women) who could be followed by OGTT in the next year were included in the present analysis. In addition, we also analyzed 107 subjects (96 men and 11 women) who were identified to have impaired fasting glucose (IFG) and/or IGT in 2004 and could be re‐examined in 2005.
Anthropometry
Anthropometric variables (height, weight and waist circumference [WC]) were measured in standing position. Body mass index (BMI) was calculated as weight (kg) divided by the square of height in meters (m2). WC at the umbilical level was measured in cm with a non‐stretchable tape in the late exhalation phase in the standing position7. Blood pressure was measured in the sitting position.
Laboratory Tests
Plasma glucose was determined by the glucose oxidase method. HbA1c was determined by high‐performance liquid chromatography. Serum uric acid (UA), total cholesterol and triglyceride concentrations were determined by enzymatic methods. LDL and HDL cholesterol were also measured by an enzymatic method after heparin and calcium precipitation.
Detailed Examination
Visceral fat area (VFA) was determined by the bioelectrical impedance analysis (BIA) method, as reported previously8. OGTT was carried out in subjects with abdominal obesity with one or more risk factor(s). In this test, plasma glucose and serum insulin concentrations were determined at 0, 30, 60 and 120 min after 75 g glucose ingestion by the glucose oxidase method and double‐antibody radioimmunoassay, respectively. Insulinogenic index (I.I.) was calculated by dividing the increment in serum insulin by the increment in plasma glucose from 0 to 30 min of the OGTT. The area under the glucose and insulin curves (AUC [glucose0–120], AUC [insulin0–120]) were calculated by the trapezoid rule. The diagnoses of diabetes, IFG, IGT and NGT were based on the criteria of the American Diabetes Association9. Adiponectin (APN) was measured using the latex particle‐enhanced turbidimetric assay10. The measurements of VFA and APN complied with the Guidelines of the Ethical Committees of Osaka University. Written informed consent was obtained from all subjects.
Statistical Analysis
Comparison of variables between groups was carried out using an unpaired Student’s t‐test. Comparisons of variables between 2004 and 2005 in the NGT group and in the IFG and/or IGT group were carried out using a paired Student’s t‐test. APN, I.I. and homeostasis model of insulin resistance (HOMA‐IR) variables were log transformed and analyzed. The area under the receiver operating characteristic (ROC) curve was used to evaluate the predictive power of various parameters. All analyses except ROC analysis were carried out using StatView, version 5 (SAS Institute, Cary, NC, USA). ROC analysis was carried out using Dr. SPSS II, standard version (SPSS, Chicago, IL, USA). Data are expressed as mean ± SD. A P‐value of <0.05 was considered statistically significant.
Results
The clinical characteristics of the subjects with NGT and IFG and/or IGT at baseline and in the next year are presented in Table 1. In 2004, there were significant differences between the NGT group and the IFG and/or IGT group in sex, age, BMI, WC, VFA, HbA1c, FPG, fasting insulin (F‐IRI) and sBP. In contrast, among the 251 NGT subjects in 2004, 26 participants converted to IFG and/or IGT and one participant developed diabetes mellitus (DM) in 2005. Furthermore, among the 107 IFG and/or IGT subjects diagnosed in 2004, 15 participants developed DM and 36 improved to NGT in 2005. In individuals with NGT in 2004, their BMI, WC, VFA, F‐IRI and dBP decreased, and HbA1c, TC and HDLC increased significantly in 2005. In individuals with IFG and/or IGT in 2004, their BMI, WC, VFA, F‐IRI, LDLC and UA decreased, and HbA1c and HDLC increased significantly in 2005.
