Abstract
Aims/Introduction: To evaluate if hemoglobin A1c (A1C) can replace the use of the oral glucose tolerance test (OGTT) to diagnose diabetes in Chinese patients.
Materials and Methods: Subjects without pre‐existing diabetes were included in this community‐based study. Each participant received a 75‐g OGTT and A1C tests.
Results: A total of 1362 subjects, 512 men and 850 women, aged 18–88 years, were enrolled. The prevalence of diabetes was 7.4 and 7.3% by OGTT and by A1C ≥ 6.5% criteria, respectively. The optimal A1C cut‐off for diabetes defined by OGTT was 6.1%. The performance of A1C ≥ 6.1% to find diabetes by OGTT was poor, with a kappa 0.50, sensitivity 80% and specificity 91%. Using current criteria of fasting plasma glucose (FPG) < 5.56 mmol/L to exclude and ≥7 mmol/L to diagnose diabetes (FPG criterion), the sensitivity, specificity and OGTT required were 77.2, 100 and 13.5%, respectively. Using A1C < 5.9% to exclude and ≥7.0% to diagnose diabetes (A1C criterion), the sensitivity, specificity and OGTT required were 89.1, 99.8 and 26.5%, respectively. However, using FPG < 5.56 mmol/L and A1C < 6.1% to exclude, and A1C ≥ 7.0% to diagnose diabetes (A1C plus FPG criterion), the sensitivity, specificity and OGTT required were 85.2, 100 and 18.9%, respectively.
Conclusions: To screen for diabetes, the A1C criterion is more sensitive than the FPG criterion, with more OGTT needed. The A1C plus FPG criterion reduced the number of OGTT needed with acceptable sensitivity. A1C can guide, but cannot replace, OGTT to diagnose diabetes. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00181.x, 2011)
Keywords: Diagnosis, Hemoglobin A1c, Oral glucose tolerance test
Abbreviations:
- 2‐h PG
2‐h plasma glucose
- A1C
hemoglobin A1c
- ADA
American Diabetes Association
- BMI
body mass index
- CI
confidence interval
- CV
coefficients of variance
- DCCT
Diabetes Control and Complications Trial
- DM
diabetes mellitus
- FPG
fasting plasma glucose, IFG, impaired fasting glucose
- IGT
impaired glucose tolerance
- IQR
interquartile range
- LR+
positive likelihood ratio
- LR−
negative likelihood ratio
- NGSP
National Glycohemoglobin Standardization Program
- NPV
negative prediction values
- OGTT
oral glucose tolerance test
- PPV
positive prediction value
- ROC
receiver–operator curve
Introduction
In January 2010, the use of A1C to diagnose DM was officially recommended by the ADA1,2. A1C has been shown to correlate with the occurrence of diabetic retinopathy in previous reports3,4. However, it was not recommended to diagnose DM at that time, mainly because of the lack of standardization of the assay2. After the years of effort, the assays for A1C have been highly standardized, at least in the USA. Therefore, the ADA revised their diagnostic criteria for DM by adopting the use of A1C2, which is more practical and time‐saving than an OGTT.
After the announcement, many studies were carried out to evaluate the diagnostic performance of A1C for DM in different populations5–10. As the optimal A1C cut‐off can vary in different races11, the performance of the currently recommended A1C cut‐off should be evaluated in different ethnic groups. For example, Araneta et al.10 reported that using the A1C cut‐off of 6.5% might delay the diagnosis of DM, especially in Asian Americans who frequently have isolated postchallenge hyperglycemia. Bao et al.9 suggested a A1C cut‐off of 6.3% for diagnosis of DM in the Chinese population, which was lower than the ADA‐proposed cut‐off. Besides, to apply A1C as a new diagnostic or screening tool, it is important to know if A1C and OGTT criteria identified a similar group of people. If these criteria highly agree with each other, A1C can simply replace the OGTT test as a simple method of diagnosis. If not, A1C could be used to decide whether OGTT should be carried out. Therefore, the aim of the present study was to investigate the following two questions: (i) can A1C criteria replace OGTT criteria to diagnose DM; and (ii) what is the screening strategy to find DM using A1C as one of the tools?
Methods
During 2006–2009, residents in Yunlin County aged 18 years and above were invited to participate in the present study, termed the Taiwan Lifestyle Study12. A questionnaire was given to discover if participants had DM or received medication for DM, with the aid of a trained nurse. Participants who reported DM or received medication for DM were excluded. Written informed consent was obtained from each individual, and the study was reviewed and approved by the Institutional Review Board.
