Abstract
Aims/Introduction: The effectiveness of incretin‐based therapies in Asian type 2 diabetes requires investigation of the secretion and metabolism of glucose‐dependent insulinotropic polypepide (GIP) and glucagon‐like peptide 1 (GLP‐1). Plasma extractions have been suggested to reduce variability in intact GLP‐1 levels among individuals by removing interference that affects immunoassays, although no direct demonstration of this method has been reported. We have evaluated the effects of ethanol and solid‐phase extractions on incretin immunoassays. We determined incretin levels during meal tolerance tests in Japanese patients with type 2 diabetes and characterized predictors for incretin secretion.
Materials and Methods: Japanese patients with type 2 diabetes (23 anti‐diabetic drug‐naïve and 18 treated with sulfonylurea [SU] alone) were subjected to meal tolerance tests, and incretin levels were determined by immunoassays with or without extraction.
Results: Intact GLP‐1 levels determined by an intact GLP‐1 immunoassay with ethanol and solid‐phase extractions were lower than those determined without extraction. Intact GLP‐1 levels determined by the extractions were highly correlated with each other, much more so than the levels with and without extraction. Total GLP‐1 was unaffected by extractions, showing that extractions remove interference only in the case of intact GLP‐1. Incretin secretion after meal ingestion was similar between drug‐naïve and SU‐treated patients. Fasting and postprandial GLP‐1 levels were correlated positively with fasting free fatty acids and negatively with dipeptidyl peptidase‐4 activity.
Conclusions: Ethanol and solid‐phase extractions remove interference for intact GLP‐1 immunoassay. SU showed little effect on incretin secretion. GLP‐1 and GIP secretion were predicted by different factors. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00141.x, 2012)
Keywords: Extraction, Immunoassay, Incretin
Introduction
Beneficial effects of the incretin hormones, glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), on glucose homeostasis through its regulation of β‐cell function suggest them as attractive therapeutic targets for diabetes1–3. On secretion from intestinal K‐ and L‐cells, both GLP‐1 and GIP undergo rapid degradation catalyzed by a serine protease, dipeptidyl peptidase‐4 (DPP‐4). GLP‐1 and GIP digested by DPP‐4 no longer act as incretin hormones. Therefore, there is a need for a reliable assay to measure intact, as well as total (i.e. intact plus DPP‐4‐digested) forms of GLP‐1 and GIP in human subjects to evaluate both secretion and processing of incretins, and the effects of DPP‐4 inhibitors, and to further develop novel incretin‐related therapies, such as small compounds that enhance secretion of incretin hormones. Immunoassays for intact GLP‐1 require specific antibodies that are not widely available. The large variability in intact GLP‐1 levels determined by the various commercially available immunoassays leads to confusion and hampers our understanding of incretin biology. We found that intact GLP‐1 levels in ethanol‐extracted plasma of Japanese subjects were greatly reduced compared with previously determined values determined by the same immunoassay, except that the plasmas were measured without extraction4. Later, a similar observation was made when intact GLP‐1 levels in ethanol‐extracted plasma of Caucasian subjects were determined5. Considering these lines of evidence, it was suggested that plasma extractions before immunoassays for intact GLP‐1 might remove interferences that cause large variations in intact GLP‐1 levels among human subjects6. In addition, plasma extractions have been suggested to improve correlations across intact GLP‐1 levels determined by various commercially available immunoassays7.
Secretion of GLP‐1 and GIP from intestinal K‐ and L‐cells is enhanced by ingestion of various nutrients, hormones and neurotransmitters2,3,8. Although the molecular mechanisms underlying enhancement of incretin secretion by such stimuli have been intensively studied in isolated K‐ and L‐cells, as well as in cultured cell lines8, the regulation of incretin secretion in human subjects is largely unknown. For example, K‐ and L‐cells express components of KATP channels, Kir6.2 and SUR1, similarly to pancreatic β‐cells, and sulfonylurea (SU) enhances secretion of GLP‐1 and GIP from these cells9,10. Enhancement of incretin hormones by SU has not been reported in humans. Furthermore, in some, but not all, patients with type 2 diabetes, reduced GLP‐1 secretion or enhanced GIP secretion has been reported previously4,11–15, but mechanistic explanations underlying such fluctuating results are missing. Knowledge of factors affecting secretion of incretin hormones in humans is required not only to understand such fluctuating results, but also to predict efficacy of the incretin‐related anti‐diabetic drugs, such as DPP‐4 inhibitors and compounds enhancing incretin secretion. To date, several predictors for secretion of GLP‐1 (fasting glucagon, fasting free fatty acid [FFA], age and bodyweight) and GIP (fasting FFA and age) have been reported in Caucasians13,15,16, but data are lacking for Asians.
Here, we report the following: (i) comparison of incretin immunoassays with or without extractions; (ii) plasma levels of incretin hormones during meal tolerance tests; and (iii) predictors of fasting and postprandial incretin secretion in Japanese patients with type 2 diabetes.
Materials and Methods
The protocol was approved by the ethics committee of Kansai Electric Power Hospital, and written informed consent was obtained from all participants. A total of 41 Japanese patients with type 2 diabetes (the Japan Diabetes Society criteria of 201017,18) participated in the current study. Of all the patients, 23 were anti‐diabetic drug‐naïve, 8 were on gliclazide and 10 were on glimepiride. Characteristics of patients are summarized in Table 1. Patients were subjected to a meal tolerance test in the morning after overnight fast. A Japanese standard meal (480 kcal; carbohydrate:protein:fat = 2.8:1:1) was ingested within 10 min. SU‐treated patients took their regular doses of SU immediately after ingestion of meals. A Saflo II 20‐gauge 1¼″ catheter (catalogue no. SR‐SFA2032; Terumo, Tokyo, Japan) was placed in a cubital vein of patients and connected to an extension tube (catalogue no. SF‐ET2527; Terumo) followed by a Discofix stopcock (catalogue no. 15898; B. Braun Melsungen AG, Melsungen, Germany). Blood samples were withdrawn from the stopcock directly into evacuated sample tubes containing relevant preservatives using Venoject II luer adaptor S (catalogue no. XX‐MN2000S; Terumo) and Venoject II tube holder D (catalogue no. XX‐VP010HD; Terumo). To prevent degradation of intact GLP‐1 by DPP‐4, blood samples were withdrawn directly into BD P700 Blood Collection Tubes (catalogue no. 366473; BD, Franklin Lakes, NJ, USA) containing a DPP‐4 inhibitor, and the tubes were kept on ice until centrifugation. Separated plasma samples were frozen and kept at −70°C for up to 6 months, until further analysis.
