Abstract
The continuous glucose monitoring system (CGM) has been used for constant checking of glucose level by measuring interstitial glucose concentrations, since the early days of the 21st century. It can potentially improve diabetes care if used carefully with the understanding of the characteristics of this system. Although there is a time lag of approximately 5–15 min between blood and interstitial glucose levels, the system is considered the most suitable device for meticulous glucose control and prevention of hypoglycemia. A large number of studies have examined its accuracy, safety and clinical effectiveness. The continuous glucose‐error grid analysis (CG‐EGA), designed by WL Clarke, evaluates the clinical accuracy of CGM. It examines ‘temporal’ characteristics of the data, analyzing pairs of reference and sensor readings as a process in time represented by a ‘bidimensional’ time series and taking into account inherent physiological time lags. Investment in CG‐EGA is clearly meaningful, even though there are other methodologies for evaluation. The use of each method complementarily is the most effective way to prove the accuracy of the device. The device has improved gradually, and real‐time CGM, which allows real‐time monitoring of blood glucose level, is already available commercially. The use of real‐time CGM could potentially lead to over‐ or undertreatment with insulin. Patient education through proper and effective handling of the new device is essential to improve diabetes care. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2012.00197.x, 2012)
Keywords: Continuous glucose monitoring system, Continuous glucose‐error grid analysis, Patient education
Introduction
The management of diabetes has seen changes in the past 30 years, which have improved the lives of patients afflicted with this disease1. Improvement in glucose measurement started with the introduction of self‐monitoring of blood glucose (SMBG) in the late 1980s, which was one of those important changes.
As the second device for measurement of blood glucose level, the continuous glucose monitoring system (CGM) was introduced to the market around the beginning of this century. It provides maximal information about fluctuations in blood glucose levels throughout the day and optimal treatment decisions to control diabetes2. In 1999, the Food and Drug Administration approved the CGM device in the USA. Since then, the device has been improved dramatically, and the real‐time CGM and insulin pump‐integrated system are already available commercially in the USA and Europe.
In Japan, the history of CGM is relatively short. The Ministry of Health, Labor and Welfare approved the CGM device in 2009, approximately 10 years after the USA. Furthermore, the second‐generation blind CGM, the MiniMed Gold of Medtronic, has been the only device approved under the government‐supported medical insurance in Japan. A new wireless, but blind CGM, is going to be in Japanese market in a very near future, at last.
With this different historical background, CGM has already been studied extensively in the USA and Europe. At this stage, it is necessary for Japanese clinicians to know what has already been investigated. In particular, we need to understand whether the continuous glucose monitoring system really is an accurate, safe and clinically effective device, or not3. In the present article, we review the literature and introduce some useful information on diabetes care in the clinical setting especially to the physicians in countries with short history of CGM.
Merits of the Continuous Glucose Monitoring System
The Diabetes Control and Complications Trial (DCCT) confirmed that patients treated intensively with insulin show better results with regard to the prevention of diabetic microvascular complications than those treated conventionally. However, we also know that the intensive insulin therapy enhances the risk of hypoglycemia. Patients who experience frequent episodes of hypoglycemia often develop various abnormalities and lose their ability to detect hypoglycemia. This is referred to as ‘hypoglycemia unawareness’ and it perpetrates ‘a vicious cycle of recurrent hypoglycemia’. However, there is strong evidence showing that minimization of the hypoglycemic episodes through more meticulous glucose control can actually reverse many of the counter‐regulatory defects4.
For meticulous glucose control and prevention of hypoglycemia, the CGM is a useful device, as it provides maximal information about fluctuating blood glucose levels throughout the day5–12. The finger stick test provides only information on blood glucose at one point in time, so even if ‘hypoglycemia unawareness’ occurs between the measured points, we are not able to detect it. With the CGM, we might accomplish both purposes – better control with the intensive insulin therapy and prevention of ‘hypoglycemia unawareness’.
