Abstract
Aims/Introduction: Although increases in urinary protein excretion generally precede a decline in the glomerular filtration rate, non‐proteinuric renal impairment is common in patients with diabetes. In the present study, we examined the relationship between indices of arterial stiffness and renal function in type 2 diabetic patients without proteinuria.
Methods: Blood sampling, 24‐h urine collection, brachial–ankle pulse wave velocity, and 24‐h ambulatory blood pressure monitoring were performed in type 2 diabetic patients without overt proteinuria. The ambulatory arterial stiffness index was calculated as (1 – the regression slope of diastolic/systolic ambulatory blood pressure). Estimated glomerular filtration rate (eGFR)was calculated using the simplified prediction equation proposed by the Japanese Society of Nephrology.
Results: Of 213 non‐proteinuric patients with type 2 diabetes, 60 (28.2%) had a reduced eGFR (<60 mL/min per 1.73 m2). Although the urinary albumin excretion rate was significantly correlated with the eGFR, 34 of 152 patients with normoalbuminuria (22.4%) had a reduced eGFR. The eGFR was significantly and negatively correlated with the ambulatory arterial stiffness index and brachial–ankle pulse wave velocity, but not with 24‐h pulse pressure. Multivariate analysis revealed that increased age and increased urinary albumin excretion were independently associated with decreased eGFR. In addition, the ambulatory arterial stiffness index, but not brachial–ankle pulse wave velocity, were found to be independently and significantly associated with eGFR.
Conclusions: Ambulatory arterial stiffness index is a marker for increased risk of renal failure in non‐proteinuric patients with type 2 diabetes. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00146.x, 2012)
Keywords: Vascular compliance, Albuminuria, Chronic kidney disease
Introduction
Although increases in urinary protein excretion generally precede a decline in renal function1, recent studies have highlighted the large number of diabetic patients with decreases in estimated glomerular filtration rate (eGFR; <60 mL/min per 1.73 m2) without increased urinary protein excretion2. Although many explanations have been proposed to account for this observation, the causes of non‐proteinuric renal impairment remain to be determined. Recently, we found that the presence of extrarenal small vessel disease, namely silent cerebral infarction (SCI), was an independent risk factor for the development of renal failure in type 2 diabetes3. Furthermore, we found that the presence of SCI did not increase the risk of progression of nephropathy (i.e., from normoalbuminuria to microalbuminuria or from microalbuminuria to overt proteinuria)3. An increased resistance index of the renal interlobar arteries has been reported to be associated with decreases in eGFR, independent of the proteinuric status4. These findings suggest that vascular damage may be a marker for reduced renal function in type 2 diabetic patients without proteinuria.
Increased arterial stiffness, recognized as an early marker of atherosclerosis5, has been reported to be associated with silent cerebral small vessel disease6. Furthermore, increases in arterial stiffness have been found to be associated with reduced renal function7,8. Aortic pulse wave velocity (aPWV) is a standard marker of arterial stiffness. However, measurement of aPWV requires complex equipment and trained personnel9. In contrast, brachial–ankle PWV (baPWV) is convenient to measure and is thus the most widely used PWV index. The ambulatory arterial stiffness index (AASI), a new index derived from 24‐h ambulatory blood pressure monitoring (ABPM), is thought to reflect dynamic arterial stiffness10,11.
The aim of the present study was to examine the associations between the indices of arterial stiffness (baPWV and AASI) and eGFR in non‐proteinuric type 2 diabetic patients.