Table 1. Characteristics of patients.
| All | No drug | SU | |
|---|---|---|---|
| n | 41 | 23 | 18 |
| Female (%) | 27.9 | 21.7 | 36.8 |
| Age (years) | 62.5 ± 8.1 | 59.1 ± 6.1 | 66.7 ± 8.0* |
| Duration (years) | 7.8 ± 7.4 | 5.3 ± 7.8 | 11.0 ± 6.1* |
| BMI (kg/m2) | 22.9 ± 2.8 | 22.9 ± 3.3 | 23.0 ± 2.4 |
| Systolic BP (mmHg) | 134.9 ± 22.0 | 128.0 ± 23.4 | 143.7 ± 18.5* |
| Diastolic BP (mmHg) | 79.4 ± 9.4 | 79.5 ± 9.7 | 79.3 ± 9.4 |
| HbA1c (%) | 7.1 ± 0.6 | 7.0 ± 0.5 | 7.3 ± 0.6 |
| Fasting plasma glucose (mg/dL) | 123.4 ± 21.2 | 121.3 ± 28.6 | 128.2 ± 13.6 |
| Fasting insulin (mU/L) | 5.1 ± 2.5 | 4.3 ± 3.2 | 6.0 ± 1.5* |
| Fasting CPR (ng/mL) | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.5 |
| Fasting glucagon (pg/mL) | 107.4 ± 33.7 | 107.1 ± 32.6 | 107.9 ± 35.3 |
| HOMA‐IR | 1.6 ± 0.8 | 1.3 ± 1.0 | 1.9 ± 0.5* |
| HOMA‐β | 33.1 ± 21.0 | 28.1 ± 20.8 | 40.0 ± 11.5* |
| SUIT | 42.2 ± 20.8 | 38.9 ± 25.2 | 46.3 ± 16.4 |
Each value represents the mean ± SD. All, both anti‐diabetic drug‐naïve patients and sulfonylureas‐treated patients; BMI, body mass index; BP, blood pressure; HOMA, homeostatic model assessment; IR, insulin resistance; No drug, anti‐diabetic drug‐naïve patients; SU, sulfonylureas‐treated patients; SUIT, secretory unit of islet in transplantation. *P < 0.05 (unpaired t‐test vs No drug).
Extraction of Plasma Samples
Aliquots of plasma were subjected to no extraction, ethanol extraction or solid‐phase extraction before incretin immunoassays. Ethanol extraction was carried out as described previously4. Briefly, ethanol was added to plasma samples at the following plasma/ethanol volumes: total GLP‐1 150 μL/350 μL, intact GLP‐1 300 μL/700 μL, and dried extracts were reconstituted in solutions supplemented with commercial immunoassay kits of the following volumes: total GLP‐1 150 μL and intact GLP‐1 250 μL. Solid phase extraction was carried out as follows: OASIS HLB Extraction Plates 30 mg/96‐well (catalogue no. WAT058951; Waters Corporation, Milford, MA, USA) were equilibrated with 1 mL of methanol and, subsequently, 1 mL of distilled water. Aliquots of plasma samples were diluted with phosphate‐buffered saline (PBS) at the following plasma/PBS volumes: total GLP‐1 150 μL/450 μL, intact GLP‐1 300 μL/900 μL and total GIP 300 μL/700 μL. The diluted plasma samples were applied to each well on the equilibrated plates. Samples were then washed twice with 1 mL of 10% methanol in distilled water and eluted twice with 0.5 mL of 0.5% NH3 (v/v) and 75% ethanol (v/v) in distilled water. Eluted samples were then dried under nitrogen gas, and reconstituted in solutions supplemented with commercial immunoassay kits of the following volumes: GLP‐1 150 μL and intact GLP‐1 250 μL. In case of total GIP, eluted samples were reconstituted in 300 μL of a 1:1 mixture of PBS with 0.05% Tween‐20 (v/v) and a solution in a commercial immunoassay kit.
Laboratory Determinations
Total GLP‐1 was measured using the Human Total GLP‐1 kit (catalogue no. K111‐FC1; Meso Scale Discovery, Gaithersburg, MD, USA) that recognizes several GLP‐1 isoforms at the following cross‐reactivity: GLP‐1 (1–37) 26%, GLP‐1 (7–37) 29%, GLP‐1 (9–37) unknown, GLP‐1 (1–36)amide 48%, GLP‐1 (7–36)amide 100% and GLP‐1 (9–36)amide 77%. Intact GLP‐1 was measured using the Glucagon Like Peptide‐1 (Active) ELISA (catalogue no. EGLP‐35K; Millipore, Billerica, MA, USA) that recognizes GLP‐1 isoforms at the following cross‐reactivity: GLP‐1 (1–37) 0.2%, GLP‐1 (7–37) 99.5%, GLP‐1 (9–37) not detected, GLP‐1 (1–36)amide 0.2%, GLP‐1 (7–36)amide 100%; GLP‐1 (9–36)amide not detected. Total GIP was measured using the Human Total GIP kit (catalogue no. EZHGIP‐54K; Millipore) that recognizes GIP isoforms at the following cross‐reactivity: GIP (1–42) 100% and GLP‐1 (3–42) 100%. Volumes of the reconstituted extracts or plasma samples used to measure incretins were as follows: total GLP‐1 25 μL, intact GLP‐1 100 μL and total GIP 20 μL. All samples were measured in duplicate. The intra‐ and interassay coefficients of variations (CV), detection limits, and recoveries of the expected concentrations for GLP‐1 and GIP added to plasmas are summarized in Table 2. Insulin was measured using Architect i2000‐Architect Insulin (Abbott Laboratories, Wiesbaden, Germany; detection limit <0.5 pmol/L; intra‐ and interassay CV <5.0%; <0.15% cross‐reactivity with 1,000,000 pg/mL of human proinsulin). C‐peptide was measured using ADVIA Centaur Immunoassay System‐C‐peptide (Siemens Healthcare Diagnostics, Germany; detection limit <0.1 ng/mL; intra‐ and interassay CV <5.0%; human proinsulin cross‐reaction 0% with 2500 pg/mL of proinsulin). Glucagon was measured using Glucagon kit ‘Daiichi‐II’ (TFB Co. Ltd., Tokyo, Japan; detection limit <30 ng/mL; intra‐ and interassay CV 5.0–10.0%). Plasma DPP‐4 activity was measured as described previously with the following, minor