The CGM is a method that provides constant checking of blood glucose level through measurement of glucose concentrations in the interstitial fluid13. The MiniMed Gold of Medtronic, which is currently available and used in Japan under the government‐based health insurance system, features a sensor inserted in the subcutaneous tissue just under the skin of the abdomen. The sensor comprises a flexible, platinum‐plated electrode housed inside a permeable membrane, which can be used for up to 72 h. Subcutaneous glucose level is measured by the glucose‐oxidase method, and the interstitial glucose level measured every 10 s is sent to a monitor that shows the average blood glucose value every 5 min. The blood glucose values are calculated using software. The MiniMed Gold calculates blood glucose values in the range of 40–400 mg/dL. Athough the user has no access to real‐time glucose levels, the measured data can be downloaded into a spreadsheet14.
One important procedure that needs to be carried out repeatedly is calibration. This is carried out at least four times a day in the MiniMed Gold, using the finger stick test. It means that self‐monitoring of blood glucose is closer to real glucose levels, the venous glucose levels, at any one point in time. It should be noted that the device has a time lag between blood and interstitial glucose levels.
Time Lag Between Blood and Interstitial Glucose Levels
In 2002, Cheyne et al.15 published their article about CGM during controlled hypoglycemia in healthy volunteers. There was no information on the performance of the sensor during sustained hypoglycemia or during recovery from hypoglycemia at that time. Therefore, they used the hyperinsulinemic glucose clamp to prove interesting findings15. Venous blood glucose levels were maintained at euglycemia for the first 60 min, then fell to 45 mg/dL for 60 min, but were finally restored to euglycemia. Blood glucose measurements were compared with interstitial values recorded by the sensor. The sensor profiles paralleled blood glucose levels at each of the three plateaus with a correlation coefficient of 0.79, and mean absolute error of 7%. The drop in glucose level measured by the sensor closely matched the drop in blood glucose, but the recovery from hypoglycemia was delayed by an average of 26 min15. The device was the first‐generation MiniMed CGM, which was probably less accurate than the second‐generation, MiniMed Gold.
In 2003, Boyne et al.16 tried to measure a time lag between the first‐generation MiniMed CGM and blood glucose, and also between the different sensors. A total of 14 patients with type 1 diabetes each had two sensors placed subcutaneously in the abdomen, acquiring data every 5 min. At the same time, blood glucose was sampled every 5 min for 8 h. The results showed that the ‘time differences between blood and interstitial glucose levels ranged from 4 to 10 min, with the interstitial glucose lagging behind blood glucose in 81% of cases. The mean (± SD) difference between the two sensors in each patient was 6.7 ± 5.1 min, representing random variation in sensor response’. The authors also showed that the lag times were statistically significant for the rise (10.1 ± 10.1 min, P < 0.001), fall (6.9 ± 8.5 min, P = 0.017) and nadir (9.4 ± 7.7 min, P < 0.001) in glucose levels. In each case, blood glucose level preceded that of interstitial glucose16.
Other groups reported similar results, although there was some variability among the different studies17–19. Thus, the main conclusions were that there is a time lag between the measured interstitial glucose levels20,21 and actual blood levels, and that the different sensors have different sensitivities in measuring interstitial glucose levels16,22.
Continuous Glucose‐Error Grid Analysis
The Clarke error grid analysis (EGA), designed by William L Clarke, was first reported in 1987. The EGA is designed for SMBG to evaluate the agreement and discrepancy between blood glucose values and sensor readings at isolated static points in time. This method was innovative, because it took into account not only the difference between the system‐generated and reference blood glucose values, but also the clinical significance of this difference23–26. In other words, Clarke et al. tried to evaluate the accuracy and clinical significance of the continuous glucose monitoring system. In 2004, they also reported an improvement to the original EGA, and introduced the CG‐EGA, the continuous glucose‐error grid analysis27.