Materials and Methods
Patients
Patients with type 2 diabetes were recruited from patients who regularly visited the outpatient clinic of the Department of Medicine, Shiga University of Medical Science, over the period 2008–2009. Patients were clinically diagnosed with type 2 diabetes in accordance with the World Health Organization criteria (http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf). Patients were excluded from the study if they had cancer, liver disease, infectious disease, collagen disease, peripheral arterial disease (PAD), or nondiabetic kidney disease confirmed by renal biopsy. Patients were considered to have PAD if the ankle–brachial index (ABI) of either leg was ≤0.90. Patients with a history of cerebrovascular events, myocardial infarction, angina treatment, heart failure, uncontrolled arrhythmias, or an implanted cardiac pacemaker were also excluded from the study. Eligible patients were informed of the study protocol both orally and in written form. Blood pressure was measured in the clinic at least twice using a mercury sphygmomanometer with the patient in the sitting position after resting for ≥5 min. Hypertension was defined as clinic‐measured systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or the current use of antihypertensive medication. Each individual provided a blood sample for biochemical analysis and underwent standard physical examination in addition to measurement of ABI, baPWV, and 24‐h ABPM. Both ABI and baPWV were measured using an automatic waveform analyzer (Colin, Komaki, Japan). The 24‐h ambulatory blood pressure was measured non‐invasively every 30 min using an automatic device (model TM‐2431; A&D, Tokyo, Japan). Twenty‐four hour SBP, DBP, and heart rate values were determined as the mean of all values recorded at 30‐min intervals over the 24‐h period. Pulse pressure (PP) was calculated as the difference between SBP and DBP. Nocturnal blood pressure was calculated as the mean of blood pressure values recorded from the time when the patient went to bed until the patient woke up, whereas daytime blood pressure was calculated as the mean of the remaining readings. For each study participant, we computed the regression slope of DBP/SBP and calculated the AASI as (1 – regression slope). The albumin excretion rate (AER) was determined by an immunoturbidimetry assay (Hitachi 7070E; Hitachi High‐Technologies, Tokyo, Japan) using a single 24‐h urine sample collected on the same day as the blood sample. On the basis of urinary AER, patients were classified as having normoalbuminuria (AER < 20 μg/min) or microalbuminuria (AER 20–200 μg/min). Patients with macroalbuminuria (>200 μg/min) were excluded from the present study. Twenty‐four hour urinary creatinine excretion was also measured in a single urine sample and creatinine clearance (Ccr) calculated. The eGFR was calculated using the simplified prediction equation proposed by the Japanese Society of Nephrology12 as follows:
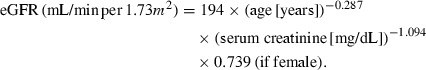 |
In the present study, according to the definition of the Japan Diabetes Society (JDS)13, the value for HbA1c (%) was estimated as an NGSP equivalent value (%) calculated using the formula HbA1c (%) = HbA1c (JDS) (%) + 0.4%.
The study protocol and the procedure for obtaining informed consent were approved by the Ethics Committee of Shiga University of Medical Science. All participants provided written informed consent before participating in the study.
Statistical Analysis
Results are expressed as the mean ± SD or as the median with the interquartile range given in parentheses. The AER was transformed logarithmically prior to analysis because of its skewed distribution. All statistical analyses were performed using JMP version 5.0 (SAS Institute, Tokyo, Japan). Mean values of continuous variables were compared between two groups using unpaired t‐tests. Comparisons of continuous variables among more than three groups were performed using one‐way anova with a Tukey–Kramer honestly significant difference (HSD) test. Comparisons of the number of classes of antihypertensive drugs were made using the Kruskal Wallis test. Frequencies were compared by χ2 analysis. Linear regression analysis was used to determine the relationship between the eGFR and continuous variables, whereas a multivariate linear regression model was applied to identify clinical independent predictive factors for decreases in eGFR. The independent variables evaluated were age, sex, body mass index, HbA1c, serum lipid levels (total cholesterol, high‐density lipoprotein–cholesterol, triglycerides), SBP, DBP, nocturnal/daytime SBP ratio, the use of inhibitors of the renin–angiotensin system (RAS), and indices of arterial stiffness (Model 1: AASI; Model 2: baPWV).
Results
Clinical Characteristics of the Patients
We analyzed 213 type 2 diabetic patients with normoalbuminuria or microalbuminuria, for whom blood sampling, 24‐h urine collection, baPWV, and 24‐h blood pressure data were all available. The AER was found to have significant negative correlations with eGFR (r = −0.23; P < 0.001; Figure 1) and with Ccr (r = −0.19; P = 0.006). Of the 152 patients with normoalbuminuria, 34 (22.4%) were found to have a reduced eGFR (i.e., <60 mL/min per 1.73 m2), compared with 26 of 61 patients (42.6%) with microalbuminuria in whom eGFR was reduced. Thus, the prevalence of a reduced eGFR was significantly higher in patients with microalbuminuria than in those with normoalbuminuria (P < 0.01).