Table 1. Clinical characteristics of the subjects.
| NGT | IFG and/or IGT | P‐value | |||||
|---|---|---|---|---|---|---|---|
| 2004 | 2005 | 2004 | 2005 | NGT 2004 vs 2005 | IFG and/or IGT 2004 vs 2005 | 2004 NGT vs IFG and/or IGT | |
| NGT/IFG and/or IGT/DM | 251/0/0 | 224/26/1 | 0/107/0 | 36/56/15 | |||
| n (male/female) | 251 (240/11) | 251 (240/11) | 107 (96/11) | 107 (96/11) | 0.0334 | ||
| Age (years) | 48.3 ± 8.6 | 52.0 ± 6.6 | <0.0001 | ||||
| BMI (kg/m2) | 26.6 ± 2.4 | 26.4 ± 2.4 | 27.4 ± 2.7 | 27.0 ± 2.8 | 0.0003 | 0.0002 | 0.0085 |
| WC (cm) | 91.6 ± 5.0 | 90.6 ± 5.2 | 93.6 ± 5.6 | 92.7 ± 6.3 | <0.0001 | 0.0023 | 0.001 |
| VFA (cm2) | 131.1 ± 23.7 | 124.4 ± 25.7 | 142.7 ± 26.0 | 133.7 ± 26.8 | <0.0001 | <0.0001 | <0.0001 |
| HbA1c (%) | 4.8 ± 0.4 | 4.9 ± 0.4 | 5.1 ± 0.4 | 5.3 ± 0.4 | <0.0001 | <0.0001 | <0.0001 |
| FPG (mg/dL) | 93.8 ± 7.0 | 94.5 ± 7.8 | 106.4 ± 10.5 | 105.5 ± 12.0 | NS | NS | <0.0001 |
| F‐IRI (μU/mL) | 7.8 ± 4.6 | 6.2 ± 3.5 | 10.0 ± 5.3 | 7.1 ± 4.2 | <0.0001 | <0.0001 | <0.0001 |
| sBP (mmHg) | 132.3 ± 14.7 | 130.7 ± 15.5 | 136.4 ± 13.4 | 135.6 ± 16.7 | NS | NS | 0.0142 |
| dBP (mmHg) | 83.9 ± 9.9 | 82.2 ± 10.6 | 85.5 ± 8.7 | 84.3 ± 11.0 | 0.0095 | NS | NS |
| TC (mg/dL) | 213.9 ± 32.6 | 221.1 ± 33.6 | 216.3 ± 34.8 | 216.9 ± 31.7 | <0.0001 | NS | NS |
| TG (mg/dL) | 180.1 ± 115.6 | 177.7 ± 150.1 | 174.3 ± 129.5 | 160.4 ± 115.9 | NS | NS | NS |
| HDLC (mg/dL) | 52.9 ± 15.2 | 56.1 ± 15.2 | 54.0 ± 15.1 | 57.9 ± 15.8 | <0.0001 | <0.0001 | NS |
| LDLC (mg/dL) | 123.6 ± 29.4 | 122.2 ± 29.7 | 127.1 ± 33.1 | 120.6 ± 32.4 | NS | 0.0038 | NS |
| UA (mg/dL) | 6.4 ± 1.3 | 6.5 ± 1.2 | 6.4 ± 1.3 | 6.2 ± 1.3 | NS | 0.021 | NS |
| Adiponectin (μg/mL) | 6.5 ± 2.7 (n = 230) | 6.3 ± 2.7 (n = 239) | 6.1 ± 2.2 (n = 94) | 6.0 ± 2.3 (n = 97) | NS | NS | NS |
Date are mean ± SD. None of the subjects in this study had taken medications for hypertension, diabetes mellitus or dyslipidemia.
BMI, body mass index; dBP, diastolic blood pressure; F‐IRI, fasting immunoreactive insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDLC, high‐density lipoprotein cholesterol; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LDLC, low‐density lipoprotein cholesterol; NGT, normal glucose tolerance; NS, not significant; sBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; VFA, visceral fat area; WC, waist circumference.