modifications19: 20 μL of plasma samples were mixed with 80 μL of 100 μmol/L H‐Gly‐Pro‐7‐amido‐4‐methyl‐coumarin (H‐Gly‐Pro‐AMC) hydrobromide (catalogue no. I‐1225; Bachem, Bubendorf, Switzerland) dissolved in a solution (25 mmol/L HEPES [pH 7.80], 140 mmol/L NaCl, 1 mg/mL bovine serum albumin [catalogue no. A3059; Sigma Aldrich, St. Louis, MO, USA], 1% dimethyl sulfoxide) and incubated for 10 min at room temperature with agitation. The reaction was stopped by adding 100 mL of 25% acetic acid (v/v), and was subjected to a microplate reader. DPP‐4 activity (nmol/min/mL) was defined as follows: AMC concentration (mmol/L) × reaction volume (100 mL)/(plasma sample volume [20 mL] × reaction time [10 min]). The assay had a detection limit of 0.050 nmol/min/mL, and intra‐ and interassay were CV <5.0%. HbA1c was measured using high performance liquid chromatography with cation‐exchange resins that separates the stable form β‐N1‐mono‐deoxyfructosyl Hb, and values are shown in National Glycohemoglobin Standardization Program values as recommended by the Japan Diabetes Society17,18. Other laboratory measurements including plasma glucose, serum triglyceride and serum free fatty acids were measured by standard assays.
Table 2. Intra‐ and interassay variations, recovery and detection limit of immunoassays with or without extractions.
| Extraction | Total GLP‐1 | Intact GLP‐1 | Total GIP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Ethanol | Solid‐phase | None | Ethanol | Solid‐phase | None | Ethanol | Solid‐phase | |
| Recovery (%) | 83.7–118.2 | 71.6–118 | 88.4–89.6 | 78.4–84.3 | 81.6–82.4 | 61.4–61.9 | 65.3–71.2 | 68.9–72.5 | 88.3–98.9 |
| Intra‐assay variations (%) | 1.00–3.94 | 1.54–8.22 | 7.11–10.2 | 4.39–8.15 | 2.43–2.85 | 4.06–14.9 | 1.98–2.55 | 5.64–13.6 | 11.1–11.3 |
| Interassay variations (%) | 4.99–10.1 | 11.3–27.1 | 9.30–10.2 | 8.09–9.71 | 10.6–12.0 | 11.1–13.2 | 4.78–13.4 | 6.92–18.0 | 10.6–18.1 |
| Detection limit (pmol/L) | 0.50 | 0.50 | 0.50 | 1.00 | 0.83 | 0.83 | 1.61 | 1.61 | 1.61 |
GIP, glucose‐dependent insulinotropic polypepide; GLP‐1, glucagon‐like peptide 1.
Calculations and Statistical Analysis
Patient characteristics and results are reported as mean ± standard deviation (SD). Area under the curve (AUC) of each measurement was calculated according to the trapezoidal rule. Homeostatic model assessment of insulin resistance (HOMA‐IR) and β‐cell function (HOMA‐β) were calculated as described20. Secretory units of islets in transplantation (SUIT) were calculated as described21. All statistical calculations were carried out using JMP for Windows ver. 8.0 (SAS Institute Inc., Cary, NC, USA) including linear regression analysis to determine the relationship between incretin secretion (AUC‐GLP‐1 and AUC‐GIP) and various parameters, as well as two‐way ANOVA for repeated measures with post‐hoc analysis to analyze time‐course curves. Values at single time‐points or AUC were compared by paired and unpaired t‐test. A P‐value <0.05 was taken to show significant differences.
Results
Immunoassays for GLP‐1 and GIP, especially those to measure their intact forms, require careful sample handling and specific antibodies, and have not been widely available. Recent studies, including the present study, have suggested the importance of ethanol and solid‐phase extractions before intact GLP‐1 immunoassays to remove interferences that generate the large variations in intact GLP‐1 levels measured among various individuals4,6,22. In Figure 1a, fasting and postprandial human plasma samples were subjected to an immunoassay for intact GLP‐1 with or without ethanol and solid‐phase extractions before the assay. Determined values were plotted to evaluate the effects of each extraction. Values of intact GLP‐1 in ethanol‐ and solid‐phase extracted samples were generally lower than those in non‐extracted samples (Figure 1a). Values of intact GLP‐1 in ethanol‐ and solid‐phase extracted samples showed a striking correlation (r = 0.978, P < 0.0001), whereas values of intact GLP‐1 in non‐extracted samples showed reduced correlations with those in ethanol‐ or solid‐phase extracted samples. Unlike intact GLP‐1, values of total GLP‐1 were not largely affected by ethanol‐ or solid‐phase extraction (Figure 1b), suggesting that ethanol and solid phase‐extractions simply exclude interferences for the intact GLP‐1 assay and do not remove GLP‐1 binding to unknown proteins of large molecular weight. Values of total GIP were not largely affected by solid‐phase extraction (Figure 1c). Effects of extractions on intact GIP levels were not studied because of lack of commercially available assays for intact GIP. Based on these results and the convenience of sample handling, solid‐phase extraction was used before carrying out all immunoassays for intact GLP‐1, total GLP‐1 and total GIP in the current study.
Figure 1.
