Abstract
Aims/Introduction: Pronounced reduction of insulin secretion in response to a rise in glucose level has been reported in Japanese patients compared with Caucasian patients, but the mean body mass index (BMI) is also lower in Japanese patients. As BMI is a determinant of insulin secretion, we examined insulin‐secretion capacity in obese and non‐obese Japanese patients.
Materials and Methods: Using the oral glucose tolerance test (OGTT), we estimated the insulin‐secreting capacity in obese (BMI ≥ 25) and non‐obese (BMI < 25) Japanese patients, including 1848 patients with normal glucose tolerance (NGT), 321 patients with impaired glucose tolerance (IGT) and 69 diabetes (DM) patients.
Results: The insulinogenic index (I.I.), calculated by dividing the increment in serum insulin by the increment in plasma glucose from 0 to 30 min during OGTT, decreased from NGT to IGT and to DM in patients with and without obesity. In patients with NGT, IGT and DM, the I.I. values of obese patients were higher than those of the non‐obese patients. The peak of insulin concentration in OGTT appeared at 60 min in NGT and at 120 min in IGT in both obese and non‐obese patients, but in DM it was observed at 120 min in obese patients and at 60 min in non‐obese patients.
Conclusions: These results show that early‐phase insulin secretion in obese Japanese patients is higher than in non‐obese patients in all stages of glucose tolerance, and delayed insulin‐secretion capacity is also conserved in obese Japanese patients, even in IGT and DM, which is similar to Caucasian patients. (J Diabetes Invest, doi:10.1111/j.2040‐1124.2011.00180.x, 2011)
Keywords: Insulin secretion, Non‐obese Japanese, Obese Japanese
Introduction
Type 2 diabetes results from the deterioration of both insulin secretion and insulin action1, but the contribution of these factors to glucose intolerance varies among various ethnic groups. In Japan, reduced insulin‐secreting capacity is considered to play a more important role than impaired insulin resistance during the transition from normal glucose tolerance (NGT) to impaired glucose tolerance (IGT), and then to overt type 2 diabetes2.
The insulinogenic index (I.I.) is calculated by dividing the increment in serum insulin from 0 to 30 min during an oral glucose tolerance test (OGTT) by the increment in plasma glucose during the same period. The I.I. is a marker of early‐phase insulin‐secretion capacity in response to a rise in blood glucose level3. A low I.I. has been described in Japanese patients, even in those with NGT or IGT, compared with Caucasian patients4. The low I.I. in Japanese patients is thought to reflect β‐cell fragility. We previously reported the positive association between body mass index (BMI) and I.I. in diabetic patients, and that BMI was an independent determinant of I.I.5. Because the average BMI is much lower in the Japanese than in Caucasians6, it is possible that the early‐phase decrease in insulin secretion in the Japanese patients is partly a result of the lower BMI. To clarify the insulin‐secretion capacity in obese and non‐obese Japanese patients, we analyzed the insulin‐secretion capacity measured by OGTT in subjects with NGT, IGT or DM, who were divided into those with and without obesity (BMI ≥ 25 or BMI < 25), and also estimated visceral fat area (eVFA) ≥ 100 cm2 or eVFA < 100 cm2.
Methods
Subjects
The study group comprised employees of Amagasaki City Office, Hyogo, Japan, an urban area, who had completed the government‐funded annual health check‐up from 2004 to 2008. Among the tests carried out during the annual examination, OGTT was carried out in those participants with one or more risk factor(s) based on the recommendation of the team physician. The risk factors included the following: (i) hypertension: systolic blood pressure (sBP) of ≥130 mmHg and/or diastolic blood pressure (dBP) of ≥85 mmHg; (ii) high plasma glucose level: fasting plasma glucose (FPG) level of ≥110 mg/dL and/or hemoglobin A1c (HbA1c) level of ≥5.9%; (iii) dyslipidemia: serum triglyceride level of ≥150 mg/dL and/or a serum high‐density lipoprotein (HDL)‐cholesterol level of <40 mg/dL, and/or a serum low‐density lipoprotein (LDL)‐cholesterol level of ≥140 mg/dL; (iv) hyperuricemia: serum uric acid level of ≥7.0 mg/dL; and (v) abdominal obesity: waist circumference equal to or >85 cm in men and 90 cm in women; these are the essential criteria of metabolic syndrome in Japan7. These patients were provided with health guidance to improve their lifestyle. Among the patients who underwent OGTT, 1848 patients were identified as having NGT from 2004 to 2007, 321 patients were identified as having IGT from 2004 to 2007, and 69 patients developed DM after IFG and/or IGT during the period from 2005 to 2008. The latter three groups were included in the present study.