Table 2 compares the 2004 clinical variables of subjects of the NGT group who showed deterioration in glucose tolerance in 2005 (worsening group) and of those who retained NGT (retaining group). WC, VFA, sBP, dBP, HbA1c, PG at 0, 30, 60 and 120 min, and AUC (glucose0–120) in OGTT were significantly higher and log (I.I.) was significantly lower in the worsening group than in the retaining group. However, BMI, TC, TG, HDLC, LDLC, UA, log (APN), IRI at 0, 30, 60 and 120 min, AUC (insulin0–120) in OGTT, and log (HOMA‐IR) were not significantly different between the two groups. The area under the ROC curve was used to evaluate the predictive power of the parameters that were significantly different between the two groups. The areas under the ROC curve of all these parameters were significantly higher than 0.5 (Table 3). Among them, the areas under the ROC curve of PG at 0 min, 60 min and AUC (glucose0–120) in OGTT were higher than approximately 0.7, showing that these parameters are significant predictors of deterioration of glucose tolerance. ROC curves of these predictors are presented in Figure 1. Next, we determined the optimal cut‐off points for these parameters to predict deterioration in glucose tolerance according to the Youden index. The optimal cut‐off points for PG at 0 min, 60 min and AUC (glucose0–120) in OGTT were 95 mg/dL, 158 mg/dL and 271 mg h/dL, respectively. The sensitivity and specificity of these cut‐off points were 0.67 and 0.64 for PG at 0 min, 0.67 and 0.75 for PG at 60 min, and 0.78 and 0.71 for AUC (glucose0–120), respectively.
Table 2. Comparison of baseline variables in normal glucose tolerance subjects who developed glucose intolerance and subjects who retained normal glucose tolerance in 2005.
| Worsening group | Retaining group | P‐value | |
|---|---|---|---|
| n (male/female) | 27 (27/0) | 224 (213/11) | |
| Age (years) | 51.3 ± 6.3 | 47.9 ± 8.8 | 0.0563 |
| WC (cm) | 93.5 ± 5.7 | 91.4 ± 4.9 | 0.0391 |
| BMI (kg/m2) | 27.1 ± 2.2 | 26.5 ± 2.4 | 0.2350 |
| VFA (cm2) | 140.6 ± 27.9 | 130.0 ± 23.0 | 0.0307 |
| sBP (mmHg) | 140.2 ± 13.6 | 131.3 ± 14.6 | 0.0029 |
| dBP (mmHg) | 88.1 ± 9.3 | 83.4 ± 9.9 | 0.0197 |
| TC (mg/dL) | 212.5 ± 32.9 | 214.0 ± 32.7 | 0.8211 |
| TG (mg/dL) | 218.8 ± 198.5 | 175.6 ± 101.6 | 0.0714 |
| HDLC (mg/dL) | 54.3 ± 18.1 | 52.7 ± 14.8 | 0.5959 |
| LDLC (mg/dL) | 117.3 ± 34.7 | 124.4 ± 28.6 | 0.2373 |
| UA (mg/dL) | 6.72 ± 0.93 | 6.36 ± 1.35 | 0.1891 |
| HbA1c (%) | 5.0 ± 0.4 | 4.7 ± 0.4 | 0.0014 |
| Log (APN) | 1.8 ± 0.4 (n = 25) | 1.8 ± 0.4 (n = 205) | 0.9964 |
| OGTT: PG (mg/dL) | |||
| 0 min | 98.4 ± 7.0 | 93.2 ± 6.9 | 0.0003 |
| 30 min | 167.1 ± 26.2 | 149.3 ± 27.7 | 0.0017 |
| 60 min | 172.4 ± 33.0 | 138.1 ± 33.9 | <0.0001 |
| 120 min | 114.0 ± 19.7 | 104.2 ± 19.1 | 0.0122 |
| OGTT: IRI (μU/mL) | |||
| 0 min | 7.5 ± 4.2 | 7.8 ± 4.7 | 0.7334 |
| 30 min | 44.1 ± 39.2 | 44.3 ± 26.6 | 0.9818 |
| 60 min | 50.6 ± 34.1 | 49.5 ± 33.2 | 0.8636 |
| 120 min | 35.3 ± 18.7 | 33.0 ± 21.2 | 0.5778 |
| log (I.I.) | −0.947 ± 0.907 | −0.569 ± 0.832 (n = 216) | 0.0284 |
| AUC (glucose0–120) | 294.5 ± 37.9 | 253.6 ± 41.0 | <0.0001 |
| AUC (insulin0–120) | 79.6 ± 46.2 | 77.7 ± 42.4 | 0.8261 |
| log (HOMA‐IR) | 0.478 ± 0.509 | 0.434 ± 0.578 | 0.7036 |
Date are mean ± SD. Worsening group consisted of those who showed deterioration of glucose tolerance in 2005.