A standard 75‐g OGTT was carried out after an 8‐h fast. Normal glycemia (FPG < 5.56 mmol/L and 2‐h PG < 7.8 mmol/L), IFG (FPG 5.56–6.9 mmol/L), IGT (2‐h PG 7.8–11.0 mmol/L) and DM (FPG ≥ 7 mmol/L and/or 2‐h PG ≥ 11.1 mmol/L) were diagnosed according to the results of OGTT by the criteria of the ADA proposed in 200313. Plasma glucose and A1C were measured by automatic analyzers (Toshiba TBA 120FR, Toshiba Medical Systems Co., Tokyo, Japan; and HLC‐723 G7 HPLC systems, Tosoh Corporation, Tokyo, Japan, respectively). The coefficients of variation (CV) for plasma glucose were 1.1–1.4% at 86 mg/dL (4.8 mmol/L) and 0.8–0.9% at 293 mg/dL (16.3 mmol/L). The CV for A1C were 1.6–1.8% at 5.5–5.7% (37–39 mmol/mol) and 0.9–1.0% at 9.7–10.1% (83–87 mmol/L). The laboratory attends and is qualified by an external quality assurance program by the Taiwan Society of Laboratory Medicine twice a year. The A1C assay was certified by the NGSP14 and standardized to the DCCT reference assay.
Kappa statistics were used to evaluate the degree of agreement between A1C cut‐offs and OGTT criteria. The performance of different criteria was assessed by ROC analysis. Optimal cut‐offs were derived from the ROC curve with the shortest distance to sensitivity = 1, and 1 − specificity = 0, and the Youden index (Y = sensitivity + specificity − 1). The statistical analyses were carried out with Stata/SE 11.0 for Windows (StataCorp LP, College Station, TX, USA).
Results
A total of 1362 participants were enrolled in the present study, including 512 (38%) men and 850 (62%) women, aged 18–88 years with a median of 50 years (IQR 41–59 years). In this community‐based Chinese population, the median for FPG, 2‐h PG and A1C were 4.94 mmol/L (IQR 4.67–5.33 mmol/L), 6.22 mmol/L (IQR 5.06–7.72 mmol/L) and 5.6% (38 mmol/mol; IQR 5.4–5.9%, 36–41 mmol/mol), respectively. Their clinical characteristics are summarized in Table 1. Among them, 101 (7%) and 100 participants (7%) were diagnosed as DM by OGTT criteria and A1C ≥ 6.5% (48 mmol/mol), respectively. Among 135 patients with DM by either criteria, there were 66 patients (48.9%) who met both OGTT and A1C criteria, 35 patients (25.9%) who met OGTT criteria only and 34 patients (25.2%) who met A1C criteria only (Figure 1a). FPG and 2‐h PG were similar in those with DM by A1C ≥ 6.5% than those with DM by abnormal OGTT results (FPG 7.2 ± 2.6 vs 7.4 ± 2.6 mmol/L, P = 0.7; 2‐h PG 13.5 ± 5.9 vs 15.0 ± 4.8 mmol/L, P = 0.054).
Table 1. Clinical characteristics of the study participants.
| All | A1C | |||
|---|---|---|---|---|
| <6.5% (48 mmol/mol) | ≥6.5% (48 mmol/mol) | P‐value | ||
| n | 1362 | 1262 | 100 | |
| Age (years) | 49.9 ± 12.8 | 49.2 ± 12.8 | 58.3 ± 9.1 | <0.001 |
| Sex (male/female) | 512/850 | 468/794 | 44/56 | 0.17 |
| FPG (mmol/L) | 5.16 ± 1.05 | 5.00 ± 0.50 | 7.22 ± 2.61 | <0.001 |
| 2‐h PG (mmol/L) | 6.88 ± 3.11 | 6.38 ± 1.94 | 13.54 ± 5.94 | <0.001 |
| A1C (%, mmol/mol) | 5.7 ± 0.8 (39 ± 9) | 5.6 ± 0.4 (38 ± 4) | 7.6 ± 1.7 (60 ± 2) | <0.001 |
| NGT (%) | 938 (69%) | 933 (74%) | 5 (5%) | <0.001 |
| IFG or IGT (%) | 323 (24%) | 294 (23%) | 29 (29%) | 0.20 |
| IFG (%) | 145 (11%) | 128 (10%) | 17 (17%) | 0.03 |
| IGT (%) | 236 (17%) | 213 (17%) | 23 (23%) | 0.12 |
| DM (%) | 101 (7%) | 35 (3%) | 66 (66%) | <0.001 |
| FPG ≥ 7 mmol/L (%) | 43 (3%) | 7 (1%) | 36 (36%) | <0.001 |
| 2‐h PG ≥ 11.1 mmol/L (%) | 97 (7%) | 32 (3%) | 65 (65%) | <0.001 |
2hPG, plasma glucose 2 h after oral glucose tolerance test; A1C, hemoglobin A1c; DM, diabetes; FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGT, normal glucose tolerance.
