Abstract
Aims/Introduction: Gastric inhibitory polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are the major incretins; their secretion after various nutrient loads are well‐evaluated in Caucasians. However, little is known of the relationship between incretin secretion and differing nutritional loading in Japanese subjects. In the present study, we evaluated GIP and GLP‐1 secretion in Japanese subjects with normal glucose tolerance (NGT) after glucose loading (75 g glucose and 17 g glucose) and meal ingestion.
Materials and Methods: A total of 10 Japanese NGT subjects participated in 75 g oral glucose tolerance test (OGTT), 17 g OGTT and meal tolerance test (MTT). Plasma glucose (PG), serum insulin (IRI), serum C‐peptide (CPR), plasma total GIP, and plasma total GLP‐1 levels during OGTT and MTT were determined.
Results: Area under the curve (AUC)‐GIP was increased in proportion to the amount of glucose, and was highest in MTT, showing that GIP secretion is also stimulated by nutrients other than glucose, such as lipid. In contrast, although the larger glucose load tended to induce a larger GLP‐1 release, AUC‐GLP‐1 was not significantly different among the three loading tests (75 g OGTT, 17 g OGTT, MTT) irrespective of the kind or amount of nutrition load.
Conclusions: Our results suggest that nutritional composition might have a greater effect on GIP secretion than that on GLP‐1 secretion in Japanese NGT subjects. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00143.x, 2012)
Keywords: Incretin, Meal tolerance test, Oral glucose tolerance test
Introduction
Oral glucose administration leads to greater insulin release from pancreatic islets than that by intravenous glucose loading yielding equivalent glucose levels. Gut hormonal substances released in response to glucose include the incretins, gastric inhibitory polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1), which are responsible for 50–60% of postprandial insulin secretion1. GIP is secreted on meal ingestion from K‐cells in the proximal small intestine, whereas GLP‐1 is secreted from L‐cells in the distal small intestine and colon, and binds to their respective receptors on the surface of pancreatic β‐cells to stimulate insulin secretion by increasing the intracellular adenosine 3′,5′‐monophosphate concentration2.
The incretin effect has been shown to be reduced in type 2 diabetic patients compared with that in normal glucose tolerance (NGT) subjects in previous studies3,4, suggesting that a reduced incretin effect might be associated with hyperglycemia after food intake and glucose loading in type 2 diabetes. Plasma GLP‐1 concentrations in type 2 diabetic patients have been reported to be reduced after meal ingestion and glucose loading4,5. However, in other studies, it was reported that GLP‐1 concentrations did not differ in NGT and type 2 diabetic patients6–8. When intravenous infusion of GIP or GLP‐1 was carried out in type 2 diabetic patients, GLP‐1 potentiated insulin secretion from pancreatic β‐cells, but GIP did not, showing that the GIP receptor (GIPR) signal is reduced in β‐cells in type 2 diabetes9. In contrast, the GIPR signal plays an important role in maintaining blood glucose levels in the non‐diabetic obese state10,11. Indeed, GIP concentrations are reported to be increased in obese rodent models and obese Caucasian subjects compared with those in lean rodents and lean Caucasian subjects, respectively12–14. In addition, we have previously shown hypersensitivity of GIPR to GIP in β‐cells of high fat‐induced obese mice11. In summary, evaluation of incretin secretion and the incretin effect in subjects with various levels of glucose tolerance is important to determine the contribution of incretin deficiency in progression from NGT to type 2 diabetes.
Type 2 diabetes is characterized by both decreased insulin secretion and reduced insulin sensitivity15–17. In Caucasians, insulin resistance is thought to play a critical role in the pathogenesis of type 2 diabetes. In contrast, insulin sensitivity in Asian subjects has been shown to be higher than that in Mexican Americans and Caucasians in previous reports18,19, which is partly because of the fact that Asians, including Japanese, are generally less obese. Thus, insulin secretion rather than insulin sensitivity is considered to be the more important factor in progression from NGT to diabetes in Japanese subjects20. Indeed, we have reported that early‐phase insulin secretion is considerably decreased even in Japanese NGT subjects with 1‐h plasma glucose levels higher than 10 mmol/L during an oral glucose tolerance test (OGTT)21.