Anthropometry and Laboratory Tests
Anthropometric variables (height, weight and waist circumference [WC]) were measured in a standing position. BMI was calculated as weight (kg) divided by the square of height in meters (m2). WC was measured at the umbilical level in cm with a non‐stretchable tape in the late exhalation phase at standing position7. Blood pressure was measured in the sitting position.
Plasma glucose was measured by the glucose oxidase method (Quick‐auto II GLU‐HKs; Shino‐Test, Tokyo, Japan). HbA1c was determined by high‐performance liquid chromatography (Rapidia Auto HbA1c‐L; TFB, Tokyo, Japan). The value of HbA1c (%) was estimated as the National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula: HbA1c (%) = HbA1c (JDS) (%) + 0.4%, considering the relational expression of HbA1c (JDS) (%), measured by the previous Japanese standard substance and measurement methods, and HbA1c (NGSP)8. Serum insulin concentration was measured by double‐antibody radioimmunoassay (Access insulin; Beckman Coulter, Brea, CA, USA) and was reported as immunoreactive insulin (IRI). Serum uric acid (UA), total cholesterol and triglyceride concentrations were measured by enzymatic methods. LDL and HDL cholesterol were also measured by enzymatic methods after heparin and calcium precipitation.
Detailed Examination
Visceral fat area (VFA) was determined by the bioelectrical impedance analysis (BIA) method, as reported previously9. In OGTT, plasma glucose (PG) and serum insulin concentrations were determined at 0, 30, 60 and 120 min after ingestion of 75 g of glucose. The I.I. was calculated by dividing the increment in serum insulin by the increment in PG from 0 to 30 min of the OGTT. Samples with I.I. <0 or ≥10 were excluded from the analysis, because they probably do not express the true early‐phase insulin secretion in response to rise in PG. The area under the insulin curve during OGTT (AUC[insulin0–120]) was calculated by the trapezoid rule. The diagnoses of diabetes, IGT and NGT were based on the criteria of the American Diabetes Association (ADA)10. Actually, NGT was defined as having FPG < 100 mg/dL and 2‐h post‐load glucose < 140 mg/dL. IGT was defined as having FPG < 126 mg/dL and 2‐h post‐load glucose 140–199 mg/dL. DM was defined as having FPG ≥ 126 mg/dL and/or 2‐h post‐load glucose ≥ 200 mg/dL. Measurement of VFA complied with the Guidelines of the Ethical Committees of Osaka University. Written informed consent was obtained from all participants.
Statistical Analysis
Data are expressed as mean ± SD. Differences in variables between groups were tested with the unpaired Student’s t‐test. Statistical significance was considered at a P‐value of <0.05.
Results
Table 1 lists the clinical characteristics of the BMI ≥ 25 and BMI < 25 groups with NGT, IGT and DM. The mean BMI of the entire BMI < 25 group was 22.9 ± 1.6 kg/m2, and that of the entire BMI ≥ 25 group was 27.6 ± 2.4 kg/m2.
Table 1. Clinical characteristics of the patients.