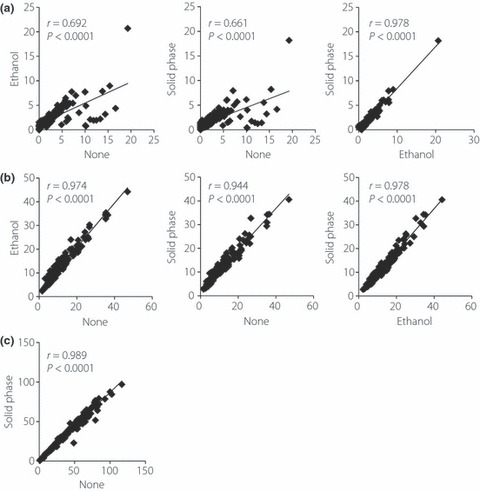
Effects of ethanol and solid‐phase extractions on immunoassays for glucagon‐like peptide 1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP). Fasting and postprandial plasma samples from Japanese subjects: 23 drug‐naïve and 18 sulfonylurea‐treated patients with type 2 diabetes during meal tolerance tests were subjected to either no extraction (none), ethanol extraction (ethanol) or solid‐phase extraction (solid phase) before immunoassays for (a) intact GLP‐1, (b) total GLP‐1 and (c) total GIP. Values determined by different extraction methods were analyzed by linear regression analyses to calculate the correlation coefficient (r) and P‐values. Ethanol extraction was not carried out before the immunoassay for total GIP, because ethanol and solid phase extraction showed a strong correlation for total and intact GLP‐1, and the effects on immunoassays for intact GIP were not evaluated, as no commercial immunoassays were available.
Levels of intact GLP‐1, total GLP‐1 and total GIP during meal‐tolerance tests were studied in Japanese type 2 diabetic patients that were drug‐naïve or treated with SU alone (Figure 2). Plasma glucose levels at 120 min after meal ingestion were significantly higher in patients treated with SU than those of drug‐naïve patients; although other factors, such as serum insulin and C‐peptide, did not show a significant difference between the two groups. Levels of intact GLP‐1, total GLP‐1 and total GIP, as well as their AUC during meal‐tolerance tests, did not differ between the two groups, suggesting that SU largely did not affect secretion of GLP‐1 and GIP in Japanese patients with type 2 diabetes.
Figure 2.
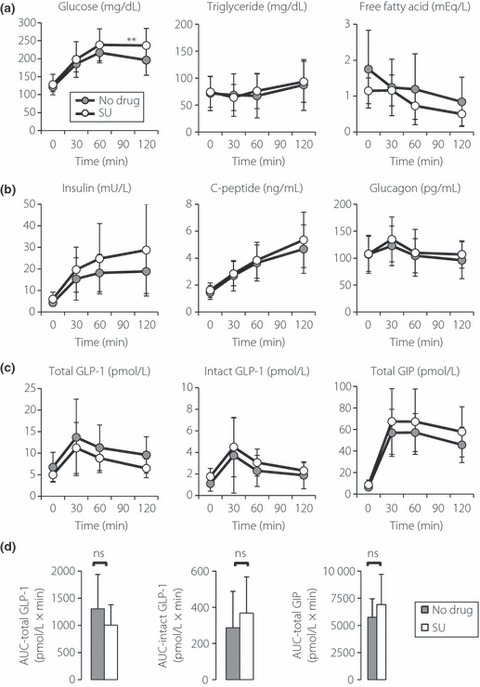
Response of glucagon‐like peptide 1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP) after meal ingestion in Japanese patients with type 2 diabetes. A total of 23 anti‐diabetic drug‐naïve (No drug) and 18 sulfonylurea‐treated (SU) patients were subjected to a meal tolerance test. (a–c) Levels of indicated measurements in each time point (gray circles, no drug; white circles, SU). Immunoassays for total GLP‐1, intact GLP‐1 and total GIP were carried out using solid‐phase extracted plasma samples. Each value represents the mean ± SD. (d) Area under the curves (AUC) for indicated measurements are shown (black bars, No drug; white bars, SU). Each value represents the mean ± SD. *P < 0.05 in an unpaired t‐test. ns, Not significant.
Predictors of fasting GLP‐1 levels are shown in Table 3 and Figure 3. There was a strong positive association between fasting levels of total GLP‐1 and FFA, and a negative association with DPP‐4 activity. These associations were also observed in drug‐naïve patients, but were not seen in SU‐treated patients. Predictors of postprandial GLP‐1 secretion after meal ingestion are shown in Table 4 and Figure 4. There was a strong positive association of GLP‐1 secretion (as assessed by AUC‐total GLP‐1) with fasting FFA, HbA1c and fasting GLP‐1. GLP‐1 secretion showed a negative association with DPP‐4 activity. These associations were also observed in drug‐naïve patients, but were not seen in SU‐treated patients. Unlike a previous report by Vollmer et al.15, postprandial GLP‐1 secretion did not show significant associations with fasting glucagon, age or body mass index (BMI; Table 4). Incremental AUC‐total GLP‐1 did not show a significant association with fasting glucagon levels, age or BMI in the current study (data not shown). Postprandial GLP‐1 secretion did not show significant associations with indices for β‐cell function (e.g. HOMA‐β and SUIT) or insulin sensitivity (e.g. HOMA‐IR) (Table 4). Postprandial GLP‐1 secretion showed a strong correlation with fasting GLP‐1 levels, but it did not show significant associations with postprandial GIP secretion and fasting GIP (Table 4).
Table 3. Predictors of fasting glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypepide (GIP) levels.