A recent study showed that, in both Caucasian NGT subjects and Caucasian type 2 diabetic patients, a meal tolerance test (MTT) elicited a significantly greater response of GIP levels than that elicited by OGTT, whereas GLP‐1 levels were not different between OGTT and MTT6. In a previous study comparing the incretin secretion measured after different amounts of glucose load in healthy Caucasian subjects and type 2 diabetic Caucasian patients, GLP‐1 and GIP were dose‐dependently increased22. Plasma GLP‐1 and GIP levels after glucose load or meal ingestion have been evaluated mainly in Caucasian subjects. In Japanese subjects, there has not been thorough elucidation, and little is known about the relationship between incretin secretion, and the kind and amount of nutrition load.
In the present study, we investigated incretin levels in association with the amount of glucose load and meal ingestion by measuring plasma GLP‐1 and GIP levels after administration of 17 or 75 g glucose or mixed meal in Japanese NGT subjects.
Materials and Methods
Subjects
A total of 10 healthy Japanese volunteers (eight male and two female) were recruited into the present study. The subjects had no history of hypertension, hyperlipidemia or kidney and liver diseases, and did not take any drugs 2 weeks before the study. The study was designed in compliance with the ethics regulations of the Helsinki Declaration and Kyoto University. Informed consent was obtained from all subjects.
Study Procedure
The subjects’ age, height and bodyweight were determined. Blood samples for measurement of liver and kidney function, HbA1c (National Glycohemoglobin Standardization Program), triglycerides (TG), total cholesterol and high‐density lipoprotein (HDL)‐cholesterol levels were drawn after an overnight fast. All subjects received 75 g OGTT, 17 g (approximately a quarter of 75 g) OGTT and a MTT. The interval between tests was 2–4 weeks. The total caloric content of the test meal was 450 kcal (carbohydrates 57.8 g, protein 17.2 g, fat 16.6 g). After the subjects fasted overnight for 10–16 h, OGTT or MTT was carried out according to the National Diabetes Data Group recommendations23. NGT was diagnosed according to World Health Organization (WHO) criteria24.
Blood samples were collected at 0, 30, 60, 120 and 180 min after glucose loadings or meal ingestion and were centrifuged at 1800 g at 4°C for 10 min. After collecting supernatant of the samples, plasma and serum were stocked at −80°C. Blood was distributed into chilled tubes containing ethylenediaminetetraacetic acid and aprotinin (500 kIU/mL blood, Trasylol; SRL Inc., Tokyo, Japan) for analyses of GLP‐1 and GIP. Plasma glucose (PG), serum insulin (IRI), serum C‐peptide (CPR), plasma total GIP and plasma total GLP‐1 were measured at the indicated times. The PG levels were measured by the glucose oxidase method. Serum IRI and CPR levels were measured by enzyme‐linked immunosorbent assay. Total GIP and total GLP‐1 levels were measured using a human GIP ELISA kit (Linco Research, St Charles, MO, USA) and human GLP‐1 ELISA kit (Meso Scale Discovery, Gaithersburg, MD, USA), respectively, as previously described25.
Calculations and Statistical Analysis
The area under the curve of PG (AUC‐PG), IRI (AUC‐IRI), CPR (AUC‐CPR), total GIP (AUC‐GIP) and total GLP‐1 (AUC‐GLP‐1) were calculated by the trapezoidal rule. Statistical analyses were carried out using anova and unpaired Student’s t‐test. P‐values <0.05 were considered statistically significant. Data are presented as mean ± standard error (SE).
Results
The profiles of the subjects are shown in Table 1. Mean age was 32.2 ± 2.0 years and mean body mass index was 22.4 ± 0.8 kg/m2. Insulinogenic index, homeostasis model assessment (HOMA)‐β and HOMA‐insulin resistance were 0.59 ± 0.10, 76.50 ± 12.60, 1.10 ± 0.19, respectively. No subjects had liver or kidney dysfunction. HbA1c, PG, TG, total cholesterol and HDL‐cholesterol levels were within normal limits in the fasting state.