Retaining group consisted of individuals who retained normal glucose tolerance (NGT) in 2005. APN, adiponectin; AUC, area under the curve; BMI, body mass index; dBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDLC, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model of insulin resistance; I.I., insulinogenic index; IRI, insulin; LDLC, low‐density lipoprotein cholesterol; NS, not significant; OGTT, oral glucose tolerance test; PG, plasma glucose; sBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; VFA, visceral fat area; WC, waist circumference.
Table 3. Areas under the receiver operating characteristic curve of various parameters in relation to worsening from normal glucose tolerance to impaired fasting glucose and/or impaired glucose tolerance and worsening from impaired fasting glucose and/or impaired glucose tolerance to diabetes mellitus.
| NGT | P | IFG and/or IGT | P | |
|---|---|---|---|---|
| WC | 0.631 | 0.027 | 0.479 | NS |
| VFA | 0.627 | 0.031 | 0.526 | NS |
| sBP | 0.688 | 0.001 | 0.536 | NS |
| dBP | 0.647 | 0.013 | 0.576 | NS |
| HbA1c | 0.681 | 0.002 | 0.676 | 0.03 |
| OGTT: PG (mg/dL) | ||||
| 0 min | 0.698 | 0.001 | 0.732 | 0.004 |
| 30 min | 0.665 | 0.005 | 0.701 | 0.013 |
| 60 min | 0.773 | <0.001 | 0.618 | NS |
| 120 min | 0.65 | 0.011 | 0.604 | NS |
| AUC (glucose0–120) | 0.773 | <0.001 | 0.666 | 0.04 |
| Log (I.I.) | 0.368 | 0.025 | 0.411 | NS |
| Age | 0.584 | NS | 0.687 | 0.02 |
AUC, area under the curve; dBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; I.I., insulinogenic index; NGT, normal glucose tolerance; NS, not significant; OGTT, oral glucose tolerance test; PG, plasma glucose; sBP, systolic blood pressure; VFA, visceral fat area; WC, waist circumference.
Figure 1.
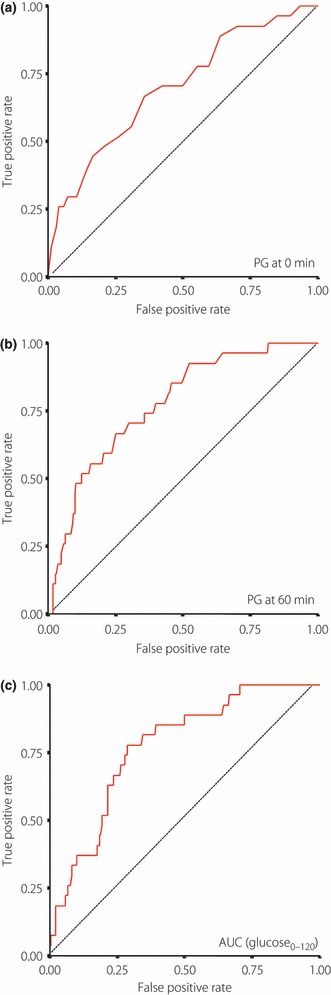
Receiver operating characteristic curves of (a) plasma glucose (PG) at 0 min, (b) 60 min and (c) area under the curve (AUC; glucose0–120) in an oral glucose tolerance test.
Table 4 compares several of the 2004 clinical variables of individuals diagnosed with IFG and/or IGT who developed DM in 2005 (worsening group 2), and those who retained IFG and/or IGT or improved to NGT (retaining or improving group 2) in 2005. Age, HbA1c, PG at 0, 30 min, AUC (glucose0–120) in OGTT were significantly higher in the worsening group 2 than in the retaining or improving group 2. The areas under the ROC curve of all these parameters were significantly higher than 0.5 (Table 3). Among them, the areas under the ROC curves of PG at 0, 30 min in OGTT were higher than 0.7, and their optimal cut‐off levels were 111 and 182 mg/dL, respectively. The sensitivity and specificity of these cut‐off points were 0.73 and 0.72 for PG at 0 min, and 0.87 and 0.49 for PG at 30 min, respectively.