| Fasting GLP‐1 (pmol/L) | Fasting GIP (pmol/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | No drug | SU | All | No drug | SU | |||||||
| r | P | r | P | r | P | r | P | r | P | R | P | |
| Age (years) | −0.107 | 0.506 | 0.089 | 0.687 | 0.121 | 0.632 | 0.049 | 0.759 | −0.283 | 0.190 | −0.068 | 0.073 |
| Duration of diabetes (years) | −0.125 | 0.437 | −0.087 | 0.692 | 0.177 | 0.482 | 0.011 | 0.943 | −0.109 | 0.621 | −0.128 | 0.614 |
| BMI (kg/m2) | 0.295 | 0.061 | 0.436 | 0.037 | 0.222 | 0.377 | −0.008 | 0.960 | −0.122 | 0.579 | 0.072 | 0.775 |
| HbA1c (%) | 0.175 | 0.274 | −0.409 | 0.053 | −0.014 | 0.956 | 0.185 | 0.246 | 0.050 | 0.822 | 0.161 | 0.524 |
| HOMA‐IR | −0.091 | 0.570 | 0.059 | 0.788 | 0.000 | 0.999 | 0.065 | 0.687 | −0.245 | 0.261 | 0.021 | 0.933 |
| HOMA‐β | −0.268 | 0.090 | −0.368 | 0.084 | −0.160 | 0.526 | 0.021 | 0.895 | −0.128 | 0.560 | −0.053 | 0.836 |
| SUIT | −0.129 | 0.423 | −0.174 | 0.426 | 0.062 | 0.808 | −0.027 | 0.869 | −0.098 | 0.657 | −0.078 | 0.758 |
| Fasting plasma glucose (mg/dL) | 0.114 | 0.479 | 0.425 | 0.043 | 0.028 | 0.914 | −0.079 | 0.625 | −0.143 | 0.517 | 0.098 | 0.698 |
| AUC‐plasma glucose (mg/dL × min) | 0.006 | 0.968 | 0.236 | 0.279 | 0.010 | 0.968 | 0.201 | 0.208 | 0.004 | 0.985 | 0.166 | 0.509 |
| Fasting glucagon (pg/mL) | 0.263 | 0.097 | 0.260 | 0.099 | 0.403 | 0.097 | −0.008 | 0.962 | 0.044 | 0.841 | −0.032 | 0.900 |
| AUC‐glucagon (pg/mL × min) | 0.278 | 0.079 | 0.277 | 0.201 | 0.519 | 0.027 | 0.111 | 0.491 | 0.059 | 0.791 | 0.123 | 0.627 |
| Fasting insulin (mU/L) | −0.147 | 0.360 | −0.097 | 0.660 | −0.021 | 0.934 | 0.054 | 0.739 | −0.216 | 0.323 | 0.011 | 0.965 |
| AUC‐insulin (mU/L × min) | −0.163 | 0.309 | −0.167 | 0.447 | −0.003 | 0.992 | 0.012 | 0.943 | −0.206 | 0.347 | −0.057 | 0.824 |
| Fasting C‐peptide (ng/mL) | 0.141 | 0.379 | 0.103 | 0.641 | 0.538 | 0.021 | −0.009 | 0.954 | −0.194 | 0.375 | 0.047 | 0.855 |
| AUC‐C‐peptide (ng/mL × min) | 0.041 | 0.798 | 0.013 | 0.954 | 0.315 | 0.203 | −0.083 | 0.607 | −0.211 | 0.333 | −0.059 | 0.817 |
| Fasting FFA (mEq/L) | 0.594 | <0.001 | 0.639 | <0.001 | 0.251 | 0.314 | −0.170 | 0.288 | −0.097 | 0.659 | −0.088 | 0.728 |
| DPP‐4 (nmol/min/mL) | −0.548 | <0.001 | −0.685 | <0.001 | −0.104 | 0.680 | −0.034 | 0.833 | −0.086 | 0.697 | −0.165 | 0.513 |
| Fasting GIP (pmol/L) | −0.106 | 0.508 | −0.094 | 0.6694 | 0.125 | 0.620 | – | – | – | – | – | – |
P‐values were calculated by linear regression analysis; r, correlation coefficient. All, both anti‐diabetic drug‐naïve patients and sulfonylureas‐treated patients; AUC, area under curve; BMI, body mass index; CPR, C‐peptide reactivity; DPP, dipeptidyl peptidase; FFA, free fatty acid; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon like polypeptide‐1; HOMA, homeostatic model assessment; IR, insulin resistance; No drug, anti‐diabetic drug‐naïve patients; SU, sulfonylureas‐treated patients; SUIT, secretory unit of islet in transplantation.
Figure 3.
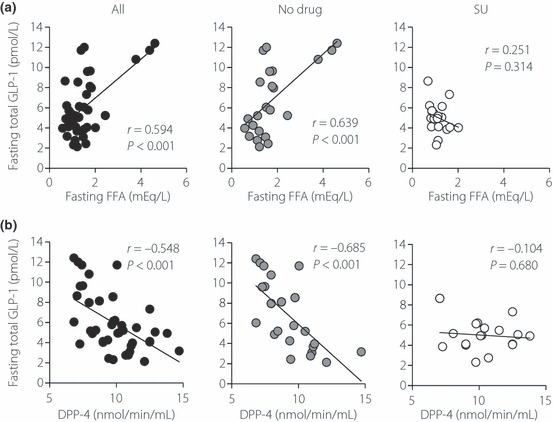
Linear regression analysis of glucagon‐like peptide 1 (GLP‐1) secretion with (a) fasting free fatty acid levels and (b) DPP‐4 activities in Japanese patients with type 2 diabetes. Linear regression analyses were carried out to calculate the correlation coefficient (r) and P‐values. All, both anti‐diabetic drug‐naïve patients and sulfonylurea‐treated patients; No drug, anti‐diabetic drug‐naïve patients; SU, sulfonylurea‐treated patients.
Table 4. Predictors of glucagon like polypeptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP) secretion (area under the curve total glucagon like polypeptide‐1 and area under the curve total glucose‐dependent insulinotropic polypeptide) after meal ingestion.