Figure 1.
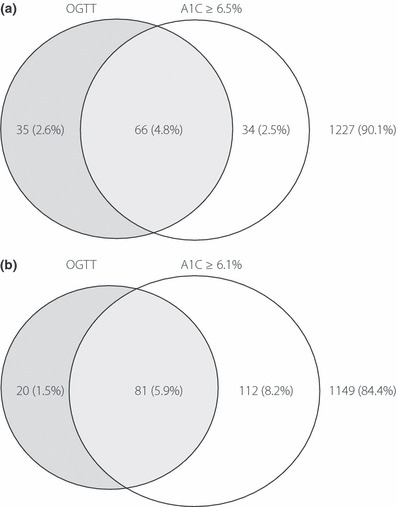
The relationship between diabetes by OGTT and A1C cut‐offs at (a) 6.5% and (b) 6.1%.
The optimal cut‐off of A1C was 6.1%. The area under the ROC curve for A1C to diagnosed DM by OGTT criteria in the present population was 0.91 (95% CI 0.87–0.95), with the optimal cut‐off of 6.1%. Among the 213 patients who had A1C ≥ 6.1% or DM by abnormal OGTT results, there were 81 subjects (38.0%) who met both criteria, 20 patients (9.4%) who met OGTT criteria only and 112 patients (52.6%) who had A1C ≥ 6.1% only (Figure 1b). As shown in Table 2, the sensitivity, specificity, PPV and NPV to diagnose DM by OGTT were 65, 97, 66 and 97% for A1C ≥ 6.5% criteria, and 80, 91, 44 and 97% for A1C ≥ 6.1% criteria, respectively. Both A1C cut‐offs showed unsatisfactory agreement with OGTT criteria for DM (kappa 0.50 and 0.63). A similar pattern was also found in both men and women (Supporting Information Table S1). As there is a nationwide FPG screening program in Taiwan, which is free for all adults older than 45 years and is provided every 3 years, we believed some, if not all, have attended the program. Therefore, we analyzed the agreement in patients younger and older than 45 years. In patients younger than 45 years, an A1C cut‐off of 6.5% showed a higher agreement with OGTT criteria (kappa 0.83) than that in those older than 45 years (kappa 0.60; Supporting Information Table S2). The performance of using ADA criteria to find patients with IFG or IGT was poor. The kappa statistics, sensitivity and specificity were 0.19, 65 and 71% for IFG, and 0.19, 54 and 72% for IGT (Table 2).
Table 2. Comparison of different criteria to diagnose diabetes, impaired fasting glucose and impaired glucose tolerance.
| Kappa (95% CI) | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | |
|---|---|---|---|---|---|---|---|
| Diabetes by OGTT† | |||||||
| A1C ≥ 6.1% | 0.50 (0.45–0.55) | 80% | 91% | 42% | 98% | 9.03 | 0.22 |
| A1C ≥ 6.5% | 0.63 (0.58–0.68) | 65% | 97% | 66% | 97% | 24.2 | 0.36 |
| Impaired fasting glucose‡ | |||||||
| A1C 5.7–6.4% | 0.19 (0.15–0.23) | 65% | 71% | 22% | 94% | 2.21 | 0.50 |
| Impaired glucose tolerance§ | |||||||
| A1C 5.7–6.4% | 0.19 (0.14–0.24) | 54% | 72% | 29% | 88% | 1.91 | 0.64 |
†Diabetes was defined as fasting plasma glucose ≥ 7 mmol/L or oral glucose tolerance test (OGTT) 2‐h plasma glucose ≥ 11.1 mmol/L. ‡Impaired fasting glucose was defined as fasting plasma glucose 5.6–6.9 mmol/L. §Impaired glucose tolerance was defined as OGTT 2‐h PG 7.8–11.0 mmol/L. CI, confidence interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; NPV, negative prediction value; PPV, positive prediction value.
There was no difference in age, sex, BMI, blood pressure and lipid profile between patients with DM diagnosed by OGTT criteria (n = 101) and by A1C ≥ 6.5% criteria, but not by OGTT criteria (n = 34). However, patients with DM by A1C criteria had lower FPG (5.5 ± 0.5 vs 7.4 ± 2.6 mmol/L, P < 0.001) and 2‐h PG (7.9 ± 2.1 vs 15.0 ± 4.8 mmol/L, P < 0.001) than patients with DM by OGTT criteria.