| NGT | P‐value (BMI < 25 vs BMI ≥ 25) | IGT | P‐value (BMI < 25 vs BMI ≥ 25) | DM | P‐value (BMI < 25 vs BMI ≥ 25) | ||||
|---|---|---|---|---|---|---|---|---|---|
| BMI < 25 | BMI ≥ 25 | BMI < 25 | BMI ≥ 25 | BMI < 25 | BMI ≥ 25 | ||||
| n | 823 | 1025 | 101 | 220 | 15 | 54 | |||
| Male/female | 714/109 | 916/109 | 93/8 | 197/23 | 13/2 | 44/10 | |||
| Age (years) | 50.0 ± 8.5 | 47.2 ± 9.5 | <0.0001 | 54.1 ± 6.6 | 51.7 ± 7.1 | 0.0056 | 55.5 ± 6.7 | 53.4 ± 6.5 | 0.2624 |
| BMI (kg/m2) | 22.9 ± 1.6 | 27.5 ± 2.3 | <0.0001 | 23.2 ± 1.5 | 28.0 ± 2.5 | <0.0001 | 23.0 ± 1.1 | 28.1 ± 2.6 | <0.0001 |
| HbA1c (%) | 5.33 ± 0.33 | 5.31 ± 0.34 | 0.3506 | 5.80 ± 0.44 | 5.83 ± 0.49 | 0.5892 | 6.25 ± 0.46 | 6.33 ± 0.51 | 0.6231 |
| FPG (mg/dL) | 89.4 ± 6.0 | 89.6 ± 5.9 | 0.4768 | 102.7 ± 10.3 | 103.2 ± 10.8 | 0.6690 | 121.8 ± 13.1 | 119.9 ± 15.3 | 0.6703 |
| 2‐h Plasma glucose | 97.5 ± 20.9 | 102.6 ± 19.8 | <0.0001 | 157.6 ± 15.1 | 161.8 ± 17.2 | 0.0378 | 201.5 ± 42.8 | 223.5 ± 31.1 | 0.0291 |
| I.I. | 0.65 ± 0.69 (795) | 0.76 ± 0.69 (990) | 0.0009 | 0.27 ± 0.25 (100) | 0.37 ± 0.34 (214) | 0.0124 | 0.12 ± 0.10 (15) | 0.26 ± 0.27 (53) | 0.0485 |
| HOMA‐IR | 0.96 ± 0.0.59 | 1.41 ± 0.77 | <0.0001 | 1.30 ± 0.71 | 2.14 ± 1.70 | <0.0001 | 1.72 ± 0.64 | 2.52 ± 1.15 | 0.0117 |
| IRI 0 min (mU/mL) | 4.3 ± 2.6 | 6.3 ± 3.3 | <0.0001 | 5.1 ± 2.7 | 8.3 ± 6.3 | <0.0001 | 5.7 ± 1.9 | 8.5 ± 3.8 | 0.0061 |
| IRI 30 min (mU/mL) | 31.1 ± 21.2 | 40.7 ± 26.7 | <0.0001 | 24.0 ± 15.3 | 34.3 ± 32.9 | 0.0029 | 15.3 ± 7.3 | 29.2 ± 19.8 | 0.0101 |
| IRI 60 min (mU/mL) | 33.1 ± 25.4 | 43.8 ± 28.5 | <0.0001 | 32.6 ± 24.2 | 51.1 ± 41.3 | <0.0001 | 29.4 ± 14.1 | 44.4 ± 21.2 | 0.0120 |
| IRI 120 min (mU/mL) | 21.7 ± 16.1 | 31.5 ± 22.8 | <0.0001 | 38.1 ± 22.6 | 56.8 ± 44.7 | <0.0001 | 28.9 ± 17.0 | 64.7 ± 47.7 | 0.0059 |
| AUC (insulin0–120) | 3138 ± 1912 | 4232 ± 2375 | <0.0001 | 3404 ± 1942 | 5155 ± 3973 | <0.0001 | 2734 ± 1104 | 4944 ± 2563 | 0.0018 |
Data are mean ± SD. The HbA1c (%) data represent the National Glycohemoglobin Standardization Program value. None of the patients in this study were receiving treatment for hypertension, diabetes mellitus or dyslipidemia. AUC, area under the curve; BMI, body mass index; DM, diabetes mellitus; FPG, fasting plasma glucose; IGT, impaired glucose tolerance; I.I., insulinogenic index; IRI, immunoreactive insulin; NGT, normal glucose tolerance.
The insulin‐secreting patterns during OGTT for the BMI ≥ 25 and BMI < 25 groups with NGT, IGT and DM are presented in Figure 1a–c, respectively. The IRI values at 0, 30, 60 and 120 min, and the AUC(insulin0–120) were all significantly higher in the BMI ≥ 25 group than in the BMI < 25 group, including those with NGT, IGT and DM (Table 1). The insulin‐secreting patterns between the BMI ≥ 25 and BMI < 25 groups with NGT, IGT or DM were also different; the peak IRI in NGT was noted at 60 min and that in IGT was recorded at 120 min in both groups, but the peak IRI in DM was observed at 120 min in the BMI ≥ 25 group, whereas at 60 min in the BMI < 25 group (Figure 1a–c). Figure 2 shows data of the patients divided according to the VFA value (eVFA ≥ 100 and eVFA < 100). The IRI at 0, 30, 60 and 120 min were significantly higher in NGT and IGT subjects with eVFA ≥ 100 than those with eVFA < 100. However, in the eVFA ≥ 100 group with DM, only the IRI at 120 min was significantly higher than that of the eVFA < 100 group (Figure 2c).