| AUC‐GLP‐1 (pmol/L × min) | AUC‐GIP (pmol/L × min) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | No drug | SU | All | No drug | SU | |||||||
| r | P | r | P | r | P | r | P | r | P | R | P | |
| Age (years) | −0.168 | 0.294 | −0.181 | 0.409 | 0.344 | 0.163 | 0.274 | 0.083 | 0.021 | 0.925 | 0.374 | 0.127 |
| Duration of diabetes (years) | −0.291 | 0.065 | −0.221 | 0.302 | −0.223 | 0.373 | 0.060 | 0.708 | 0.179 | 0.798 | −0.327 | 0.185 |
| BMI (kg/m2) | −0.04 | 0.806 | 0.096 | 0.660 | −0.241 | 0.336 | 0.058 | 0.720 | −0.191 | 0.384 | 0.008 | 0.993 |
| HbA1c (%) | 0.425 | 0.006 | 0.682 | <0.001 | −0.285 | 0.251 | 0.256 | 0.109 | 0.257 | 0.237 | 0.150 | 0.553 |
| HOMA‐IR | −0.107 | 0.505 | 0.221 | 0.311 | −0.098 | 0.698 | −0.073 | 0.650 | −0.342 | 0.110 | −0.132 | 0.601 |
| HOMA‐β | −0.278 | 0.078 | −0.228 | 0.295 | −0.090 | 0.722 | −0.006 | 0.969 | −0.564 | 0.005 | 0.068 | 0.787 |
| SUIT | −0.089 | 0.582 | −0.174 | 0.428 | −0.032 | 0.899 | −0.004 | 0.981 | −0.518 | 0.011 | 0.190 | 0.451 |
| Fasting plasma glucose (mg/dL) | 0.001 | 0.993 | 0.437 | 0.037 | −0.044 | 0.863 | 0.003 | 0.983 | 0.331 | 0.122 | −0.176 | 0.486 |
| AUC‐plasma glucose (mg/dL × min) | 0.032 | 0.845 | 0.643 | 0.001 | 0.231 | 0.357 | 0.308 | 0.050 | 0.538 | 0.008 | 0.107 | 0.674 |
| Fasting glucagon (pg/mL) | 0.111 | 0.491 | −0.001 | 0.911 | 0.345 | 0.160 | −0.079 | 0.622 | −0.317 | 0.140 | 0.107 | 0.671 |
| AUC‐glucagon (pg/mL × min) | 0.056 | 0.726 | −0.065 | 0.770 | 0.361 | 0.141 | 0.052 | 0.747 | −0.244 | 0.263 | 0.244 | 0.330 |
| Fasting insulin (mU/L) | −0.193 | 0.227 | 0.071 | 0.746 | −0.062 | 0.808 | −0.061 | 0.704 | −0.457 | 0.028 | −0.054 | 0.832 |
| AUC‐insulin (mU/L × min) | 0.147 | 0.359 | −0.070 | 0.749 | 0.225 | 0.216 | 0.037 | 0.819 | −0.269 | 0.214 | 0.067 | 0.790 |
| Fasting C‐peptide (ng/mL) | 0.078 | 0.628 | 0.071 | 0.747 | 0.103 | 0.683 | −0.127 | 0.427 | −0.400 | 0.059 | −0.012 | 0.961 |
| AUC‐C‐peptide (ng/mL × min) | 0.049 | 0.761 | −0.021 | 0.925 | 0.544 | 0.020 | −0.026 | 0.874 | −0.304 | 0.159 | 0.135 | 0.593 |
| Fasting FFA (mEq/L) | 0.526 | <0.001 | 0.598 | 0.003 | −0.233 | 0.352 | −0.096 | 0.550 | 0.111 | 0.614 | −0.310 | 0.211 |
| DPP‐4 (nmol/min/mL) | −0.422 | 0.006 | −0.547 | 0.007 | −0.030 | 0.906 | 0.162 | 0.311 | 0.132 | 0.549 | 0.080 | 0.752 |
| Fasting GLP‐1 (pmol/L) | 0.633 | <0.001 | 0.654 | <0.001 | 0.579 | 0.008 | 0.087 | 0.588 | 0.035 | 0.873 | −0.143 | 0.579 |
| Fasting GIP (pmol/L) | −0.008 | 0.962 | −0.054 | 0.807 | −0.324 | 0.164 | 0.538 | <0.001 | 0.284 | 0.179 | 0.326 | 0.548 |
| AUC‐GIP (pmol/L × min) | 0.128 | 0.426 | −0.205 | 0.3484 | −0.287 | 0.248 | – | – | – | – | – | – |
AUC, area under curve; BMI, body mass index; CPR, C‐peptide reactivity; DPP, dipeptidyl peptidase; FFA, free fatty acid; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon like polypeptide‐1; HOMA, homeostatic model assessment; IR, insulin resistance; SUIT, secretory unit of islet in transplantation. P values were calculated by linear regression analysis; r, correlation coefficient. All, both anti‐diabetic drug‐naïve patients and sulfonylureas‐treated patients; No drug, anti‐diabetic drug‐naïve patients; and SU, sulfonylureas‐treated patients.
Figure 4.
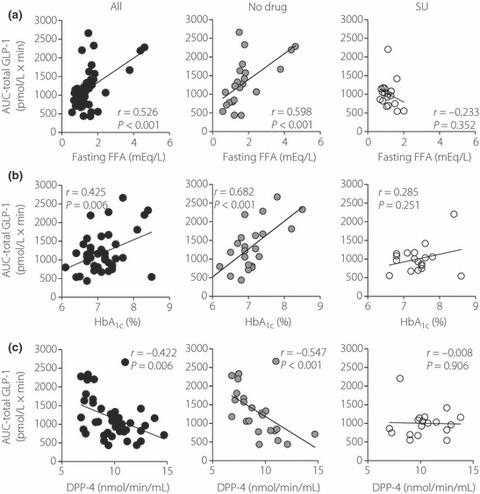
Linear regression analysis of glucagon‐like peptide 1 (GLP‐1) secretion with (a) fasting free fatty acid levels, (b) HbA1c and (c) DPP‐4 activities in Japanese patients with type 2 diabetes. GLP‐1 secretion was assessed by area under curve of total GLP‐1 levels in Figure 2. Linear regression analyses were carried out to calculate the correlation coefficient (r) and P‐values. All, both anti‐diabetic drug‐naïve patients and sulfonylurea‐treated patients; No drug, anti‐diabetic drug‐naïve patients; SU, sulfonylurea‐treated patients.
Predictors of fasting GIP and postprandial GIP secretion were also studied (Tables 3 and 4). There was a strong positive association between postprandial GIP secretion (as assessed by AUC‐total GIP) and fasting GIP. Unlike the Vollmer study, GIP secretion did not show a significant association with fasting FFA levels15. In drug‐naïve patients, GIP secretion showed negative associations with indices for fasting insulin levels and β‐cell function (e.g. HOMA‐β and SUIT), but these associations were not seen in SU‐treated patients. GIP secretion did not show a significant association with insulin sensitivity (HOMA‐IR).