Table 1. Clinical characteristics of the subjects.
| n (Male/female) | 10 (8/2) |
|---|---|
| Age (years) | 32.2 ± 2.0 |
| BMI (kg/m2) | 22.4 ± 0.8 |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.2 |
| HbA1c (%) | 5.3 ± 0.1 |
| Triglycerides (mg/dL) | 79.4 ± 10.5 |
| Total cholesterol (mg/dL) | 169.2 ± 6.1 |
| HDL‐cholesterol (mg/dL) | 61.5 ± 5.3 |
| LDL‐cholesterol (mg/dL) | 93.0 ± 9.2 |
Data represent the mean ± SD. BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
The profiles of PG, IRI and CPR in 75 g OGTT, 17 g OGTT and MTT are shown in Figure 1. Judging by the results of 75 g OGTT, all the subjects were diagnosed with NGT according to WHO criteria with fasting plasma glucose and 2 h glucose levels below 6.1 and 7.8 mmol/L, respectively. Fasting concentrations of PG, IRI and CPR were not different among the two OGTT and MTT. In OGTT studies, AUC‐PG, AUC‐IRI and AUC‐CPR measured by the 75 g OGTT were significantly larger than those measured by the 17 g OGTT (Figure 1d–f). At 30 min after glucose ingestion, the levels of PG, IRI and CPR in the 75 g OGTT and those in the 17 g OGTT were not significantly different. Between MTT and the two OGTT, AUC‐PG, AUC‐IRI and AUC‐CPR in MTT were significantly higher than those in the 17 g OGTT. AUC‐PG, AUC‐IRI and AUC‐CPR in the 75 g OGTT and in MTT were not significantly different.
Figure 1.
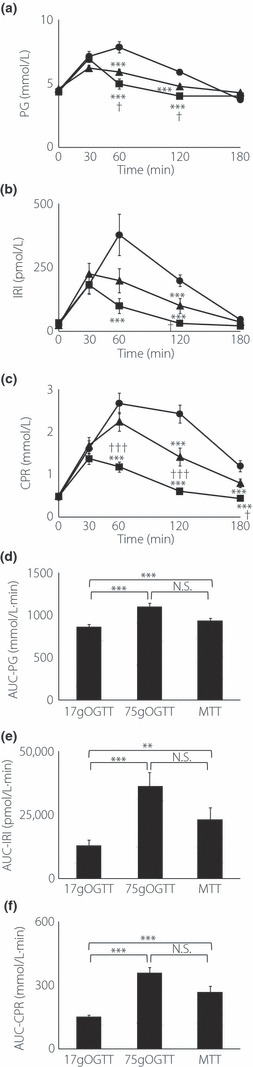
Concentrations of (a) plasma glucose (PG), (b) serum insulin (IRI) and (c) serum C‐peptide (CPR) during the 75 g oral glucose tolerance test (OGTT; closed circle), 17 g OGTT (closed square) and meal tolerance test (MTT; closed triangle) in 10 Japanese subjects. Asterisks indicate significant differences vs 75 g OGTT at individual time‐points (*P < 0.05, **P < 0.01, ***P < 0.001); daggers indicate significant differences vs MTT at individual time‐points (†P < 0.05, ††P < 0.01, †††P < 0.001). (d) Area under the curve (AUC)‐PG, (e) AUC‐IRI, (f) AUC‐CPR were calculated by the trapezoidal rule. Asterisks indicate significant differences at individual time‐points (*P < 0.05, **P < 0.01, ***P < 0.001). Statistical analyses were carried out using anova and unpaired Student’s t‐test. P‐values <0.05 were considered statistically significant. Data are presented as mean ± standard error. N.S., not significant.