CG‐EGA was specifically designed to evaluate the clinical accuracy of continuous glucose monitoring in terms of precision of both blood glucose readings and blood glucose rate of change. Unlike the original EGA, the CG‐EGA examines ‘temporal’ characteristics of the data, analyzing pairs of reference and sensor readings as a process in time represented by a ‘bidimensional’ time series and taking into account inherent physiological time lags27. In this method, they introduced a new concept of ‘rate‐error grid analysis (R‐EGA)’ in addition to modifying the traditional EGA into a new ‘point‐error grid analysis (P‐EGA)’ that reflects the temporal characteristics of blood glucose.
The R‐EGA is a rate‐error grid analysis that assesses ‘the sensor’s ability to capture the direction and rate of blood glucose fluctuations’. For each pair of RBG (reference blood glucose) readings (RBG [t1], RBG [t2]) taken at times t1 and t2, the RBG rate is computed as ΔBG divided by the elapsed time. The RBG rate of change (mg/dL/min) = (RBG [t2] – RBG [t1])/(t2 – t1). Similarly, for each sensor blood glucose (SBG) pair (SBG [t1], SBG [t2]), SBG rate is computed as SBG rate of change (mg/dL/min) = (SBG [t2] – SBG [t1]/[t2 – t1]). Then, the SBG rate is plotted against the RBG rate (Figure 1). The P‐EGA is a point‐error grid analysis that evaluates the sensor’s accuracy in terms of ‘correct representation of blood glucose values’. Point accuracy reflects the difference between two paired samples at one point in time (Figure 2)27.
Figure 1.
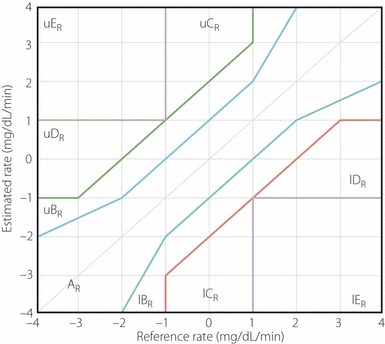
The rate‐error grid analysis (R‐EGA) divided into AR, BR, CR, DR and ER for sensor blood glucose (SBG) rate vs reference blood glucose (RBG) rate. The R‐EGA zones extend theoretically to infinity. l, Lower; R, rate; u, upper.
Figure 2.
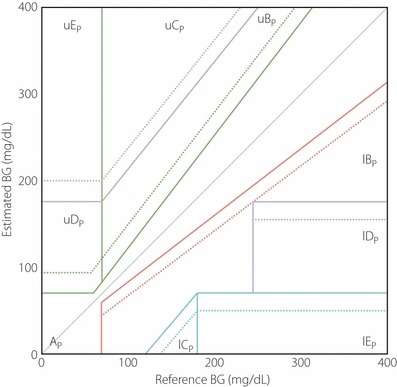
The point‐error grid analysis (P‐EGA) divided into AP, BP, CP, DP and EP for sensor blood glucose (SBG) vs reference blood glucose (RBG). The P‐EGA zones are defined based on the reference rate of changes in blood glucose. l, Lower; p, point; u, upper.
Both the R‐EGA and P‐EGA divide the glucose rate or glucose ranges into clinically meaningful zones: ‘zone A, corresponding to clinically accurate reading; zone B, corresponds to benign errors; zone C, signifies overcorrection errors; zone D, indicates failure to detect clinically significant rate of change in blood glucose; and zone E, indicates an erroneous reading’28. The P‐EGA zones are defined depending on the reference rate of BG changes. Also, the R‐EGA zones theoretically extend to infinity.
The CG‐EGA recognizes that the clinical meaning of rate accuracy depends greatly on the absolute blood glucose level, with different blood glucose levels requiring different interpretations of the combination R‐EGA + P‐EGA. For this reason, the CG‐EGA computes the combined accuracy of R‐EGA + P‐EGA in three clinically relevant regions: hypoglycemia (blood glucose ≤70 mg/dL), euglycemia and hyperglycemia (blood glucose >180 mg/dL; Figure 3)27. As the CG‐EGA is intended for software application, most of these parameters could be user selectable. For example, the time lag between blood and interstitial glucose has a default value of 7 min, based on literature data. If a device has a longer technical lag, then the software would allow the time lag used by the P‐EGA to be changed27.