Tables 3–5 show the prevalence, PPV, NPV, false positive and false negative percentages by using different cut‐offs of FPG, A1C and FPG with A1C to screen DM by OGTT. In finding criteria to rule out DM, NPV were similar with different A1C cut‐offs. In contrast, as the A1C cut‐off increased, the prevalence and false negative percentage increased. In other words, as the A1C cut‐off increased, there were fewer people who had to receive the OGTT, in the expanse of a higher false negative percentage. In finding criteria to rule in DM, the main concern was PPV, as these subjects might be treated as having DM.
Table 3. Prevalence, positive and negative predictive value, false positive and false negative by different cut‐offs of fasting plasma glucose to screen diabetes.
| Rule out | Rule in | |||||
|---|---|---|---|---|---|---|
| Prevalence (<cut‐off) | NPV | False negative† | Prevalence (≥cut‐off) | PPV | False positive‡ | |
| 5.0 | 51.0% | 99.0% | 6.93% | 49.1% | 14.1% | 45.52% |
| 5.1 | 60.2% | 98.8% | 9.90% | 39.8% | 16.8% | 35.77% |
| 5.2 | 68.0% | 98.7% | 11.88% | 32.0% | 20.4% | 27.52% |
| 5.3 | 75.0% | 98.8% | 11.88% | 25.0% | 26.1% | 19.98% |
| 5.4 | 80.0% | 98.6% | 14.85% | 20.0% | 31.5% | 14.83% |
| 5.5 | 81.6% | 98.6% | 15.84% | 18.4% | 33.9% | 13.16% |
| 5.56 | 83.3% | 98.3% | 18.8% | 16.7% | 36.1% | 11.5% |
| 5.6 | 85.1% | 98.3% | 19.80% | 14.9% | 39.9% | 9.67% |
| 5.7 | 87.7% | 98.1% | 22.77% | 12.3% | 46.4% | 7.14% |
| 5.8 | 90.2% | 97.9% | 25.74% | 9.8% | 56.0% | 4.68% |
| 5.9 | 91.5% | 97.7% | 28.71% | 8.5% | 62.1% | 3.49% |
| 6.0 | 92.0% | 97.6% | 29.70% | 8.0% | 65.1% | 3.01% |
| 6.1 | 93.2% | 97.3% | 33.66% | 6.8% | 72.0% | 2.06% |
| 6.2 | 94.4% | 97.1% | 37.62% | 5.6% | 82.9% | 1.03% |
| 6.3 | 95.0% | 96.8% | 40.59% | 5.0% | 88.2% | 0.63% |
| 6.4 | 95.2% | 96.6% | 43.56% | 4.8% | 87.7% | 0.63% |
| 6.5 | 95.3% | 96.5% | 44.55% | 4.7% | 87.5% | 0.63% |
| 6.6 | 95.6% | 96.5% | 45.54% | 4.4% | 91.7% | 0.40% |
| 6.7 | 96.0% | 96.2% | 49.50% | 4.0% | 94.4% | 0.24% |
| 6.8 | 96.2% | 96.1% | 50.50% | 3.8% | 96.2% | 0.16% |
| 6.9 | 96.7% | 95.7% | 56.44% | 3.3% | 97.8% | 0.08% |
| 7.0 | 96.8% | 95.6% | 57.43% | 3.2% | 100.0% | 0.00% |
†False negative values among patients with diabetes by oral glucose tolerance test are shown. ‡False positive values among patients without diabetes by oral glucose tolerance test are shown. NPV, negative predictive value; PPV, positive predictive value.
Table 4. Prevalence, positive and negative predictive value, false positive and false negative by different cutoffs of A1C to screen diabetes.
| Rule out | Rule in | |||||
|---|---|---|---|---|---|---|
| Prevalence (<cut‐off) | NPV | False negative† | Prevalence (≥cut‐off) | PPV | False positive‡ | |
| 5.3 | 15.3% | 99.0% | 2.0% | 84.7% | 8.6% | 83.6% |
| 5.4 | 23.1% | 99.1% | 3.0% | 76.9% | 9.4% | 75.3% |
| 5.5 | 31.1% | 99.1% | 4.0% | 68.9% | 10.3% | 66.7% |
| 5.6 | 52.9% | 98.8% | 8.9% | 47.1% | 14.3% | 43.6% |
| 5.7 | 61.5% | 98.8% | 9.9% | 38.5% | 17.4% | 34.3% |
| 5.8 | 61.5% | 98.8% | 9.9% | 38.5% | 17.4% | 34.3% |
| 5.9 | 69.8% | 98.8% | 10.9% | 30.2% | 21.9% | 25.5% |
| 6.0 | 77.2% | 98.5% | 15.8% | 22.8% | 27.4% | 17.8% |
| 6.1 | 85.8% | 98.3% | 19.8% | 14.2% | 42.0% | 8.9% |
| 6.2 | 88.5% | 98.0% | 23.8% | 11.5% | 49.4% | 6.3% |
| 6.3 | 88.5% | 98.0% | 23.8% | 11.5% | 49.4% | 6.3% |
| 6.4 | 90.9% | 97.7% | 27.7% | 9.1% | 58.9% | 4.0% |
| 6.5 | 92.7% | 97.2% | 34.7% | 7.3% | 66.0% | 2.7% |
| 6.6 | 94.4% | 97.0% | 38.6% | 5.6% | 81.6% | 1.1% |
| 6.7 | 95.7% | 96.2% | 48.5% | 4.3% | 88.1% | 0.6% |
| 6.8 | 95.7% | 96.2% | 48.5% | 4.3% | 88.1% | 0.6% |
| 6.9 | 96.0% | 96.2% | 49.5% | 4.0% | 92.7% | 0.3% |
| 7.0 | 96.3% | 96.0% | 52.5% | 3.7% | 96.0% | 0.2% |
†False negative values among patients with diabetes by oral glucose tolerance test are shown. ‡False positive values among patients without diabetes by oral glucose tolerance test are shown. NPV, negative predictive value; PPV, positive predictive value.