Figure 1.
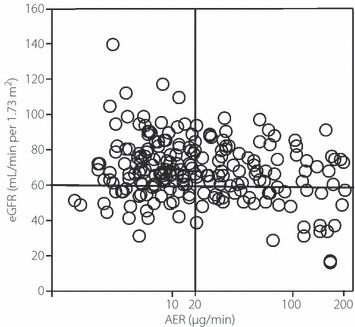
Correlation between the estimated glomerular filtration rate (eGFR) and albumin excretion rate (AER) in type 2 diabetic patients without proteinuria (r = −0.23; P = 0.0008).
The baseline clinical characteristics of the study population, grouped according to eGFR and albuminuria, are compared in Table 1. When compared with patients with normoalbuminuria and conserved renal function (Group 1), patients with microalbuminuria and reduced eGFR (Group 4) were found to be significantly older and to have higher 24‐h PP and AASI. Even among patients with normoalbuminuria, AASI was significantly higher in those with reduced eGFR (Group 2) than in those with conserved renal function (Group 1). Patients with reduced eGFR were treated more aggressively with antihypertensive medications. Finally, Ccr was found to differ significantly between the four groups (Table 1).
Table 1. Patient characteristics.
| Normoalbuminuria | Microalbuminuria | |||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |
| n | 118 | 34 | 35 | 26 |
| Age (years) | 64.5 ± 10.9 | 68.4 ± 10.4 | 63.8 ± 7.8 | 70.2 ± 6.9* |
| Sex (male/female) | 59/59 | 17/17 | 25/10 | 17/9 |
| BMI (kg/m2) | 24.3 ± 4.2 | 25.0 ± 3.2 | 24.9 ± 3.3 | 24.2 ± 3.5 |
| HbA1c (%) | 7.5 ± 1.0 | 7.4 ± 0.7 | 7.5 ± 0.8 | 7.6 ± 0.9 |
| TChol (mg/dL) | 199 ± 30 | 184 ± 24* | 192 ± 25 | 202 ± 32 |
| TG (mg/dL) | 105 ± 79 | 93 ± 29 | 119 ± 69 | 120 ± 45 |
| HDL‐C (mg/dL) | 58 ± 16 | 55 ± 16 | 53 ± 12 | 55 ± 16 |
| No. hypertensives (%) | 89 (75.4) | 30 (88.2) | 31 (88.6) | 22 (84.6) |
| 24‐h Mean blood pressure | ||||
| SBP (mmHg) | 137 ± 14 | 135 ± 12 | 142 ± 14 | 140 ± 12 |
| DBP (mmHg) | 78 ± 9 | 76 ± 7 | 81 ± 9 | 76 ± 8 |
| PP | 58 ± 10 | 59 ± 11 | 62 ± 9 | 65 ± 10* |
| Nocturnal/daytime SBP ratio | 0.88 ± 0.09 | 0.87 ± 0.16 | 0.91 ± 0.12 | 0.91 ± 0.09 |
| Ccr (mL/min per 1.73 m2) | 99 ± 22 | 89 ± 26* | 95 ± 23 | 73 ± 26*† |
| AASI | 0.50 ± 0.12 | 0.57 ± 0.12* | 0.56 ± 0.13 | 0.60 ± 0.09* |
| baPWV (cm/s) | 1615 ± 311 | 1665 ± 328 | 1726 ± 186 | 1721 ± 228 |
| No. antihypertensive drugs (range) | 1 (0–2) | 2 (1–3) | 1 (0–2) | 2* (1–3) |
Data are given as the mean ± SD, the number of patients with percentages in parentheses, or as the median, with 25th–75th interquartile range in parentheses, as appropriate. *P < 0.05 compared with Group 1; †P < 0.05 compared with Group 3. Group 1, estimated glomerular filtration rate (eGFR) ≥60 mL/min per 1.73 m2; Group 2, eGFR < 60 mL/min per 1.73 m2; Group 3, eGFR ≥60 mL/min per 1.73 m2; Group 4, eGFR < 60 mL/min per 1.73 m2; BMI, body mass index; TChol, total cholesterol; TG, triglycerides; HDL‐C, high‐density lipoprotein–cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; Ccr, creatinine clearance; AASI, ambulatory arterial stiffness index; baPWV, brachial–ankle pulse wave velocity.