Figure 3.
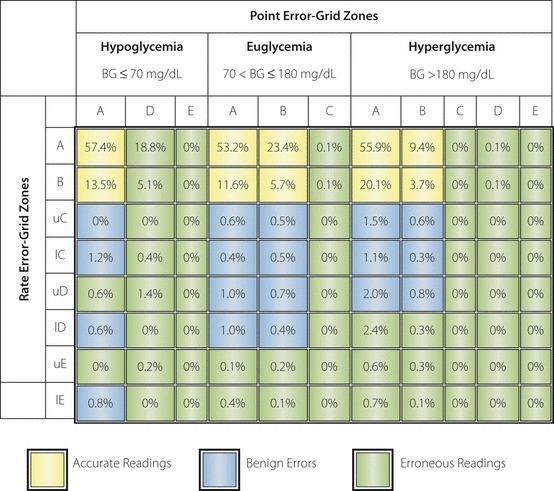
The continuous glucose‐error grid analysis (CG‐EGA) computes the accuracy of the combination of rate‐error grid analysis (R‐EGA) plus point‐error grid analysis (P‐EGA) into three clinically relevant regions: hypoglycemia (blood glucose ≤70 mg/dL), euglycemia and hyperglycemia (blood glucose >180 mg/dL). BG, blood glucose; l, lower; u, upper.
Clarke et al. used the CG‐EGA to evaluate the continuous glucose monitoring system, TheraSense Freestyle Navigator27. Using the CG‐EGA, they reported that the accuracy of the Navigator, measured as a percentage of accurate readings plus benign errors, was significantly different at hypoglycemia (73.5%), euglycemia (99%) and hyperglycemia (95.4%). Failure to detect hypoglycemia was recognized to be the most common error27.
Pros and Cons of the CG‐EGA
Wentholt et al.29 explored the CG‐EGA by comparing it with classical accuracy assessment methods, such as correlation, linear regression and mean absolute difference (MAD), using data reported in a previous study that compared two different continuous glucose sensors in type 1 diabetic patients. They found differences in the accuracy of MAD and CG‐EGA, and that the CG‐EGA was not reliable. In contrast, Clarke et al. responded by showing that the methods used by Wentholt unfortunately failed to take into account the basic structure of continuous glucose monitoring data, which represents a time series analysis30,31. They reported that it is impossible to compare the different methods altogether, and approached the issue of accuracy by applying several different methods for ‘each’ different purpose28. Using the different methods complementarily is the most effective way to prove the accuracy of the device.
The investment in CG‐EGA, which has been widely used by scholars until now, is really meaningful for assessing the accuracy of CGM. By using multiple established assessment methods for evaluation, we can reach the true accuracy of CGM, thus ensuring the safety and clinical effectiveness of the device.
Importance of Patient Education
In the symposium, Wolpert published some important points4. As we already know, there is a lag time between interstitial and blood glucose. The lag time ranges from 5 to 15 min depending on the rate of glucose change. Increasing glucose levels are reflected first in capillary blood. However, detecting decreases in glucose after insulin injection or exercise might be confounded by the placement of the CGM sensor. For example, if the CGM sensor is placed next to an insulin‐sensitive tissue, the glucose level might decrease first in the interstitial fluid before it is reflected in capillary blood glucose. Also, Wolpert pointed out that the sensor needs to be calibrated when glucose is ‘in a steady state’. If the patient carried out the calibration when glucose is rising at 3 mg/dL/min and there is a 10‐min lag, then there will be a discrepancy of approximately 30 mg/dL between the capillary glucose measurement and the interstitial glucose measurement. This will shift the curve upwards and make the sensor less accurate4.