Table 5. Prevalence, positive and negative predictive value, false positive and false negative by FPG criteria and different cut‐offs of A1C to screen diabetes.
| Rule out† | Rule in‡ | |||||
|---|---|---|---|---|---|---|
| Prevalence (<cut‐off) | NPV | False negative | Prevalence (≥cut‐off) | PPV | False positive | |
| FPG 5.56 mmol/L/A1C 5.3% | 14.8% | 99.5% | 1.0% | 16.1% | 37.0% | 10.9% |
| FPG 5.56 mmol/L/A1C 5.4% | 22.3% | 99.3% | 2.0% | 15.8% | 37.7% | 10.6% |
| FPG 5.56 mmol/L/A1C 5.5% | 29.8% | 99.5% | 2.0% | 15.4% | 38.3% | 10.2% |
| FPG 5.56 mmol/L/A1C 5.6% | 49.9% | 99.3% | 5.0% | 13.7% | 41.7% | 8.6% |
| FPG 5.56 mmol/L/A1C 5.7% | 57.8% | 99.2% | 5.9% | 12.9% | 44.3% | 7.8% |
| FPG 5.56 mmol/L/A1C 5.8% | 57.8% | 99.2% | 5.9% | 12.9% | 44.3% | 7.8% |
| FPG 5.56 mmol/L/A1C 5.9% | 65.1% | 99.2% | 6.9% | 12.0% | 47.9% | 6.7% |
| FPG 5.56 mmol/L/A1C 6.0% | 71.2% | 98.9% | 10.9% | 10.7% | 53.1% | 5.4% |
| FPG 5.56 mmol/L/A1C 6.1% | 77.4% | 99.0% | 10.9% | 8.2% | 65.2% | 3.1% |
| FPG 5.56 mmol/L/A1C 6.2% | 79.3% | 98.9% | 11.9% | 7.4% | 69.3% | 2.5% |
| FPG 5.56 mmol/L/A1C 6.3% | 79.3% | 98.9% | 11.9% | 7.4% | 69.3% | 2.5% |
| FPG 5.56 mmol/L/A1C 6.4% | 80.8% | 98.6% | 14.9% | 6.6% | 76.7% | 1.7% |
| FPG 5.56 mmol/L/A1C 6.5% | 81.9% | 98.6% | 15.8% | 5.9% | 78.8% | 1.4% |
| FPG 5.56 mmol/L/A1C 6.6% | 82.9% | 98.4% | 17.8% | 5.1% | 87.1% | 0.7% |
| FPG 5.56 mmol/L/A1C 6.7% | 83.1% | 98.4% | 17.8% | 4.1% | 91.1% | 0.4% |
| FPG 5.56 mmol/L/A1C 6.8% | 83.1% | 98.4% | 17.8% | 4.1% | 91.1% | 0.4% |
| FPG 5.56 mmol/L/A1C 6.9% | 83.3% | 98.3% | 18.8% | 4.0% | 92.7% | 0.3% |
| FPG 5.56 mmol/L/A1C 7.0% | 83.3% | 98.3% | 18.8% | 3.7% | 96.0% | 0.2% |
†Fasting plasma glucose (FPG) < 5.56 mmol/L and A1C < cut‐off. ‡FPG ≥ 5.56 mmol/L and A1C ≥ cut‐off. NPV, negative predictive value; PPV, positive predictive value.