Table 4. Comparison of baseline variables in subjects with impaired fasting glucose and/or impaired glucose tolerance who developed diabetes mellitus and those who retained impaired fasting glucose and/or impaired glucose tolerance or improved to normal glucose tolerance in 2005.
| Worsening group 2 | Retaining or improving group 2 | P | |
|---|---|---|---|
| n (male/female) | 15 (13/2) | 92 (83/9) | |
| Age (years) | 55.3 ± 3.0 | 51.4 ± 6.9 | 0.0367 |
| WC (cm) | 93.1 ± 5.7 | 93.7 ± 5.6 | 0.7192 |
| BMI (kg/m2) | 27.4 ± 2.7 | 27.3 ± 2.8 | 0.9016 |
| VFA (cm2) | 143.7 ± 27.4 | 142.6 ± 26.0 | 0.8790 |
| sBP (mmHg) | 138.1 ± 14.3 | 136.1 ± 13.3 | 0.5863 |
| dBP (mmHg) | 87.9 ± 9.8 | 85.2 ± 8.5 | 0.2644 |
| TC (mg/dL) | 211.0 ± 31.5 | 217.1 ± 35.4 | 0.5308 |
| TG (mg/dL) | 194.5 ± 118.7 | 171.0 ± 131.5 | 0.5184 |
| HDLC (mg/dL) | 56.1 ± 24.4 | 53.7 ± 13.2 | 0.5722 |
| LDLC (mg/dL) | 114.0 ± 39.4 | 129.2 ± 31.7 | 0.0986 |
| UA (mg/dL) | 6.50 ± 1.60 | 6.33 ± 1.30 | 0.6465 |
| HbA1c (%) | 5.3 ± 0.4 | 5.0 ± 0.4 | 0.0461 |
| log(APN) | 1.7 ± 0.4 | 1.8 ± 0.4 (n = 79) | 0.5546 |
| OGTT: PG (mg/dL) | |||
| 0 min | 113.3 ± 8.9 | 105.2 ± 10.3 | 0.0054 |
| 30 min | 205.6 ± 30.6 | 184.8 ± 29.3 | 0.0126 |
| 60 min | 221.3 ± 36.2 | 202.1 ± 41.2 | 0.0917 |
| 120 min | 150.7 ± 24.9 | 150.6 ± 24.5 | 0.9880 |
| OGTT: IRI (μU/mL) | |||
| 0 min | 9.9 ± 3.9 | 10.0 ± 5.5 | 0.9654 |
| 30 min | 29.4 ± 16.4 | 39.7 ± 28.5 | 0.1752 |
| 60 min | 47.0 ± 22.0 | 60.9 ± 39.2 | 0.1854 |
| 120 min | 46.6 ± 19.8 | 62.0 ± 45.0 | 0.1968 |
| Log (I.I.) | −1.800 ± 1.395 (n = 13) | −1.260 ± 0.903 (n = 91) | 0.0648 |
| AUC (glucose0–120) | 372.5 ± 44.0 | 345.6 ± 46.3 | 0.0384 |
| AUC (insulin0–120) | 75.8 ± 28.9 | 99.0 ± 60.6 | 0.1483 |
| log (HOMA‐IR) | 0.952 ± 0.409 | 0.797 ± 0.609 | 0.3741 |
Date are mean ± SD. Worsening group consisted of subjects who developed DM in 2005.
Retaining or improving group consisted of subjects who retained IFG and/or IGT or improved to NGT in 2005. APN, adiponectin; AUC, area under the curve; BMI, body mass index; dBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDLC, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model of insulin resistance; I.I., insulinogenic index; IRI, insulin; LDLC, low‐density lipoprotein cholesterol; NS, not significant; OGTT, oral glucose tolerance test; PG, plasma glucose; sBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; VFA, visceral fat area; WC, waist circumference.