Another important point relates to the education of the patient. In the USA and Europe, the real‐time CGM are already available on the market, and many articles have been published on the device32–37. The real‐time CGM wireless type is a very convenient and useful device, but it can potentially lead to excess treatment based on the measured data. If patients overcompensate for rising glucose levels using frequent bolus injections of insulin, they are at increased risk of hypoglycemia. Also, patients could reduce or discontinue basal insulin therapy in order to avoid hypoglycemia. This occurs more commonly in patients who lack the understanding of the concept of the lag time between capillary and interstitial glucose, thus considering their glucose to be much lower than it actually is. We need to educate these patients about insulin pharmacodynamics, as well as the factors that affect postprandial glucose patterns. They must also be taught not to rely on interstitial glucose data alone to assess hypoglycemia4.
Meta‐Analysis and Guideline about CGM
Some meta‐analyses of randomized trials about CGM and real‐time CGM have already been published. There are some variabilities about the conclusions among the different meta‐analyses. A group at the Mayo clinic concluded that the device was associated with a significant reduction in mean glycated hemoglobin (HbA1c). It was true for adults with type 1 diabetes as well as type 2 diabetes. No significant effect was noted in children and adolescents. There was no significant difference in HbA1c reduction between studies of real‐time vs non‐real‐time devices, if the devices were appropriately used. Data for the incidence of severe or nocturnal hypoglycemia were sparse and imprecise38. The meta‐analysis by Mayo Clinic is important, because it was newly published in 2011, and the number of the trials providing the data, 19, was the biggest among the meta‐analyses about CGM3,38–41.
The safety of the device was reported in another meta‐analysis40. Data of adverse events were available only in a few trials and comprised mainly mild reactions, such as redness and itching, at the sensor implantation site. Individual cases of skin abscess formation or cellulitis were infrequently reported. The device errors of data storage, and alarms going off infrequently occurred.
In October 2011, ‘Continuous Glucose Monitoring: An Endocrine Society Clinical Practice Guideline’ was published42. Even if there are various conclusions in meta‐analyses about CGM, the Task Force evaluated ‘three potential uses of CGM’: (i) real‐time CGM in an adult hospital setting; (ii) real‐time CGM in children and adolescent outpatients; and (iii) real‐time CGM in adult outpatients. The authors used the available data to develop evidence‐based recommendations for good control of glycemia and limiting the risk of hypoglycemia. In that guideline, the blind CGM is not strongly recommended, and evaluated as ‘an alternative for patients who cannot safely and effectively take advantage of the information provided to them’ by real‐time CGM42, even though there are some studies concluding that there was no significant difference in HbA1c reduction between real‐time vs non‐real‐time devices38. However, it might be an appropriate statement in countries where the real‐time CGM is the first choice CGM. Also the guideline approves the efficacy of the device for children.
Conclusion
By reviewing the literature on CGM, we understand that this device has the potential to improve diabetes care in the clinical setting, provided we take into consideration its pros and cons. There is a need for more knowledge about its accuracy, safety and clinical effectiveness through daily medical examination. Furthermore, the introduction of the more developed devices, such as real‐time CGM available in the USA and Europe, under the Japan government‐sponsored health‐care system should be encouraged.
Acknowledgment
T Hirose and H Watada have received grant support from Takeda, Nippon Eli Lilly, and MSD. T Hirose and H Watada have also acted as spokespeople for Takeda, Nippon Eli Lilly, and Sanofi Aventis. J Sato declares no conflict of interest.