In the 17 g OGTT, the total GLP‐1 level peaked at 30 min and rapidly decreased to the baseline at 60 min after the glucose load. The total GLP‐1 level peaked at 30 min after the meal load and was sustained for up to 180 min. In the 75 g OGTT, the GLP‐1 level peaked at 60 min and gradually decreased with time, but the level was still higher than baseline even at 180 min. The level of total GLP‐1 at 60 min after the 75 g glucose load was significantly higher than that after the 17 g glucose load (Figure 2a). Although a larger glucose load tended to induce a larger GLP‐1 release, total AUC‐GLP‐1 measured by the 75 g OGTT, 17 g OGTT and MTT were not significantly different (Figure 2b).
Figure 2.
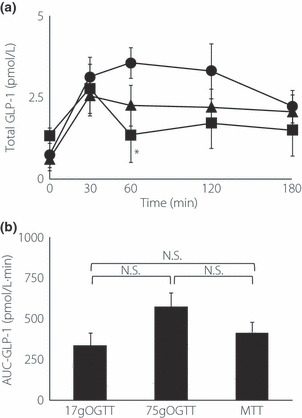
(a) Concentrations of total glucagon‐like peptide‐1 (GLP‐1) during the 75 g oral glucose tolerance test (OGTT; closed circle), 17 g OGTT(closed square) and meal tolerance test (MTT; closed triangle) in 10 Japanese subjects. Asterisks indicate significant differences vs 75 g OGTT at individual time‐points (*P < 0.05, **P < 0.01, ***P < 0.001); daggers indicate significant differences vs MTT at individual time‐points (†P < 0.05, ††P < 0.01, †††P < 0.001). (b) Area under the curve (AUC)‐GLP‐1 was calculated by the trapezoidal rule. Asterisks indicate significant differences at individual time‐points (*P < 0.05, **P < 0.01, ***P < 0.001). Statistical analyses were carried out using anova and unpaired Student’s t‐test. P‐values <0.05 were considered statistically significant. Data are presented as mean ± standard error. N.S., not significant.
The baseline levels of GIP were approximately 10 pmol/L. The GIP level rapidly increased, peaked at 30 min after the meal load and gradually decreased with time, but the level was still higher than baseline even at 180 min. In the 75 g OGTT, the GIP level significantly increased at 30 min after the glucose load, peaked at 120 min and were maintained up to 180 min. In the 17 g OGTT, the total GIP level peaked at 30 min after glucose load and gradually decreased to baseline at 180 min. At 30 min after ingestion, total GIP levels in the 75 g OGTT and those in the 17 g OGTT were not significantly different (Figure 3a).
Figure 3.
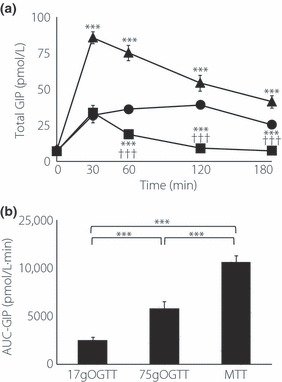
(a) Concentrations of total gastric inhibitory polypeptide (GIP) during 75 g oral glucose tolerance test (OGTT; closed circle), 17 g OGTT (closed square) and meal tolerance test (MTT; closed triangle) in 10 Japanese subjects. Asterisks indicate significant differences vs 75 g OGTT at individual time‐points (*P < 0.05, **P < 0.01, ***P < 0.001); daggers indicate significant differences vs MTT at individual time‐points (†P < 0.05, ††P < 0.01, †††P < 0.001). (b) Area under the curve (AUC)‐GIP was calculated by the trapezoidal rule. Asterisks indicate significant differences at individual time‐points (*P < 0.05, **P < 0.01, ***P < 0.001). Statistical analyses were carried out using anova and unpaired Student’s t‐test. P‐values <0.05 were considered statistically significant. Data are presented as mean ± standard error.
AUC‐GIP was significantly higher in the 75 g OGTT than that in the 17 g OGTT. Unlike GLP‐1, the peak levels of GIP and the AUC‐GIP measured in the MTT were significantly higher than those measured in the 75 g OGTT and 17 g OGTT (Figure 3b).
Discussion
In the present study, incretin levels were estimated after glucose loading or meal ingestion in Japanese NGT subjects.