Finally, we investigated whether changes in VFA over the 1‐year period were associated with changes in glucose tolerance in subjects with NGT. For this purpose, we divided the subjects into three groups according to the mean ± 1 SD of changes in VFA and calculated the incidence rates of worsening of glucose tolerance. As shown in Figure 2, the rate of worsening of glucose tolerance in NGT subjects did not decrease with decreases in VFA over 1 year. In contrast, the rate of development of DM in IFG and/or IGT subjects tended to decrease with decreases in VFA over 1 year (Figure 2), although not significantly (Table 5). A similar analysis was carried out for data of subjects of the NGT with PG at 0 min of >95 mg/dL or PG at 60 min of >158 mg/dL or AUC (glucose0–120) of >271 mg h/dL, who were at high risk of deterioration of glucose tolerance according to the results obtained in the present study. The rate of worsening of glucose tolerance tended to decrease with decrease in VFA in these subjects (Figure 3), although not significantly (Table 5). Because all subjects of the worsening group were male, we focused on the data in male subjects and re‐calculated the rate of worsening of glucose tolerance, and we obtained a similar results (data not shown).
Figure 2.
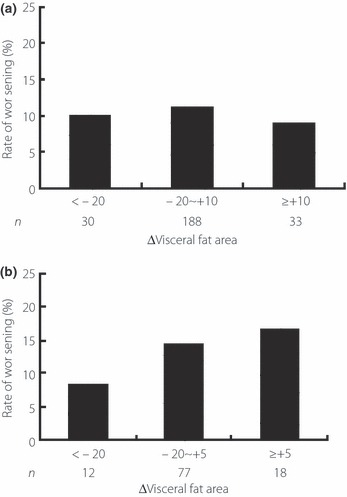
Rates of worsening of glucose tolerance in the three study groups divided according to the mean ± 1 SD of changes in visceral fat area over the 1‐year period of the study. (a) Normal glucose tolerance subjects, (b) impaired fasting glucose and/or impaired glucose tolerance subjects in 2004. ΔVisceral fat area indicates the increment in visceral fat area from 2004 to 2005 in each subject. n, number of subjects.
Table 5. Odds ratio and 95% confidence intervals of worsening of glucose tolerance in relation to Δvisceral fat area.
| n | OR | 95% CI | P‐value | |
|---|---|---|---|---|
| NGT | ||||
| Mean − SD > ΔVFA | 30 | 1 | Reference | |
| Mean + SD > ΔVFA ≥ mean − SD | 188 | 1.132 | 0.316–4.058 | 0.8493 |
| ΔVFA ≥ mean + SD | 33 | 0.900 | 0.167–4.843 | 0.9023 |
| IFG and/or IGT | ||||
| Mean − SD > ΔVFA | 12 | 1 | Reference | |
| Mean + SD > ΔVFA ≥ mean − SD | 77 | 1.833 | 0.215–15.653 | 0.5796 |
| ΔVFA ≥ mean + SD | 18 | 2.200 | 0.201–24.092 | 0.5185 |
| NGT PG at 0 min ≥95 | ||||
| Mean − SD > ΔVFA | 12 | 1 | Reference | |
| Mean + SD > ΔVFA ≥ mean − SD | 91 | 2.347 | 0.282–19.497 | 0.4297 |
| ΔVFA ≥ mean + SD | 11 | 2.444 | 0.189–31.534 | 0.4933 |
| NGT PG at 60 min ≥158 | ||||
| Mean − SD > ΔVFA | 11 | 1 | Reference | |
| Mean + SD > ΔVFA ≥ mean − SD | 59 | 3.111 | 0.365–26.484 | 0.2989 |
| ΔVFA ≥ mean + SD | 8 | 6.000 | 0.490–73.470 | 0.1609 |
| NGT AUC (glucose0–120) ≥ 271 | ||||
| Mean − SD > ΔVFA | 11 | 1 | Reference | |
| Mean + SD > ΔVFA ≥ mean − SD | 63 | 2.857 | 0.336–24.282 | 0.3362 |
| ΔVFA ≥ mean + SD | 13 | 8.571 | 0.836–87.847 | 0.0704 |
AUC, area under the curve; IFG, impaired fasting glucose, IGT, impaired glucose tolerance;NGT, normal glucose tolerance; VFA, visceral fat area.
