Abstract
Anti‐diabetic agent‐related hypoglycemia is a serious complication in type 2 diabetic patients on hemodialysis. Therefore, we assessed the efficacy and tolerability of 24 weeks of monotherapy with vildagliptin, a dipeptidyl peptidase four inhibitor, which is a new class of antidiabetic agent. This open‐label, single‐arm clinical trial was performed on 26 patients on hemodialysis. The primary assessments were changes in postprandial glucose level and glycated albumin (GA). During the study, three patients dropped out, and data from 23 patients were analyzed. Significant reductions were seen in postprandial glucose (−2.60 ± 3.80 mmol/L, P < 0.001) and GA (−2.59 ± 2.33%, P < 0.001) levels. No serious drug‐related adverse events were observed. Vildagliptin monotherapy can be recommended for glycemic control in type 2 diabetic patients on hemodialysis. This trial was registered with the University Hospital Medical Information Network (no. UMIN000003661). (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00169.x, 2011)
Keywords: DPP‐4, Hemodialysis, Vildagliptin
Introduction
Strict glycemic control has beneficial effects on the prognosis of diabetic patients both with and without renal dysfunction1,2. Although intensive treatment with insulin or oral agents is used for glycemic control, the management of diabetes in patients with renal dysfunction presents unique challenges. Drug‐induced hypoglycemia is of particular concern in patients with renal dysfunction, being a serious complication that limits therapeutic choices and leads to unsatisfactory outcomes3. Thus, additional effective and well‐tolerated treatment options are needed for diabetic patients with renal dysfunction.
Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone, which stimulates insulin secretion and suppresses glucagon secretion. Inhibitors of the enzyme dipeptidyl peptidase‐4 (DPP‐4), which degrades GLP‐1, have shown beneficial effects on glycemic control along with a low risk of hypoglycemia4,5. Of the DPP‐4 inhibitors introduced to date, vildagliptin can be used for patients with renal dysfunction, because its main metabolites are pharmacologically inactive, although systemic exposure to vildagliptin is slightly increased in patients with renal dysfunction6 (Cmax 8–66%; AUC 32–134%). However, there are limited data for its use in patients on hemodialysis, because vildagliptin was recently approved and its efficacy has only been examined in patients without end‐stage renal disease7–9. In this study, therefore, we assessed the efficacy and tolerability of vildagliptin monotherapy in type 2 diabetic patients on hemodialysis.
Materials and Methods
This was a 24‐week, open‐label, single‐arm clinical study conducted at Otowa Memorial Hospital and Shiga University of Medical Science in Japan. The study enrolled hemodialysis patients with drug‐naïve type 2 diabetes with postprandial plasma glucose (PG) >11.1 mmol/L, glycated albumin (GA) >20%, or HbA1c >6.5%, as well as patients treated with an oral anti‐diabetic agent. Patients who had been treated with an oral agent before the study were switched to vildagliptin without a wash‐out period. Exclusion criteria included patients on insulin therapy, and those with liver disease, stroke, coronary artery disease, or evidence of malignancy or infectious disease.
After a 2‐week screening period, eligible patients received vildagliptin monotherapy (50 mg daily) for 24 weeks. Postprandial (2 h after a meal) blood samples were collected before starting hemodialysis. Postprandial PG and GA levels were measured at screening, and at weeks 0, 2, 4, 8, 12, 16, 20 and 24. Postprandial plasma active GLP‐1 and glucagon were assessed at weeks 0, 4, 8, 16 and 24. All adverse events were recorded. To measure active GLP‐1 or glucagon, plasma was collected in tubes containing either the DPP‐4 inhibitor or aprotinin. GLP‐1 and glucagon were measured by ELISA and RIA, respectively (Linco Research Inc., St Charles, MO, USA). The value for HbA1c (%) is estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula HbA1c (%) = HbA1c Japan Diabetes Society (JDS) (%) + 0.4%, considering the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods and HbA1c (NGSP)10.
Data were analyzed using SPSS version 17.0 (SPSS Inc., Tokyo, Japan). The distribution of variables was analyzed by checking histograms and normal plots of the data, and normality was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Statistical analyses were conducted by anova followed by Tukey’s test or Student’s t‐test to compare between time‐points. Pearson’s or Spearman’s rank correlation coefficients were calculated to determine correlations between variables. Values are expressed as means ± SD and P < 0.05 was considered statistically significant.
All participants provided written informed consent. The protocol was approved by an independent ethics committee/institutional review board.
Results
Of 26 patients enrolled in this study, three patients dropped out because of fatigue, worsening of glycemic control after switching to vildagliptin from mitiglinide, and severe pneumonia. Therefore, we analyzed data from 23 patients (male/female = 9/14) who completed the study. The mean age and duration of hemodialysis were 73.4 and 4.6 years, respectively. The mean postprandial PG and GA levels at baseline were 11.4 mmol/L and 23.8%. Sixteen patients were drug‐naïve, five patients had been previously treated with voglibose (0.9 mg/day) and two patients had been previously treated with mitiglinide (10 mg/day).