References
- 1.Deeb LC. Diabetes technology during the past 30 years: a lot of changes and mostly for the better. Diabetes Spectr 2008; 21: 78–83 [Google Scholar]
- 2.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care 2005; 28: 1231–1239 [DOI] [PubMed] [Google Scholar]
- 3.Golicki DT, Golicka D, Groele L, et al. Continuous glucose monitoring system in children with type 1 diabetes mellitus: a systematic review and meta‐analysis. Diabetologia 2008; 51: 233–240 [DOI] [PubMed] [Google Scholar]
- 4.Wolpert HA. Use of continuous glucose monitoring in the detection and prevention of hypoglycemia. J Diabetes Sci Technol 2007; 1: 146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachedina N, Pickup JC. Performance assessment of the Medtronic‐MiniMed continuous glucose monitoring system and its use for measurement of glycaemic control in type 1 diabetic subjects. Diabet Med 2003; 20: 1012–1015 [DOI] [PubMed] [Google Scholar]
- 6.De Block C, Manuel‐Y‐Keenoy B, Van Gaal L, et al. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care 2006; 29: 1750–1756 [DOI] [PubMed] [Google Scholar]
- 7.Augstein P, Vogt L, Kohnert KD, et al. Outpatient assessment of Karlsburg diabetes management system‐based decision support. Diabetes Care 2007; 30: 1704–1708 [DOI] [PubMed] [Google Scholar]
- 8.Rhee SY, Chon S, Koh G, et al. Clinical experience of an iontophoresis based glucose measuring system. J Korean Med Sci 2007; 22: 70–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein RL, Schwartz SL, Brazg RL, et al. Accuracy of the 5‐day freestyle navigator continuous glucose monitoring system: comparison with frequent laboratory reference measurements. Diabetes Care 2007; 30: 1125–1130 [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Li H, Ran X, et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care 2009; 32: 1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care 2010; 33: 1297–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group , Wilson DM, Xing D, et al. Hemoglobin A1c and mean glucose in patients with type 1 diabetes: analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care 2011; 34: 540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogh‐Andersen N, Altura BM, Altura BT, et al. Composition of interstitial fluid. Clin Chem 1995; 41: 1522–1525 [PubMed] [Google Scholar]
- 14.Corstjens AM, Ligtenberg JJ, van der Horst IC, et al. Accuracy and feasibility of point‐of‐care and continuous blood glucose analysis in critically ill ICU patients. Crit Care 2006; 10: R135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheyne EH, Cavan DA, Kerr D. Performance of a continuous glucose monitoring system during controlled hypoglycemia in healthy volunteers. Diabetes Technol Ther 2002; 4: 607–613 [DOI] [PubMed] [Google Scholar]
- 16.Boyne MS, Silver DM, Kaplan J, et al. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003; 52: 2790–2794 [DOI] [PubMed] [Google Scholar]
- 17.Kulcu E, Tamada JA, Reach G, et al. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care 2003; 26: 2405–2409 [DOI] [PubMed] [Google Scholar]
- 18.Klonoff DC. The need for separate performance goals for glucose sensors in the hypoglycemic, normoglycemic, and hyperglycemic ranges. Diabetes Care 2004; 27: 834–836 [DOI] [PubMed] [Google Scholar]
- 19.Nielsen JK, Djurhuus CB, Gravholt CH, et al. Continuous glucose monitoring in interstitial subcutaneous adipose tissue and skeletal muscle reflects excursions in cerebral cortex. Diabetes 2005; 54: 1635–1639 [DOI] [PubMed] [Google Scholar]
- 20.Stout PJ, Racchini JR, Hilgers ME. A novel approach to mitigating the physiological lag between blood and interstitial fluid glucose measurements. Diabetes Technol Ther 2004; 6: 635–644 [DOI] [PubMed] [Google Scholar]
- 21.Schrot RJ, Patel KT, Foulis P. Evaluation of inaccuracies in the measurement of glycemia in the laboratory, by glucose meters, and through measurement of hemoglobin A1c. Clin Diabetes 2007; 25: 43–49 [Google Scholar]
- 22.Wentholt IM, Vollebregt MA, Hart AA, et al. Comparison of a needle‐type and a microdialysis continuous glucose monitor in type 1 diabetic patients. Diabetes Care 2005; 28: 2871–2876 [DOI] [PubMed] [Google Scholar]