Figure 3.
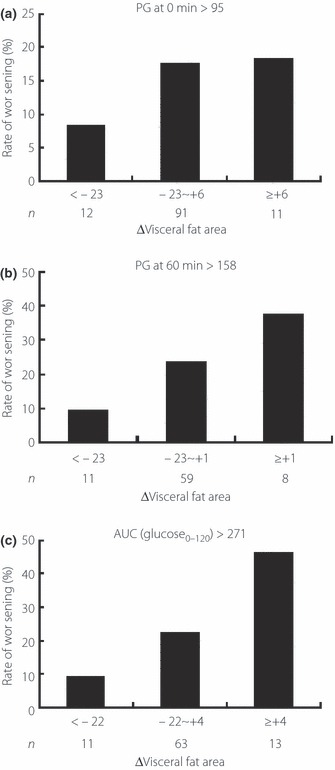
Rates of worsening of glucose tolerance in subjects with (a) normal glucose tolerance and plasma glucose (PG) at 0 min of >95 mg/dL, (b) PG at 60 min of >158 mg/dL, (c) and area under the curve (AUC; glucose0–120) of >271 mg h/dL in an oral glucose tolerance test. ΔVisceral fat area indicates the increment in VFA from 2004 to 2005 in each subject. n, number of subjects.
Discussion
In the present study, we found that WC, VFA, sBP, dBP, HbA1c, PG at 0, 30, 60 and 120 min, AUC (glucose0–120) of OGTT and log (I.I.) can predict deterioration of glucose tolerance over 1 year in NGT subjects with abdominal obesity, and that the power of PG at 0, 60 min and AUC (glucose0–120) was relatively the strongest among these variables. Furthermore, we calculated the optimal cut‐off values of these parameters: 95 mg/dL for PG at 0 min, 158 mg/dL for PG at 60 min, and 271 mg h/dL for AUC (glucose0–120). It has already been reported that the PG at 60 min during OGTT is a strong predictor of future risk of type 2 diabetes with a cut‐off value of 155 mg/dL11. Although the report was based on data about the risk of type 2 diabetes during a 7–8‐year follow‐up period, the cut‐off value of PG at 60 min described in the aforementioned study was almost similar to that computed in the present study. In addition, the same study also showed that NGT subjects with PG at 60 min of >155 mg/dL, who also fulfilled the criteria for the metabolic syndrome, were at greater risk of developing diabetes. Together, with the aforementioned study, the present results suggest that NGT subjects with abdominal obesity with PG at 60 min of >155–158 mg/dL are at high risk of deterioration of glucose tolerance over both a short and long period.
The present results also showed that the rate of worsening of glucose tolerance in NGT subjects did not decrease with decreases in VFA over the 1‐year period, suggesting that reducing visceral fat over 1 year had no beneficial effect on glucose tolerance in NGT. In contrast, the rate of developing DM in IFG and/or IGT subjects tended to decrease with decreases in VFA over a 1‐year period. These results are in agreement with those of Schäfer et al.12, who showed that moderate weight loss under a lifestyle intervention program with reduction in visceral fat improved glucose tolerance in individuals with IGT, but not with NGT. Their follow‐up period was 7–11 months, and was as short as ours. It is possible that the beneficial effects of reduction of visceral fat in NGT might not become apparent over a short period of time and that such intervention in NGT might prevent future deterioration of glucose tolerance over a longer period of time. In fact, the F‐IRI decreased significantly, similar to VFA, over the 1‐year period, even in NGT subjects in the present study (Table 1), which should lead to conservation of future insulin secretion capacity. We also addressed the question of whether reductions in visceral fat have beneficial effects in NGT subjects with PG at 0 min of >95, PG at 60 min of >158, or AUC (glucose0–120) of >271, who are at high risk of worsening of glucose tolerance based on the results of the present study. The results showed that these subjects benefit from such reduction, similar to persons with IFG and/or IGT. We propose that the individuals with these parameters over the cut‐off values should receive a lifestyle intervention program aimed at decreasing visceral fat, even in NGT.