Between the OGTT studies, AUC‐PG, AUC‐IRI and AUC‐CPR in the 75 g OGTT were larger than those in the 17 g OGTT. Regarding incretins, AUC‐GIP was significantly larger in the 75 g OGTT than in the 17 g OGTT. In contrast, AUC‐GLP‐1 was not significantly different between the 75 g OGTT and the 17 g OGTT. Previous studies showed that a larger amount of oral glucose load elicited more GIP and GLP‐1 secretion1,22, whereas a recent study also reported that the secretory response of GIP was more sensitive than that of GLP‐1 to changes in intestinal carbohydrate content26. The present study also showed that while GLP‐1 level was not increased, GIP level was increased dose‐dependently in response to glucose load, showing higher sensitivity of GIP to changes of administered nutrient dose.
Between the 75 g OGTT and MTT studies, AUC‐PG, AUC‐IRI and AUC‐CPR were not significantly different. AUC‐GIP was significantly larger in MTT than that in the 75 g OGTT. In contrast, there was no significant difference in AUC‐GLP‐1 among the MTT and the two OGTT. By comparing the results of the three loading tests (75 g OGTT, 17 g OGTT, MTT), we speculate that AUC‐GIP is more susceptible to the contents of each loading test than AUC‐GLP‐1 is. Vollmer et al.6 reported that GIP responses were significantly higher in MTT than in OGTT, whereas GLP‐1 levels were similar in both tests in Caucasian NGT, IGT and type 2 diabetic subjects. Because the mixed meal contains not only carbohydrates but also fat, which has been reported to stimulate GIP secretion27–29, it is likely that the increased GIP concentrations after MTT were largely as a result of the fat content, which might have had no additional impact on GLP‐1 secretion.
There are two previous reports that evaluate the incretin levels in both OGTT and MTT in Japanese NGT subjects8,30. However, they compared the incretin levels in 75 g glucose or meal load between NGT and type 2 diabetic subjects, but did not compare the incretin levels between 75 g glucose and meal load directly. The present study directly compared the incretin levels in the two OGTT and MTT. Our data clearly show that GIP responses were significantly higher in MTT than those in the two OGTT, whereas GLP‐1 levels were not different between the two OGTT and MTT in Japanese NGT subjects.
According to the study by Yabe et al.8, AUC‐GIP is similar between the OGTT and MTT group in Japanese control subjects. It should be noted that the difference between GIP secretion after meal load and that after glucose load was far greater in the present study than that in the study by Yabe et al. The total caloric content of the test meal used in their study was 480 kcal (carbohydrates 58.4%, protein 20.8%, fat 20.8%) and that in the present study was 450 kcal (carbohydrates 51.4%, protein 15.3%, fat 33.3%). Therefore, it is possible that the higher amount of contained fat in the test meal used in the present study led to the greater response of GIP secretion in the MTT.
Fasting and peak total GLP‐1 concentrations in the present study were approximately 1 pmol/L and 3.5 pmol/L, respectively, and seemed to be lower than those in some published results8,31. However, in other reports, total GLP‐1 levels after glucose and meal load were not very different from those in the present study. Rijkelijkhuizen et al.32 measured the total GLP‐1 concentration with radioimmunoassay, and in their results, the fasting and peak total GLP‐1 concentrations in the MTT were approximately 1 pmol/L and 4.5 pmol/L, respectively. In addition, Villareal et al.33 evaluated total GLP‐1 concentrations by the same method that we used in the present study, and reported that the fasting and peak total GLP‐1 concentrations in OGTT were approximately 1.5 and 6 pmol/L, respectively. Judging by the data in these reports, it is not necessarily the case that total GLP‐1 concentrations were extremely low in the present study.