Based on these data, the proposed two‐step screening strategies are summarized in Figure 2. Using IFG criteria (FPG < 5.56 mmol/L to rule out DM and FPG ≥ 7 mmol/L to rule in DM), DM could be excluded in 83.3%, confirmed in 3.2, and 13.5% of the whole population required OGTT (Figure 2a). Although the NPV to rule out DM was high (98.3%), there were 18.8% of diabetic patients who were missed (false negative). The sensitivity and specificity of this strategy to diagnose DM by OGTT were 77.2% and 100%, respectively. In Figure 2b, A1C < 5.9% was chosen to be the cut‐off to rule out DM, in order to reduce the false negative percentage to an acceptable range (10.9%), with good NPV (98.8%). A1C ≥ 7.0% was used to rule in DM to maximize PPV (96%). By these criteria, there were 26.5% of the patients who had to receive the OGTT to confirm DM. The sensitivity and specificity to diagnose DM by OGTT were 89.1 and 99.8%, respectively. In Figure 2c, both FPG and A1C were applied to screen DM. FPG < 5.56 mmol/L and A1C < 6.1% were used to exclude DM, in order to reduce the false negative percentage (10.9%) while having good NPV (99%). There were 18.9% of the patients who had to receive OGTT to confirm DM. FPG ≥ 5.56 mmol/L and A1C ≥ 7% were used to rule in DM, with high PPV (96%) and a very low percentage of false positive percentages (0.2% of total non‐diabetic subjects). The sensitivity and specificity to diagnose DM by OGTT were 85.2 and 100%, respectively. The performance of combining FPG ≥ 5.56 mmol/L and A1C ≥ 7% to rule in DM is identical to that of A1C alone, suggesting that HbA1c ≥ 7% alone did rule in diabetes, regardless of FPG levels.
Figure 2.
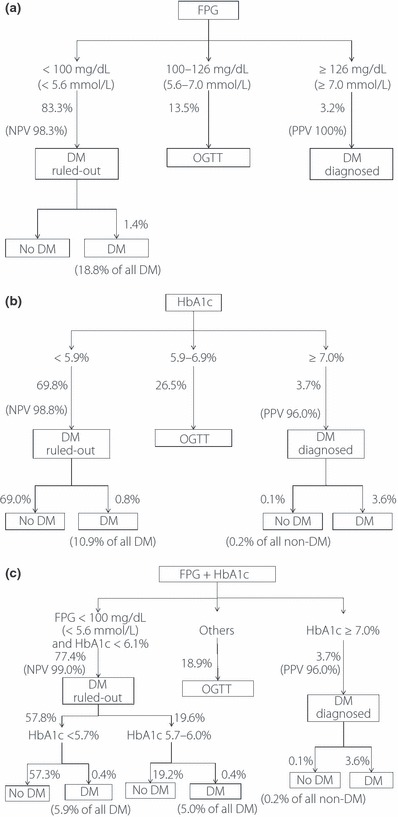
Different screening strategies to find diabetes by OGTT. (a) By impaired fasting glucose criteria, that is, fasting plasma glucose (FPG) 5.56–7.0 mmol/L. The sensitivity and specificity for this strategy were 77.2 and 100%, respectively. (b) By A1C cut‐offs at 5.9 and 7%. The sensitivity and specificity for this strategy were 89.1 and 99.8%, respectively. (c) By FPG cut‐off at 5.56 mmol/L and A1C cut‐offs at 6.1 and 7%. The sensitivity and specificity for this strategy were 85.2 and 100%, respectively.
Discussion
In the present study, the agreement between OGTT and A1C criteria to diagnose DM was poor; neither the ADA‐proposed cut‐off of 6.5% nor the optimal cut‐off of 6.1% derived from our population. In the Korean and Chinese population, the optimal cut‐off for A1C to find DM defined by OGTT was also 6.1%15,16. Similar to the present results, the sensitivity and specificity for A1C were not good enough to find DM by OGTT (Korean population 81.8 and 84.9%, Chinese population 81 and 81%). These observations suggest that A1C criteria identified different groups of people with DM. In the present report, the agreement between OGTT with A1C cut‐off of 6.5% to diagnose DM is higher (kappa 0.83) in those younger than 45 years than that in those older than 45 years. Consistently, Mannucci et al.17 reported that screening of DM with A1C is more sensitive in a younger population. Motta et al.18 also found that the performance of A1C to diagnose DM is poorer than FPG in the elderly. Taken together, these results show that A1C cannot replace OGTT to diagnose DM, although its use in a younger population might be acceptable.
We found that A1C could be used as a screening tool for the diagnosis of DM. Two different strategies were provided (Figure 2). If fasting is the main obstacle in screening DM, A1C followed by OGTT strategy is recommended (Figure 2b). Compared with IFG criteria, use of A1C cut‐offs at 5.9 and 7.0% improved overall sensitivity and reduced the false negative percentage, from 18.8 to 10.9%, with similar false positive and false negative percentages (Figure 2a,b). However, more people will be recommended to receive OGTT (IFG criteria 13.5%, A1C criteria 26.5%). In the Australian population, Lu et al.7 suggested cut‐offs of A1C ≥ 7.0% to rule in DM and ≤5.5% to exclude DM. Following their criteria, up to 65.2% of patients who had A1C values between 5.5 and 7.0% were recommended to have OGTT, which can be practically difficult and might be not cost‐effective.