Association Between Indices of Arterial Stiffness and eGFR
As shown in Figure 2, eGFR was inversely correlated with AASI and baPWV. Conversely, 24‐h PP was not significantly correlated with eGFR (P = 0.065). Using multivariate linear regression to evaluate whether indices of arterial stiffness (AASI and baPWV) are independently associated with an increased risk of decreases in eGFR, we found that AER and AASI were independently associated with eGFR in Model 1 (including AASI), whereas only age and AER were independently associated with eGFR in Model 2 (including baPWV; Table 2).
Figure 2.
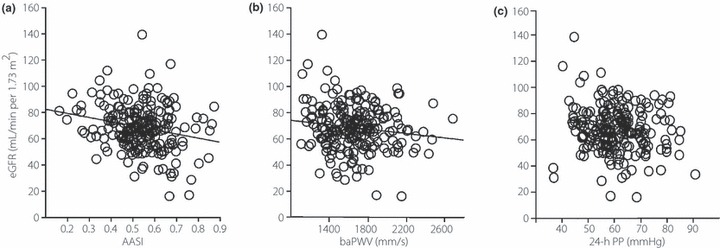
Correlations between estimated glomerular filtration rate (eGFR) and (a) ambulatory arterial stiffness index (AASI; r = −0.22; P = 0.001), (b) brachial–ankle PWV (baPWV; r = −0.17; P = 0.044) and (c) 24‐h pulse pressure (PP; r = −0.13; P = 0.065) in type 2 diabetic patients without proteinuria.
Table 2. Multivariate linear regression analysis to identify variables independently associated with estimated glomerular filtration rate.
| Model 1* | Model 2† | |||
|---|---|---|---|---|
| β | P | β | P | |
| Age | −0.325 | <0.001 | −0.343 | <0.001 |
| AER | −0.194 | 0.007 | −0.225 | 0.002 |
| AASI | −0.152 | 0.025 | – | – |
*r2 = 0.216; †r2 = 0.198. The independent variables evaluated were age, sex, body mass index, HbA1c, serum lipid levels (total cholesterol, high‐density lipoprotein–cholesterol, triglycerides), systolic blood pressure, nocturnal/daytime systolic blood pressure ratio, the use of renin–angiotensin system inhibitors, and either ambulatory arterial stiffness index (AASI; Model 1) or brachial–ankle pulse wave velocity (baPWV; Model 2).
Discussion
The results of the present cross‐sectional study revealed that AASI, a conventional index of arterial stiffness, is independently associated with reduced renal function in non‐proteinuric type 2 diabetic patients. In addition, AER was also associated with eGFR. These results suggest that albuminuria and atherosclerosis are independently associated with reduced renal function in type 2 diabetic patients.
Increased aortic stiffness is a well‐known predictor of cardiovascular morbidity and mortality, and is considered to be an early sign of atherosclerosis14. In addition, arterial stiffness has been reported to be independently associated with eGFR and albuminuria8,15. The carotid–femoral PWV is a well‐established index of aortic stiffness. However, the accurate measurement of this index involves a delicate procedure to manipulate the measurement probe and usually takes >20 min, which may cause ischemic events in patients with carotid atherosclerosis by dislodging any plaques. Recently, the baPWV was developed as a new method to evaluate arterial stiffness using an oscillometer. However, because the baPWV is influenced by blood pressure, an increased baPWV may be due to elevated blood pressure at the time of measurement rather than by arterial stiffness per se16. New indices derived from 24‐h ABPM, namely 24‐h PP and AASI, have been proposed to reflect dynamic arterial stiffness10,11. The AASI is strongly influenced by PP because increases in PP are associated with a fall in compliance of the arterial circulation and pulse wave reflection17. Because wave reflection occurs throughout the arterial tree at each arterial branch, the AASI and 24‐h PP may be influenced by peripheral vascular resistance. However, 24‐h PP may also be influenced by elevated blood pressure at the time of measurement rather than by arterial stiffness per se. Therefore, AASI may be a more sensitive predictor of cardiovascular events than 24‐h PP. In fact, AASI has been reported to predict cardiovascular and stroke mortality independent of 24‐h PP and other risk factors in a Japanese population18.