The present study has limitations. The number of subjects included in the present analysis was approximately two‐thirds of those identified as NGT in 2004, and we could not follow the rest of the subjects by OGTT in 2005. When we compared the clinical data of the rest of the subjects with those of the present study, VFA in both 2004 and 2005 were significantly different between the two groups (120.3 ± 21.8 vs 131.1 ± 23.7 in 2004, 106.2 ± 26.3 vs 124.4 ± 25.7 in 2005). It might be that we analyzed subjects with relatively severe abdominal obesity.
Although we found various factors, including WC, VFA and log (I.I.), were involved in significant predictors of deterioration of glucose tolerance from NGT to IFG/IGT, only HbA1c, PG at 0 and 30 min, and AUC (glucose0–120) in OGTT were significantly higher in the worsening groups from IFG/IGT to DM. We speculate that WC and VFA dropped out from such factors in IFG/IGT, probably because visceral fat is likely to decrease by intervention in larger WC and VFA individuals, leading to an improvement in glucose tolerance. Thus, we might not be able to predict deterioration of glucose tolerance by only using these parameters in the IFG/IGT group. Regarding log (I.I.), the P‐value was 0.0648, suggesting that it might be defined as a significant predictor if evaluated in a larger sample size.
In conclusion, the present study identified certain predictors in NGT subjects with abdominal obesity for deterioration of glucose tolerance over a 1‐year period, these include PG at 0 and 60 min, and AUC (glucose0–120) in OGTT. The results also showed that lifestyle intervention that results in reduction of visceral fat does not prevent deterioration of glucose tolerance in NGT, although such a program seems beneficial in subjects with the aforementioned predictors at levels higher than the cut‐off points. We propose that individuals with PG at 0 and 60 min, and AUC (glucose0–120) higher than the cut‐off values, including NGT, should receive a lifestyle intervention aimed at reducing visceral fat. Further studies of larger population samples and longer follow‐up periods are warranted.
Acknowledgements
The authors thank Dr. Tetsuya Ohira for his helpful comments. The authors declare no financial support or relationship that may pose conflict of interest.
References
- 1.Yamasaki Y, Kawamori R, Matsushima H, et al. Asymptomatic hyperglycaemia is associated with increased intimal plus medial thickness of the carotid artery. Diabetologia 1995; 38: 585–591 [DOI] [PubMed] [Google Scholar]
- 2.Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999; 22: 920–924 [DOI] [PubMed] [Google Scholar]
- 3.Su Y, Liu XM, Sun YM, et al. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract 2008; 62: 877–882 [DOI] [PubMed] [Google Scholar]
- 4.Nichols GA, Arondekar B, Herman WH. Complications of dysglycemia and medical costs associated with nondiabetic hyperglycemia. Am J Manag Care 2008; 14: 791–798 [PubMed] [Google Scholar]
- 5.Matsuzawa Y. Metabolic syndrome – definition and diagnostic criteria in Japan. J Atheroscler Thromb 2005; 12: 301 [DOI] [PubMed] [Google Scholar]
- 6.Okauchi Y, Nishizawa H, Funahashi T, et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care 2007; 30: 2392–2394 [DOI] [PubMed] [Google Scholar]
- 7.Tokunaga K, Matsuzawa Y, Ishikawa K, et al. A novel technique for the determination of body fat by computed tomography. Int J Obes 1983; 7: 437–445 [PubMed] [Google Scholar]
- 8.Ryo M, Maeda K, Onda T, et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 2005; 28: 451–453 [DOI] [PubMed] [Google Scholar]
- 9.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21: 518–524 [DOI] [PubMed] [Google Scholar]
- 10.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle‐enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta 2006; 371: 163–168 [DOI] [PubMed] [Google Scholar]
- 11.Abdul‐Ghani MA, Lyssenko V, Tumoi T, et al. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 2009; 32: 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schäfer S, Kantartzis K, Machann J, et al. Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur J Clin Invest 2007; 37: 535–543 [DOI] [PubMed] [Google Scholar]