- 23.Clarke WL, Cox D, Gonder‐Frederick LA, et al. Evaluating clinical accuracy of systems for self‐monitoring of blood glucose. Diabetes Care 1987; 10: 622–628 [DOI] [PubMed] [Google Scholar]
- 24.Cox DJ, Gonder‐Frederick LA, Kovatchev BP, et al. Understanding error grid analysis. Diabetes Care 1997; 20: 911–912 [DOI] [PubMed] [Google Scholar]
- 25.Parkes JL, Slatin SL, Pardo S, et al. A New Consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000; 23: 1143–1148 [DOI] [PubMed] [Google Scholar]
- 26.Clarke WL. The original Clarke error grid analysis (EGA). Diabetes Technol Ther 2005; 7: 776–779 [DOI] [PubMed] [Google Scholar]
- 27.Kovatchev BP, Gonder‐Frederick LA, Cox DJ, et al. Evaluating the accuracy of continuous glucose‐monitoring sensors/Continuous glucose‐error grid analysis illustrated by TheraSense freestyle navigator data. Diabetes Care 2004; 27: 1922–1928 [DOI] [PubMed] [Google Scholar]
- 28.Kovatchef B, Anderson S, Heinemann L, et al. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care 2008; 31: 1160–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wentholt IM, Hoekstra JB, DeVries JH. A Critical appraisal of the continuous glucose‐error grid analysis. Diabetes Care 2006; 29: 1805–1811 [DOI] [PubMed] [Google Scholar]
- 30.Clarke WL, Gonder‐Frederick L, Cox D, et al. A critical appraisal of the continuous glucose‐error grid analysis: response to Wentholt et al. Diabetes Care 2007; 30: 449–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wentholt IM, Hoekstra JB, DeVries JH. A critical appraisal of the continuous glucose‐error grid analysis: response to Clarke et al. Diabetes Care 2007; 30: 450–451 [DOI] [PubMed] [Google Scholar]
- 32.Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real‐time continuous glucose sensor: a randomized controlled trial. Diabetes Care 2006; 29: 44–50 [DOI] [PubMed] [Google Scholar]
- 33.O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient‐led use of sensor‐guided pump therapy in type 1 diabetes: a randomized controlled trial. Diabetologia 2009; 52: 1250–1257 [DOI] [PubMed] [Google Scholar]
- 34.Holzinger U, Warszawska J, Kitzberger R, et al. Real‐time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care 2010; 33: 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins AJ, Krishnamurthy B, Best JD, et al. Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real‐time continuous glucose levels: a randomized controlled trial. Diabetes Care 2010; 33: 1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ly TT, Hewitt J, Davey RJ, et al. Improving epinephrine responses in hypoglycemia unawareness with real‐time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care 2011; 34: 50–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigersky RA, Fonda SJ, Chellappa M, et al. Short‐ and long‐term effects of real‐time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012; 35: 32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi GY, Kovalaske M, Kudva Y, et al. Efficacy of continuous glucose monitoring in improving glycemic control and reducing hypoglycemia: a systematic review and meta‐analysis of randomized trials. J Diabetes Sci Technol 2011; 5: 952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chetty VT, Alumulla A, Odueyungbo A, et al. The effect of continuous subcutaneous glucose monitoring (CGMS) vs intermittent whole blood finger‐stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in Type 1 diabetic patients: a systematic review. Diabetes Res Clin Pract 2008; 81: 79–87 [DOI] [PubMed] [Google Scholar]
- 40.Wojciechowski P, Ryś P, Lipowska A, et al. Efficacy and safety comparison of continuous glucose monitoring and self‐monitoring of blood glucose in type 1 diabetes: systematic review and meta‐analysis. Pol Arch Med Wewn 2011; 121: 333–343 [PubMed] [Google Scholar]
- 41.Hoekes LB, Greven WL, de Valk HW. Real‐time continuous glucose monitoring system for treatment of diabetes: a systemic review. Diabet Med 2011; 28: 386–394 [DOI] [PubMed] [Google Scholar]
- 42.Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 2968–2979 [DOI] [PubMed] [Google Scholar]