It is well established that an increase in arterial stiffness is associated with reduced renal function7,8. It was also reported that AASI is negatively correlated with eGFR in hypertensive subjects19–21. Because intrarenal vascular lesions are one of the main features of hypertension‐associated nephropathy, increased arterial stiffness in hypertensive subjects may be closely associated with renal function. However, in diabetic patients without proteinuria, it remains unclear whether elevated arterial stiffness is an independent risk factor for a decline in renal function. In the present study, AASI was independently correlated with eGFR. These findings support the hypothesis that small artery lesions in the kidney play an important role in the development of renal failure in type 2 diabetes. The association between impaired kidney function and magnetic resonance imaging (MRI)‐detected cerebral infarction was reported previously in several cross‐sectional studies22,23. In addition, in an earlier study we showed that the presence of SCI was associated with poor renal prognosis, but not with the progression of nephropathy, which was defined on the basis of levels of albuminuria3. A unique feature of the kidneys and brain is that they are continually exposed to pulsatile circumferential stress because their vascular resistance is very low24. In the Atherosclerosis Risk in Communities (ARIC) study, MRI evidence of the presence of white matter lesions, a small vessel disease in the brain, was associated with PP25. These findings indicate that small artery disease contributes to the worsening of renal function and brain damage, and that AASI may be a suitable marker for poor renal prognosis in type 2 diabetic patients.
The urinary AER is the best available non‐invasive clinical predictor of diabetic nephropathy, although recent clinical studies have shown that renal insufficiency can occur in the absence of microalbuminuria2,26. In addition, albuminiuria was reported to be associated with arterial stiffness15. In fact, in the present study, AER was associated with AASI (r = 0.22; P = 0.001) and 24‐h PP (r = 0.23; P < 0.001), but not with baPWV. However, the present study showed that AER and AASI were inversely and independently correlated with eGFR. These results have prompted the suggestion that glomerular lesions and renal vessel diseases both independently play important roles in the development of renal failure in type 2 diabetes.
Vascular damage is closely associated with renal function and lipids may be involved in the progression of renal disease. Although elevated low‐density lipoprotein–cholesterol (LDL‐C) is a well‐established risk factor for cardiovascular disease, few studies have found a relationship between LDL‐C and the progression of chronic kidney disease (CKD)27. In the present study, total cholesterol and LDL‐C levels calculated using the Friedewald equation were not independently associated with eGFR. Long‐term observations and randomized trials are needed to determine whether elevated LDL affects renal function and whether modifying the LDL levels may delay the progression of CKD.
The present study has some limitations. First, although patients with a history of cerebrovascular events, myocardial infarction, angina treatment, or heart failure and PAD were excluded, we did not evaluate renal artery diseases. Indeed, atherosclerotic renal artery stenosis may be responsible for the decreased eGFR in some patients. In addition, we did not measure aortic PWV and indicators reflecting renal tubular damage. Finally, the present cross‐sectional study could not address whether the risk of future end‐stage renal failure is higher in patients with elevated 24‐h PP. Further prospective studies are needed to determine whether the conventional indices of arterial stiffness, such as AASI, can predict future cardiovascular events, progression of diabetic nephropathy, and the decline in renal function.
In conclusion, the present study has revealed that AASI is independently associated with reduced renal function and that 24‐h ABPM provides valuable information with which to assess cardiovascular morbidity and mortality (including blood pressure, the circadian rhythm of blood pressure, and arterial stiffness). Therefore, 24‐h ABPM seems to be a useful tool to aid the early detection of diabetic vascular complications, including diabetic nephropathy, and/or to evaluate the risk of progression of those complications in type 2 diabetic patients.
Acknowledgement
The authors declare no conflict of interest.