There are some reports showing that GLP‐1 secretion is dependent on meal size, especially on carbohydrate and glucose loads. Schirra et al.34 reported that GLP‐1 plasma levels rose from basal levels to fourfold after 50 g glucose ingestion and to eightfold after 100 g glucose ingestion. Rijkelijkhuizen et al.32 showed that GLP‐1 secretion is increased by the amount of carbohydrate (75 and 109 g) and not by the quantity of the meal. In the present study, however, AUC‐GLP‐1 was not significantly different among the three loading tests (75 g OGTT, 17 g OGTT, MTT) irrespective of kinds or amounts of nutrition load, although larger glucose load tended to induce a larger GLP‐1 release. The most notable difference between the previous studies and the present study was the amount of glucose load. We compared GLP‐1 secretion after administration of 17 g glucose, 75 g glucose and 57.8 g of carbohydrate contained in the meal that we used. The amount of glucose and carbohydrate load in the present study were relatively lower than those in the previous studies. It is possible that evaluation of GLP‐1 secretion after larger glucose loads could be more appropriate to show the glucose dependency of GLP‐1 secretion.
It is also noteworthy that the levels of PG, IRI, CPR, GIP and GLP‐1 at 30 min after the 75 g OGTT and 17 g OGTT were similar to each other. In addition, the levels of IRI, CPR and GLP‐1 at 30 min after MTT and the two OGTT were not significantly different. By contrast, the GIP level at 30 min after MTT was much higher than those after the 17 g OGTT and 75 g OGTT. Given the similar plasma glucose levels at 30 min after the 17 g OGTT and 75 g OGTT, it is likely that under physiological conditions, the rate at which ingested glucose emptied into the duodenum is regulated finely enough to prevent an abrupt increase in plasma glucose levels irrespective of the amount of ingested glucose. Previous studies have shown that GLP‐1 secretion after a test meal or oral glucose load is associated with the rate of gastric emptying, whereas GIP secretion seems to be dependent on nutrient absorption rather than on rate of gastric emptying34. Accordingly, a finely regulated rate of gastric emptying might account for the similar levels of GLP‐1 at 30 min after MTT, 17 g OGTT and 75 g OGTT. In contrast, the level of total GIP at 30 min after the MTT was much higher than those after the two OGTT, probably because of the presence of fat in the duodenal lumen, as fat is a forcible stimulant of GIP, as discussed earlier.
The present results clearly show that the secretion of GIP and GLP‐1 are regulated by different nutrient factors. On the basis of our data, it is also suggested that nutritional composition might have a greater effect on GIP secretion than on GLP‐1 secretion in Japanese NGT subjects.
Acknowledgements
We thank Dr Yutaka Seino (Kansai Electric Power Hospital) for his helpful suggestions. This study was supported by Scientific Research Grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by Kyoto University Global COE Program ‘Center for Frontier Medicine’, and also by Novo Nordisk Pharma Ltd.
References
- 1.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C‐peptide responses. J Clin Endocrinol Metab 1986; 63: 492–498 [DOI] [PubMed] [Google Scholar]
- 2.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormone: similarities and difference. J Diabetes Invest 2010; 1: 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non‐insulin‐dependent) diabetes. Diabetologia 1986; 29: 46–52 [DOI] [PubMed] [Google Scholar]
- 4.Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57: 1340–1348 [DOI] [PubMed] [Google Scholar]
- 5.Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon‐like peptide 1 in type 2 diabetic patients. Diabetes 2001; 50: 609–613 [DOI] [PubMed] [Google Scholar]
- 6.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687 [DOI] [PubMed] [Google Scholar]
- 7.Faerch K, Vaag A, Holst JJ, et al. Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 2008; 51: 853–861 [DOI] [PubMed] [Google Scholar]