Figure 1.
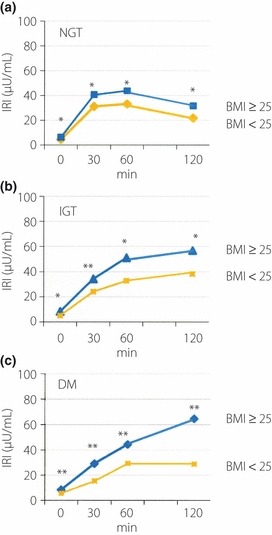
Insulin secretion patterns during oral glucose tolerance test (OGTT) in obese (body mass index [BMI] ≥ 25) and non‐obese (BMI < 25) patients with (a) normal glucose tolerance (NGT), (b) impaired glucose tolerance (IGT) and (c) diabetes mellitus (DM). *P < 0.0001 vs BMI < 25; **P < 0.05 vs BMI < 25.
Figure 2.
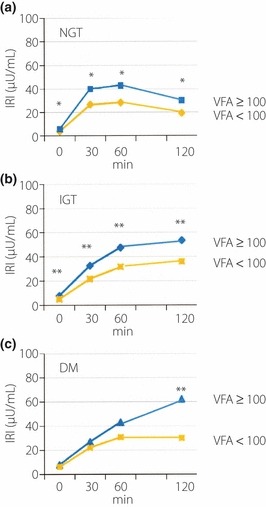
Insulin secretion patterns during oral glucose tolerance test (OGTT) in patients with abdominal obesity (estimated visceral fat area [eVFA] ≥ 100) and non‐abdominal obesity (eVFA < 100) with (a) normal glucose tolerance (NGT), (b) impaired glucose tolerance (IGT) and (c) diabetes mellitus (DM). *P < 0.0001 vs VFA < 100; **P < 0.05 vs VFA < 100.
Next, we examined I.I. and homeostasis model of insulin resistance (HOMA‐IR) in the BMI ≥ 25 and BMI < 25 groups with NGT, IGT and DM. The I.I. decreased from NGT to IGT and to DM in both the BMI ≥ 25 and BMI < 25 groups (Figure 3a). The I.I. values of the BMI ≥ 25 groups were significantly higher than those of the BMI < 25 groups in each stage of glucose tolerance (Table 1). The HOMA‐IR increased from NGT to IGT and to DM in both the BMI ≥ 25 and BMI < 25 groups (Figure 3b). Furthermore, HOMA‐IR values of the BMI ≥ 25 groups with NGT, IGT and DM were significantly higher than those of the BMI < 25 groups (Table 1).
Figure 3.
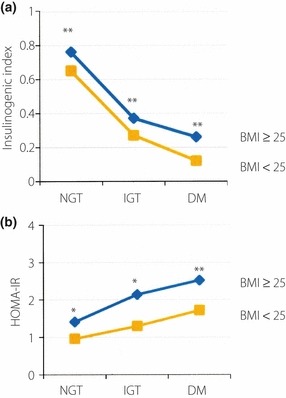
(a) Insulinogenic index (I.I.) and (b) homeostasis model of insulin resistance (HOMA‐IR) of obese (body mass index [BMI] ≥ 25) and non‐obese (BMI < 25) patients with normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and diabetes mellitus (DM). *P < 0.0001 vs BMI < 25; **P < 0.05 vs BMI < 25.
The I.I. values also decreased from NGT to IGT and to DM in both the eVFA ≥ 100 and eVFA < 100 groups (Figure 4a). Furthermore, the I.I. values of the eVFA ≥ 100 group were significantly higher than those of the eVFA < 100 with NGT and IGT (NGT: 0.76 ± 0.70 vs 0.58 ± 0.64, P < 0.0001; IGT: 0.36 ± 0.33 vs 0.20 ± 0.16, P = 0.0016). The HOMA‐IR values increased from NGT to IGT and to DM in both the eVFA ≥ 100 and eVFA < 100 groups (Figure 4b). Furthermore, the HOMA‐IR values of the eVFA ≥ 100 group were significantly higher than those of the eVFA < 100 group with NGT and IGT (NGT: 1.33 ± 0.77 vs 0.87 ± 0.46, P < 0.0001; IGT: 1.98 ± 1.58 vs 1.29 ± 0.86, P = 0.0035). In patients with DM, the I.I. and HOMA‐IR values of the eVFA ≥ 100 group tended to be higher than those of the eVFA < 100 group, albeit insignificantly (I.I.: 0.24 ± 0.26 vs 0.16 ± 0.13, P = 0.3270; HOMA‐IR: 2.42 ± 1.11 vs 1.94 ± 1.03, P = 0.2054).