Discussion
Measuring circulating hormones by immunoassay is sometimes difficult as a result of the presence of interferences in the blood samples. For example, circulating somatostatin levels determined by immunoassay differ with or without extractions before the assay, and the values of somatostatin levels are much decreased by extractions that remove such interference23,24. This was also the case with intact GLP‐1. Ethanol and solid‐phase extractions have recently been suggested to improve variability of intact GLP‐1 levels among individual human subjects6. Similar to immunoassays for somatostatin, the levels of intact GLP‐1 were much decreased by extraction procedures that remove interference of unknown identity6. In our preliminary experiments, a solid‐phase extraction significantly improved variability in intact GLP‐1 levels among Japanese subjects; fasting plasma levels of intact GLP‐1 were reduced by extraction of more than 10 pmol/L in 5 out of 29 healthy volunteers, and the difference between values of intact GLP‐1 in extracted‐ and non‐extracted samples was as large as 50 pmol/L (data not shown). There was a striking correlation between intact GLP‐1 levels in ethanol and solid‐phase extracted samples, whereas no such correlations were found between intact GLP‐1 levels in non‐extracted and extracted samples (Figure 1a), suggesting that both ethanol and solid‐phase extractions remove the same interference from plasma samples. In addition, such extractions show little effect on values of total GLP‐1 levels that were only three to fourfold higher than those of intact GLP‐1 levels (Figure 1b). These lines of evidence suggest that extractions simply remove interference irrelevant to GLP‐1 and do not exclude GLP‐1 forming a complex with unknown GLP‐1‐binding proteins. Therefore, extractions are recommended to measure intact GLP‐1 levels in human plasma samples.
In the current study, fasting and postprandial levels of incretins in Japanese patients with type 2 diabetes were determined (Figure 2). Little enhancement of meal‐induced GLP‐1 response in Japanese patients with type 2 diabetes, which we reported previously4,25, was confirmed in the present study, although absolute values of GLP‐1 and GIP were not directly compared in our previous report as a result of the usage of different assays. As the meal‐induced GIP response was maintained, understanding the mechanisms underlying the reduced postprandial GLP‐1 response in Japanese patients might reveal the regulatory machinery of incretin secretion in intestinal K‐ and L‐cells. Regarding such regulatory machinery in K‐ and L‐cells, a potential action of SU on incretin secretion has been investigated intensively in cultured cells8. Recent studies have confirmed expression of the KATP channel subunits Kir6.2 and SUR1 in purified mouse K‐ and L‐cells, and SU‐stimulated secretion of GLP‐1 and GIP from primary cultures9,10. Expression of Kir6.2 was also detected in human K‐ and L‐cells, suggesting that SU might stimulate secretion of GLP‐1 and GIP in humans26. As shown in Figure 2, fasting and postprandial incretin levels did not differ with or without SU treatment. Multiple regression analysis taking SU usage as a nominal variable also did not show significant associations of SU usage with GLP‐1 and GIP levels (Supplemental Table S1). Although further studies are definitely needed to evaluate the effects of SU on incretin secretion in humans, the current study suggests that SU has little effect on incretin secretion in Japanese patients with type 2 diabetes.
Characterization of predictors for GLP‐1 secretion showed that postprandial GLP‐1 levels positively correlate with fasting FFA and HbA1c, and negatively correlate with DPP‐4 activity in Japanese patients with type 2 diabetes (Table 4 and Figure 4). Although fasting GLP‐1 also showed strong associations with fasting FFA and DPP‐4 (Table 3), incremental AUC‐total GLP‐1 did not show a significant correlation with fasting FFA (r = 0.196 and P = 0.219) and DPP‐4 activity (r = −0.100 and P = 0.535), suggesting that fasting FFA and DPP‐4 activity could predict basal, but not meal‐induced, GLP‐1 secretion in Japanese patients with type 2 diabetes. In contrast, incremental AUC‐total GLP‐1 show a significant correlation with HbA1c (r = 0.406 and P = 0.008), whereas fasting GLP‐1 did not (Table 3), suggesting that HbA1c levels could predict meal‐induced GLP‐1 secretion in Japanese patients with type 2 diabetes. Further studies are, of course, needed to understand the physiological processes underlying associations of GLP‐1 secretion with fasting FFA, HbA1c and DPP‐4 activity.
Regarding hormonal regulation of postprandial GLP‐1 secretion, it was previously suggested that GLP‐1 secretion was enhanced by GIP27. However, the present study in Japanese patients with type 2 diabetes does not support the notion that GIP enhances GLP‐1 secretion, as no significant association was observed. Glucagon is another potential regulator of postprandial GLP‐1 secretion. In Caucasians, not only does administration of exogenous GLP‐1 decrease fasting plasma glucagon levels28, but administration of exendin (9–39)amide, a potent GLP‐1 antagonist, elevates fasting plasma glucagon levels29. Thus, GLP‐1 at physiological levels has glucagonostatic actions, and a reverse relationship between GLP‐1 and glucagon might exist. However, it has been recently shown that administration of glucagon does not affect postprandial GLP‐1 secretion30, and, thus, the mechanism underlying the association of fasting glucagon with postprandial GLP‐1 secretion observed by Vollmer et al. is still unknown. Unlike the Vollmer study, the current study did not show strong associations of fasting and postprandial glucagon with postprandial GLP‐1 secretion, suggesting some difference in regulation of GLP‐1 secretion between Japanese and Caucasian patients with type 2 diabetes.
Characterization of predictors for GIP secretion showed that postprandial GIP secretion, in drug‐naïve patients but not in SU‐treated patients, positively correlated with fasting glucose and negatively correlated with fasting insulin, HOMA‐β and SUIT (Table 4). Other parameters, including fasting FFA, did not show significant associations with pre‐ and postprandial GIP secretion, unlike the Vollmer study15. The Japanese patients in the present study had much lower BMI, but their age and HbA1c were similar to those of Caucasian patients with type 2 diabetes in the Vollmer study. Further studies are needed to understand the differences in predictors of GLP‐1 and GIP secretion between Japanese and Caucasian patients; unequal dependency of postprandial GIP and GLP‐1 secretion in the current study confirms that secretion of the two incretin hormones is regulated by separate factors.
In conclusion, the present study has shown the effects of extractions on immunoassays to measure GLP‐1 and GIP. Using incretin immunoassays with solid‐phase extraction, we have shown that SU‐treatment has little effect on pre‐ and postprandial secretion of GLP‐1 and GIP in Japanese patients. Furthermore, we found that secretion of GLP‐1 and GIP is predicted by different factors.