- 8.Yabe D, Kuroe A, Lee S, et al. Little enhancement of meal‐induced glucagon‐like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Invest 2010; 1: 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon‐like peptide 1 [7‐36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. J Clin Invest 1993; 91: 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyawaki K, Yamada Y, Yano H, et al. Glucose intolerance caused by a defect in the entero‐insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 1999; 96: 14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada N, Yamada Y, Tsukiyama K, et al. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic beta‐cells in obese mice. Am J Physiol Endocrinol Metab 2008; 294: E61–E68 [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002; 8: 738–742 [DOI] [PubMed] [Google Scholar]
- 13.Flatt PR, Bailey CJ, Kwasowski P, et al. Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes 1983; 32: 433–435 [DOI] [PubMed] [Google Scholar]
- 14.Creutzfeldt W, Ebert R, Willms B, et al. Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia 1978; 14: 15–24 [DOI] [PubMed] [Google Scholar]
- 15.Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992; 326: 22–29 [DOI] [PubMed] [Google Scholar]
- 16.Haffner SM, Stern MP, Hazuda HP, et al. Increased insulin concentrations in nondiabetic offspring of diabetic parents. N Engl J Med 1988; 319: 1297–1301 [DOI] [PubMed] [Google Scholar]
- 17.Saad MF, Knowler WC, Pettitt DJ, et al. A two‐step model for development of non‐insulin‐dependent diabetes. Am J Med 1991; 90: 229–235 [PubMed] [Google Scholar]
- 18.Chiu KC, Chuang LM, Yoon C. Comparison of measured and estimated indices of insulin sensitivity and beta cell function: impact of ethnicity on insulin sensitivity and beta cell function in glucose‐tolerant and normotensive subjects. J Clin Endocrinol Metab 2001; 86: 1620–1625 [DOI] [PubMed] [Google Scholar]
- 19.Mandavilli A, Cyranoski D. Asia’s big problem. Nat Med 2004; 10: 325–327 [DOI] [PubMed] [Google Scholar]
- 20.Seino Y, Ikeda M, Yawata M, et al. The insulinogenic index in secondary diabetes. Horn Metab Res 1975; 7: 323–335 [Google Scholar]
- 21.Harada N, Fukushima M, Toyoda K, et al. Factors responsible for elevation of 1‐h postchallenge plasma glucose levels in Japanese men. Diabetes Res Clin Pract 2008; 81: 284–289 [DOI] [PubMed] [Google Scholar]
- 22.Bagger JI, Knop FK, Lund A, et al. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 737–745 [DOI] [PubMed] [Google Scholar]
- 23.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979; 28: 1039–1057 [DOI] [PubMed] [Google Scholar]
- 24.Alberi KG, Zimmeret PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 54: 539–553 [DOI] [PubMed] [Google Scholar]
- 25.Harada N, Hamasaki A, Yamane S, et al. Plasma GIP and GLP‐1 levels after glucose loading are associated with different factors in Japanese subjects. J Diabetes Invest 2011; 2: 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoder SM, Yang Q, Kindel TL, et al. Differential responses of the incretin hormones GIP and GLP‐1 to increasing doses of dietary carbohydrate but not dietary protein in lean rats. Am J Physiol Gastrointest Liver Physiol 2010; 299: G476–G485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JC, Dryburgh JR, Ross SA, et al. Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res 1975; 31: 487–532 [DOI] [PubMed] [Google Scholar]
- 28.Falko JM, Crockett SE, Cataland S, et al. Gastric inhibitory polypeptide (GIP) stimulated by fat ingestion in man. J Clin Endocrinol Metab 1975; 41: 260–265 [DOI] [PubMed] [Google Scholar]
- 29.Pederson RA, Schubert HE, Brown JC. Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes 1975; 24: 1050–1056 [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Yabe D, Nohtomi K, et al. Intact glucagon‐like peptide‐1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J 2010; 57: 119–126 [DOI] [PubMed] [Google Scholar]
- 31.Kozawa J, Okita K, Imagawa A, et al. Similar incretin secretion in obese and non‐obese Japanese subjects with type 2 diabetes. Biochem Biophys Res Commun 2010; 393: 410–413 [DOI] [PubMed] [Google Scholar]
- 32.Rijkelijkhuizen JM, McQuarrie K, Girman CJ, et al. Effects of meal size and composition on incretin, alpha‐cell, and beta‐cell responses. Metabolism 2010; 59: 502–511 [DOI] [PubMed] [Google Scholar]
- 33.Villareal DT, Robertson H, Bell GI, et al. TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes 2010; 59: 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996; 97: 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]