Figure 4.
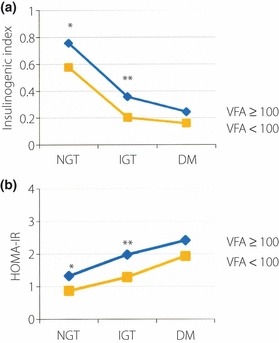
(a) Insulinogenic index (I.I.) and (b) homeostasis model of insulin resistance (HOMA‐IR) of patients with abdominal obesity (estimated visceral fat area [eVFA] ≥ 100) and non‐abdominal obesity (eVFA < 100) according to glucose tolerance (normal glucose tolerance [NGT], impaired glucose tolerance [IGT] and diabetes mellitus [DM]). *P < 0.0001 vs VFA < 100; **P < 0.005 vs VFA < 100.
We also analyzed 230 isolated IGT in our patients in order to compare them with those in the Botnia study, because that study showed only the data in isolated IGT, not in total IGT. Isolated IGT was defined as having FPG < 110 mg/dL and 2‐h post‐load glucose of 140–199 mg/dL according to the diagnostic criteria of the ADA guideline 1997. The insulin‐secreting patterns were similar in all IGT subjects; that is, the peak IRI was recorded at 120 min in both the BMI ≥ 25 and BMI < 25 groups (57.4 ± 47.8 mU/mL vs 39.1 ± 23.4 mU/mL, P = 0.0017). The I.I. values of the BMI ≥ 25 and the BMI < 25 groups in our isolated IGT were 0.39 ± 0.37 and 0.29 ± 0.28 (BMI ≥ 25 vs BMI < 25, P = 0.0374), respectively. The I.I. value in obese Japanese patients with isolated IGT was lower than that in the Caucasian patients with isolated IGT from the literature11 (0.39 ± 0.37 vs 0.77 ± 0.21, P < 0.01), although the mean BMI were not significantly different (28.0 ± 2.6 vs 27.4 ± 4.5). The HOMA‐IR values of the BMI ≥ 25 and the BMI < 25 groups in our isolated IGT were 1.85 ± 1.71 and 1.25 ± 0.70 (BMI ≥ 25 vs BMI < 25, P = 0.0035), respectively. The HOMA‐IR value in obese Japanese with isolated IGT was significantly lower than that in the Caucasian patients with isolated IGT (1.85 ± 1.71 vs 2.36 ± 2.00, P < 0.01).
Discussion
Reduction in insulin‐secretion capacity is considered to play a more important role than insulin resistance in the development to DM in Japanese patients. This is mainly supported by two facts. First, serum insulin levels during OGTT are lower in patients with IGT than those with NGT, and even lower in DM patients4. Second, the I.I. values are low throughout the development of glucose tolerance from NGT to IGT and to DM compared with Caucasians4. In the present study, we analyzed these issues in patients divided according to their BMI, and also according to the VFA value.
Our results showed significantly higher IRI values at 0, 30, 60 and 120 min, I.I. and AUC(insulin0–120) in obese (BMI ≥ 25) than in non‐obese (BMI < 25) patients with all stages of glucose tolerance. As shown in Table 1, in the BMI ≥ 25 group, the AUC(insulin0–120) of IGT was 1.22‐fold higher than that of the NGT patients (5155 ± 3973 vs 4232 ± 2375) and that of the DM patients was almost 1.17‐fold (4944 ± 2563) that of the NGT subjects. In addition, the peak level of IRI during OGTT in DM was noted at 120 min in the BMI ≥ 25 patients and 60 min in the BMI < 25 group. These observations show that the total and late insulin secretion during OGTT in obese patients does not decrease as much as in non‐obese patients with NGT to IGT and to DM, and it is similar to the pattern in Caucasians. The previous report4 that insulin concentrations during OGTT are lower in Japanese patients with IGT than those with NGT, and even lower in DM patients, does not match our obese group. These observations are also true when subjects are divided according to eVFA. It is not clear what causes the differences of insulin‐secretion patterns between the obese and non‐obese groups, but we believe they are β‐cell capacity and visceral fat volume. Individuals who have a larger β‐cell capacity could easily be developing visceral fat by overeating, leading to insulin resistance, deterioration of glucose tolerance and delayed hyperinsulin secretion in OGTT. In contrast, individuals who have a smaller β‐cell capacity might not be developing so much visceral fat, but easily developing a deterioration of glucose tolerance owing to the decompensation of early‐phase and then delayed‐phase insulin secretion when overeating.