Supplementary Material
Table S1 Multiple regression analysis of potential predictors of fasting GLP‐1 and GIP levels in Japanese patients with type 2 diabetes
Supporting info item
Acknowledgements
No potential conflicts of interest relevant to this article were reported. The authors thank Tsutomu Hashimoto and Iino Yukari of Mitsubishi Chemical Medience Corporation (Tokyo, Japan) and Takeshi Murakami and Takuro Yamaguchi of Kansai Electric Power Hospital for technical support. We also thank Aya Sanagi at Kansai Electric Power Hospital for secretarial assistance. The present study was partly supported by grants from the Japan Diabetes Foundation and the Diabetes Masters Conference.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–165 [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev 2007; 87: 1409–1439 [DOI] [PubMed] [Google Scholar]
- 3.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Invest 2010; 1: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabe D, Kuroe A, Lee S, et al. Little enhancement of meal‐induced GLP‐1 secretion in Japanese: comparison of type 2 diabetes and healthy controls. J Diabetes Invest 2010; 1: 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaboe K, Knop FK, Vilsboll T, et al. Twelve weeks treatment with the DPP‐4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non‐glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2010; 12: 323–333 [DOI] [PubMed] [Google Scholar]
- 6.Deacon CF, Holst JJ. Immunoassays for the incretin hormones GIP and GLP‐1. Best Pract Res Clin Endocrinol Metab 2009; 23: 425–432 [DOI] [PubMed] [Google Scholar]
- 7.LaMarca CJ, Perregaux DG, Preston GM, et al. Comparison and validation of multiple commercially available methods for active GLP‐1 quantification: sample extraction is a ubiquitous requirement. In: ENDD2009. Washington, D.C. 2009.
- 8.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient‐stimulated incretin secretion. Expert Rev Mol Med 2010; 12: e1 [DOI] [PubMed] [Google Scholar]
- 9.Parker HE, Habib AM, Rogers GJ, et al. Nutrient‐dependent secretion of glucose‐dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009; 52: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reimann F, Habib AM, Tolhurst G, et al. Glucose sensing in L cells: a primary cell study. Cell Metab 2008; 8: 532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross SA, Brown JC, Dupre J. Hypersecretion of gastric inhibitory polypeptide following oral glucose in diabetes mellitus. Diabetes 1977; 26: 525–529 [DOI] [PubMed] [Google Scholar]
- 12.Takemura J, Seino Y, Tsuda K, et al. Hypersecretion of gastric inhibitory polypeptide induced by glucose ingestion in diabetes mellitus. Endocrinol Jpn 1981; 28: 17–21 [DOI] [PubMed] [Google Scholar]
- 13.Toft‐Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon‐like peptide‐1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001; 86: 3717–3723 [DOI] [PubMed] [Google Scholar]
- 14.Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon‐like peptide 1 in type 2 diabetic patients. Diabetes 2001; 50: 609–613 [DOI] [PubMed] [Google Scholar]
- 15.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687 [DOI] [PubMed] [Google Scholar]
- 16.Nauck MA, Vardarli I, Deacon CF, et al. Secretion of glucagon‐like peptide‐1 (GLP‐1) in type 2 diabetes: what is up, what is down? Diabetologia 2010; 54: 10–18 [DOI] [PubMed] [Google Scholar]
- 17.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Diabetol Int 2010; 1: 2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Tamvakopoulos C, Xie D, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide‐(1‐38). J Biol Chem 2003; 278: 22418–22423 [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 21.Yamada Y, Fukuda K, Fujimoto S, et al. SUIT, secretory units of islets in transplantation: an index for therapeutic management of islet transplanted patients and its application to type 2 diabetes. Diabetes Res Clin Pract 2006; 74: 222–226 [DOI] [PubMed] [Google Scholar]
- 22.Dai H, Gustavson SM, Preston GM, et al. Non‐linear increase in GLP‐1 levels in response to DPP‐IV inhibition in healthy adult subjects. Diabetes Obes Metab 2008; 10: 506–513 [DOI] [PubMed] [Google Scholar]
- 23.Conlon JM, Bridgeman M, Alberti KG. The nature of big plasma somatostatin: implications for the measurement of somatostatin‐like immunoreactivity in human plasma. Anal Biochem 1982; 125: 243–252 [DOI] [PubMed] [Google Scholar]
- 24.Tsuda K, Sakurai H, Seino Y, et al. Somatostatin‐like immunoreactivity in human peripheral plasma measured by radioimmunoassay following affinity chromatography. Diabetes 1981; 30: 471–474 [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Yabe D, Nohtomi K, et al. Intact glucagon‐like peptide‐1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J 2010; 57: 119–126 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen LB, Ploug KB, Swift P, et al. Co‐localisation of the Kir6.2/SUR1 channel complex with glucagon‐like peptide‐1 and glucose‐dependent insulinotrophic polypeptide expression in human ileal cells and implications for glycaemic control in new onset type 1 diabetes. Eur J Endocrinol 2007; 156: 663–671 [DOI] [PubMed] [Google Scholar]
- 27.Roberge JN, Brubaker PL. Regulation of intestinal proglucagon‐derived peptide secretion by glucose‐dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 1993; 133: 233–240 [DOI] [PubMed] [Google Scholar]
- 28.Ritzel R, Orskov C, Holst JJ, et al. Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP‐1 [7‐36 amide] after subcutaneous injection in healthy volunteers. Dose‐response‐relationships. Diabetologia 1995; 38: 720–725 [DOI] [PubMed] [Google Scholar]
- 29.Schirra J, Sturm K, Leicht P, et al. Exendin(9‐39)amide is an antagonist of glucagon‐like peptide‐1(7‐36)amide in humans. J Clin Invest 1998; 101: 1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meier JJ, Ueberberg S, Korbas S, et al. Diminished glucagon suppression after beta‐cell reduction is due to impaired alpha‐cell function rather than an expansion of alpha‐cell mass. Am J Physiol Endocrinol Metab 2011; 300: E717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Multiple regression analysis of potential predictors of fasting GLP‐1 and GIP levels in Japanese patients with type 2 diabetes
Supporting info item