References
- 1.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 2.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004; 27: 195–200 [DOI] [PubMed] [Google Scholar]
- 3.Uzu T, Kida Y, Shirahashi N, et al. Cerebral microvascular disease predicts renal failure in type 2 diabetes. J Am Soc Nephrol 2010; 21: 520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacIsaac RJ, Panagiotopoulos S, McNeil KJ, et al. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care 2006; 29: 1560–1566 [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241 [DOI] [PubMed] [Google Scholar]
- 6.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small‐vessel disease in hypertensive patients. Hypertension 2008; 52: 1120–1126 [DOI] [PubMed] [Google Scholar]
- 7.Tetzner F, Scholze A, Wittstock A, et al. Impaired vascular reactivity in patients with chronic kidney disease. Am J Nephrol 2008; 28: 218–223 [DOI] [PubMed] [Google Scholar]
- 8.Madero M, Wassel CL, Peralta CA, et al. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol 2009; 20: 1086–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Bortel LM, Duprez D, Starmans‐Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 2002; 15: 445–452 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Wang JG, Dolan E, et al. Ambulatory arterial stiffness index derived from 24‐hour ambulatory blood pressure monitoring. Hypertension 2006; 47: 359–364 [DOI] [PubMed] [Google Scholar]
- 11.Palmas W, Pickering TG, Teresi J, et al. Ambulatory blood pressure monitoring and all‐cause mortality in elderly people with diabetes mellitus. Hypertension 2009; 53: 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo S, Imai E, Horio M, et al. Revised equations for estimating glomerular filtration rate (GFR) from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 13.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Diabetol Int 2010; 1: 2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blacher J, Asmar R, Djane S, et al. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117 [DOI] [PubMed] [Google Scholar]
- 15.Smith A, Karalliedde J, De Angelis L, et al. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol 2005; 16: 1069–1075 [DOI] [PubMed] [Google Scholar]
- 16.Choi JC, Lee JS, Kang SY, et al. Limitation of brachial–ankle pulse wave velocity in assessing the risk of stroke: importance of instantaneous blood pressure. Cerebrovasc Dis 2009; 27: 417–425 [DOI] [PubMed] [Google Scholar]
- 17.Dart AM, Kingwell BA. Pulse pressure: a review of mechanisms and clinical relevance. J Am Coll Cardiol 2001; 37: 975–984 [DOI] [PubMed] [Google Scholar]
- 18.Kikuya M, Staessen JA, Ohkubo T, et al. Ambulatory arterial stiffness index and 24‐hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke 2007; 38: 1161–1166 [DOI] [PubMed] [Google Scholar]
- 19.Ratto E, Leoncini G, Viazzi F, et al. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens 2006; 24: 2033–2038 [DOI] [PubMed] [Google Scholar]
- 20.Mulè G, Cottone S, Cusimano P, et al. Inverse relationship between ambulatory arterial stiffness index and glomerular filtration rate in arterial hypertension. Am J Hypertens 2008; 21: 35–40 [DOI] [PubMed] [Google Scholar]
- 21.García‐García A, Gómez‐Marcos MA, Recio‐Rodriguez JI, et al. Relationship between ambulatory arterial stiffness index and subclinical target organ damage in hypertensive patients. Hypertens Res 2011; 34: 180–186 [DOI] [PubMed] [Google Scholar]
- 22.Seliger SL, Gillen DL, Tirschwell D, et al. Risk factors for incident stroke among patients with end‐stage renal disease. J Am Soc Nephrol 2003; 14: 2623–2631 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S, Ikeda T, Moriya H, et al. Asymptomatic cerebral lacunae in patients with chronic kidney disease. Am J Kidney Dis 2004; 44: 35–41 [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46: 200–204 [DOI] [PubMed] [Google Scholar]
- 25.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology 1997; 16: 149–162 [DOI] [PubMed] [Google Scholar]
- 26.Kramer HJ, Nguyen QD, Curhan G, et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289: 3273–3277 [DOI] [PubMed] [Google Scholar]
- 27.Muntner P, Coresh J, Smith JC, et al. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int 2000; 58: 293–301 [DOI] [PubMed] [Google Scholar]