In order to examine whether it is also true in obese Japanese patients that early‐phase insulin secretion is lower than Caucasian patients, we compared the I.I. in Japanese obese patients with isolated IGT with that in Caucasian patients with isolated IGT. The I.I. value in obese Japanese patients with isolated IGT was lower than that of the Caucasian patients with isolated IGT, although the mean BMI of them were not significantly different, suggesting decreased early‐phase insulin secretion in obese, as well as non‐obese, Japanese patients compared with Caucasian patients. In addition, the HOMA‐IR value in obese Japanese patients with isolated IGT was lower than that in the Caucasian patients with isolated IGT. Together, with the lower I.I., these findings suggest that decreased early‐phase insulin‐secretion capacity plays a more important role than insulin resistance in the transition of IGT to DM, even in Japanese obese patients.
The present study had some study limitations. First, in screening patients, OGTT was carried out in subjects with one or more risk factor(s) as mentioned in the Methods section. Therefore, individuals with metabolic syndrome showing insulin resistance might be over represented in the present study, even in NGT. Actually, of the 1848 NGT patients in the present study, the number of individuals with BMI ≥ 25 was 1025 and the number of individuals with eVFA ≥ 100 was 1324. However, the mean HOMA‐IR of non‐obese patients in the NGT group was 0.96 and they showed no insulin resistance. We believe that non‐obese patients in our NGT group could represent a control population. The second limitation was the comparison with the Botnia study. In the Botnia study, patients with known type 2 diabetes and their available family members were included. The study population was basically different from the present study population, including the male and female ratio. It might be that we cannot say the population of the Botnia Study is an appropriate one as a comparative study with the present study. In addition, comparison of mean BMI with Caucasian patients in the Botnia study might not be reasonable, as they did not separate the subjects with and without obesity. A comparative study with Caucasian patients in appropriate populations with matched BMI will be necessary in the future.
In conclusion, the present study showed that early‐phase insulin secretion in obese Japanese patients is higher than in non‐obese patients in all stages of glucose tolerance and the delayed insulin‐secretion capacity is also conserved in obese Japanese patients, even in IGT and DM, which is similar to Caucasian patients.
Acknowledgements
We thank Word‐Medex Pty Ltd for help in the preparation of this manuscript. This study was partly supported by the Health and Labour Science Research Grants (Research in intractable diseases) to Dr Hirano, Yamashita, Ikuno, Iwahashi, Ohishi, Mano and Ishihara. The authors declare no conflict of interest.
References
- 1.Kahn SE. The relative contributions of insulin resistance and beta‐cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3–19 [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto K, Miyake S, Yano M, et al. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care 1997; 20: 1562–1568 [DOI] [PubMed] [Google Scholar]
- 3.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2002; 44: 929–945 [DOI] [PubMed] [Google Scholar]
- 4.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66S: S37–S43 [DOI] [PubMed] [Google Scholar]
- 5.Fukuda‐Akita E, Okita K, Okauchi Y, et al. Impaired early insulin secretion in Japanese type 2 diabetes with metabolic syndrome. Diabetes Res Clin Pract 2008; 79: 482–489 [DOI] [PubMed] [Google Scholar]
- 6.Mandavilli A, Cyranoski D. News Feature: Asia’s big problem. Nat Med 2004; 10: 325–327 [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa Y. Metabolic syndrome‐ definition and diagnostic criteria in Japan. J Atheroscler Thromb 2005; 12: 301 [DOI] [PubMed] [Google Scholar]
- 8.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol Int 2010; 1: 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryo M, Maeda K, Onda T, et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 2005; 28: 451–453 [DOI] [PubMed] [Google Scholar]
- 10.The expert Committee on the Diagnosis and Classifications of Diabetes Mellitus . Follow‐up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care 2003; 26: 3160–3167 [DOI] [PubMed] [Google Scholar]
- 11.Insulin secretion and insulin sensitivity in relation to glucose tolerance . Lessons from the Botnia Study. Diabetes 2000; 49: 975–980 [DOI] [PubMed] [Google Scholar]