In contrast, if fasting is not a concern, the present results showed that A1C could improve the performance of FPG as the first‐step screening tool (Figure 2c). Compared with IFG criteria (Figure 2a), FPG and A1C criteria improved overall sensitivity, reduced the false negative percentage from 18.8 to 10.9%, had similarly good PPV and NPV, and had a little more OGTT required (IFG criteria 13.5%, FPG and A1C criteria 18.9%). Similarly, combining FPG and A1C as a screening tool has been suggested to improve the diagnostic performance and to reduce the need of OGTT16,19,20. In the Chinese population, Ko et al.20 suggested that patients with a FPG range of 5.6–7.7 mmol/L and A1C ≥ 5.5% should receive OGTT to confirm DM. In a multi‐ethnic study, FPG ≥ 5.7 mmol/L and A1C ≥ 5.9% yielded a sensitivity of 78.6% and a specificity of 95.9% for the Chinese population (n = 307)19. Hu et al.16 also reported a high specificity of 96.3% combining FPG ≥ 6.1 mmol/L or A1C ≥ 6.1% to screen DM. Most previous studies provided a single cut‐off of A1C instead of using two different cut‐offs to rule in and rule out DM. In the UK and the Australian populations, Manley et al. provided an algorithm to diagnose DM using FPG and A1C as initial screening tools. DM was confirmed if FPG ≥ 7 mmol/L, whereas DM was excluded if FPG < 7 mmol/L and A1C < 6.0%. Those with FPG < 7 mmol/L and A1C ≥ 6.0% were recommended to receive the OGTT21. These criteria are similar to our suggestions in Figure 2c.
In the present study, patients who met the A1C criteria, but not the OGTT criteria, had lower FPG and 2‐h PG, suggesting that the addition of A1C criteria helps to identify people with hyperglycemia earlier in their time course. Although adding A1C criteria as screening costs more money, early identification of these patients and subsequent early intervention might reduce diabetic complications. The cost–benefit issue should be studied in longitudinal follow‐up studies.
A very low agreement among A1C ranged 5.7–6.4%, IFG and IGT were found in the present study. The test performance of A1C criteria against IFG or IGT was also poor, which is consistent with previous studies in the USA22, Australian 23 and Chinese populations16. Therefore, the A1C criteria of 5.7–6.4% might identify a different population at risk of DM from IFG and IGT criteria, and should not be viewed as a replacement for OGTT.
There were some limitations in the present study. First, there were differences in the distribution of age and sex between the present study subjects and whole Taiwanese population. Although age, but not sex, is a risk factor for isolated postprandial hyperglycemia24, using only one criterion is more practical than using different criteria in different age groups. Indeed, there is only one criterion for the diagnosis of diabetes and prediabetes, by both the ADA and the EASD. Therefore, we think it’s acceptable to use current cut‐offs and the flow charts in the present report. If age and other risk factors are of concern, we have also provided four different risk scores to determine if OGTT is needed in a previous report24. Second, we did not assess if the subjects had hemoglobinopathies in the present study. Although this is more likely to be the real clinical situation, care should be taken in applying the findings of the present study.
In conclusion, using A1C < 5.9% to exclude and A1C ≥ 7.0% to diagnose DM by OGTT is more sensitive than using FPG < 5.56 mmol/L to exclude and FPG ≥ 7.0 mmol/L to diagnose DM, with more OGTT needed. Using A1C < 6.1% plus FPG < 5.56 mmol/L to exclude and A1C ≥ 7.0% to diagnose DM by OGTT reduced the number of OGTT needed with acceptable sensitivity. However, there is no single cut‐off for A1C to replace OGTT to diagnose DM, IFG and IGT.
Supplementary Material
Table S1 Comparison of different criteria to diagnose diabetes, impaired fasting glucose, and impaired glucose tolerance in male and female subjects
Table S2 Comparison of different criteria to diagnose diabetes, impaired fasting glucose, and impaired glucose tolerance in subjects older or younger than 45 years old
Supporting info item
Acknowledgements
This work is supported in part by grants from the National Science Council, Taiwan (NSC95‐2314‐B‐041‐002 and NSC96‐2628‐B‐041‐002‐MY3). None of the authors have conflicts of interest.
References
- 1.ADA . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl. 1): S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Expert Committee . International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu QZ, Pettitt DJ, Hanson RL, et al. Glycated haemoglobin, plasma glucose and diabetic retinopathy: cross‐sectional and prospective analyses. Diabetologia 1993; 36: 428–432 [DOI] [PubMed] [Google Scholar]
- 4.Cheng YJ, Gregg EW, Geiss LS, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population. Diabetes Care 2009; 32: 2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riet E, Alssema M, Rijkelijkhuizen JM, et al. Relationship between A1C and glucose levels in the general Dutch population. Diabetes Care 2010; 33: 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Steffes MW, Gregg E, et al. Performance of A1C for the classification and prediction of diabetes. Diabetes Care 2011; 34: 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu ZX, Walker KZ, O’Dea K, et al. HbA1c for screening and diagnosis of Type 2 diabetes in routine clinical practice. Diabetes Care 2010; 33: 817–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson AP, Reynolds K, Fonseca VA, et al. Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 2010; 33: 95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao Y, Ma X, Li H, et al. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. BMJ 2010; 340: c2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araneta MRG, Grandinetti A, Chang HK. A1C and diabetes diagnosis among Filipino Americans, Japanese Americans, and Native Hawaiians. Diabetes Care 2010; 33: 2626–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett CM, Guo M, Dharmage SC. HbA1c as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med 2007; 24: 333–343 [DOI] [PubMed] [Google Scholar]
- 12.Li HY, Wei JN, Lin MS, et al. Serum vascular adhesion protein‐1 is increased in acute and chronic hyperglycemia. Clin Chim Acta 2009; 404: 149–153 [DOI] [PubMed] [Google Scholar]
- 13.Expert Committee on the Diagnosis Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003; 26(Suppl. 1): S5–S20 [DOI] [PubMed] [Google Scholar]
- 14.Little RR, Rohlfing CL, Wiedmeyer H‐M, et al. The national glycohemoglobin standardization program: a five‐year progress report. Clin Chem 2001; 47: 1985–1992 [PubMed] [Google Scholar]
- 15.Kim KS, Kim SK, Lee YK, et al. Diagnostic value of glycated haemoglobin HbA1c for the early detection of diabetes in high‐risk subjects. Diabet Med 2008; 25: 997–1000 [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Liu W, Chen Y, et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol 2009; 47: 231–236 [DOI] [PubMed] [Google Scholar]
- 17.Mannucci E, Ognibene A, Sposato I, et al. Fasting plasma glucose and glycated haemoglobin in the screening of diabetes and impaired glucose tolerance. Acta Diabetol 2003; 40: 181–186 [DOI] [PubMed] [Google Scholar]
- 18.Motta M, Bennati E, Cardillo E, et al. The value of glycosylated hemoglobin (HbA1c) as a predictive risk factor in the diagnosis of diabetes mellitus (DM) in the elderly. Arch Gerontol Geriatr 2010; 50: 60–64 [DOI] [PubMed] [Google Scholar]
- 19.Anand SS, Razak F, Vuksan V, et al. Diagnostic strategies to Detect glucose intolerance in a multiethnic population. Diabetes Care 2003; 26: 290–296 [DOI] [PubMed] [Google Scholar]
- 20.Ko GT, Chan JC, Yeung VT, et al. Combined use of a fasting plasma glucose concentration and HbA1c or fructosamine predicts the likelihood of having diabetes in high‐risk subjects. Diabetes Care 1998; 21: 1221–1225 [DOI] [PubMed] [Google Scholar]
- 21.Manley SE, Sikaris KA, Lu ZX, et al. Validation of an algorithm combining haemoglobin A1c and fasting plasma glucose for diagnosis of diabetes mellitus in UK and Australian populations. Diabet Med 2009; 26: 115–121 [DOI] [PubMed] [Google Scholar]
- 22.Saydah SH, Byrd‐Holt D, Harris MI. Projected impact of implementing the results of the diabetes prevention program in the U.S. population. Diabetes Care 2002; 25: 1940–1945 [DOI] [PubMed] [Google Scholar]
- 23.Colagiuri S, Hussain Z, Zimmet P, et al. Screening for Type 2 diabetes and impaired glucose metabolism. Diabetes Care 2004; 27: 367–371 [DOI] [PubMed] [Google Scholar]
- 24.Li H‐Y, Lin M‐S, Shih S‐R, et al. The performance of risk scores and hemoglobin A1c to find undiagnosed diabetes with isolated postload hyperglycemia. Endocr J 2011; 58: 441–448 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison of different criteria to diagnose diabetes, impaired fasting glucose, and impaired glucose tolerance in male and female subjects
Table S2 Comparison of different criteria to diagnose diabetes, impaired fasting glucose, and impaired glucose tolerance in subjects older or younger than 45 years old
Supporting info item