Several parameters remained unchanged at endpoint (Table 1), and no hypoglycemic events were recorded in the 23 analyzed patients. Vildagliptin elicited significant reductions in parameters of glycemic control by week 2, including postprandial PG and GA, which is a better marker for glycemic control than HbA1c in dialysis patients11 (Figure 1a,b). At the end of the study, the change from baseline was –2.60 ± 3.80 mmol/L (P < 0.001) for postprandial PG and −2.59 ± 2.33% (P < 0.001) for GA. Vildagliptin significantly increased postprandial plasma GLP‐1 levels (Figure 1c) and decreased postprandial plasma glucagon levels (Figure 1d). The relative change in plasma GLP‐1 was significantly and inversely correlated with that of GA (Figure 1e). Although not statistically significant, vildagliptin tended to decrease the plasma IL‐6 level, a prognostic marker for death and cardiovascular events in hemodialysis patients12 (Table 1).
Table 1. Characteristics of all participants at baseline and at the end of the study.
| Variables | Baseline | Study end (24 weeks) | P‐value |
|---|---|---|---|
| Creatinine (μmol/L) | 680.5 ± 293.8 | 682.3 ± 316.8 | 0.938 |
| Blood urea nitrogen (mmol/L) | 19.9 ± 6.54 | 17.6 ± 5.21 | 0.109 |
| Albumin (g/L) | 33.8 ± 3.2 | 34.1 ± 3.2 | 0.575 |
| Hematocrit (%) | 31.4 ± 4.1 | 31.7 ± 3.0 | 0.690 |
| Hemoglobin (g/L) | 99.0 ± 13.0 | 100.0 ± 9.0 | 0.569 |
| Alanine transferase (ALT) (IU/L) | 12.4 ± 4.7 | 13.0 ± 3.0 | 0.368 |
| Triglyceride (mmol/L) | 1.62 ± 0.70 | 1.70 ± 0.65 | 0.557 |
| Total cholesterol (mmol/L) | 4.47 ± 1.24 | 4.59 ± 0.96 | 0.538 |
| High density lipoprotein cholesterol (mmol/L) | 1.06 ± 0.29 | 1.03 ± 0.23 | 0.750 |
| Immunoreactive insulin (IRI) (pmol/L) | 145.1 ± 160.4 | 127.0 ± 86.3 | 0.504 |
| C‐peptide (nmol/L) | 3.31 ± 1.49 | 3.42 ± 1.33 | 0.659 |
| Interleukin 6 (pg/mL) | 6.21 ± 1.67 | 5.58 ± 1.93 | 0.101 |
| Erythropoietin dose (U/week) | 4532.6 ± 3193.8 | 3913.0 ± 2568.0 | 0.302 |
| Body weight (dry weight) (kg) | 51.3 ± 14.6 | 51.3 ± 14.8 | 0.928 |
| Intradialysis weight gain (kg) | 2.62 ± 1.64 | 2.56 ± 1.39 | 0.664 |
| Systolic blood pressure (mmHg) (before starting hemodialysis) | 146.3 ± 19.2 | 144.2 ± 19.7 | 0.637 |
| Diastolic blood pressure (mmHg) (before starting hemodialysis) | 73.0 ± 11.9 | 72.5 ± 13.8 | 0.833 |
Paired Student’s t test was used to compare between time‐points. Values are expressed as means ± SD and P < 0.05 was considered statistically significant.
Figure 1.
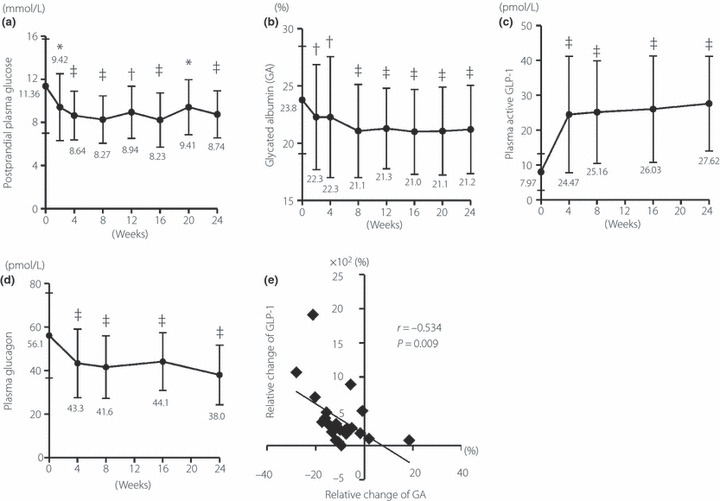
Time‐course of changes in (a) postprandial plasma glucose, (b) glycated albumin, (c) plasma GLP‐1 and (d) plasma glucagon levels. *P < 0.05 vs week 0; †P < 0.01 vs week 0; ‡P < 0.001 vs week 0. (e) Correlation between relative changes in plasma active GLP‐1 and glycated albumin levels (n = 23).
We next analyzed the data in two groups of patients according to prior treatment. In the drug‐naïve patients, the results were similar to those for all patients (Figure 2a–d). In patients treated with anti‐diabetic agents before the study, GA and glucagon levels decreased significantly while GLP‐1 increased significantly after switching to vildagliptin. In contrast, postprandial PG did not change significantly (Figure 2a–d).
Figure 2.
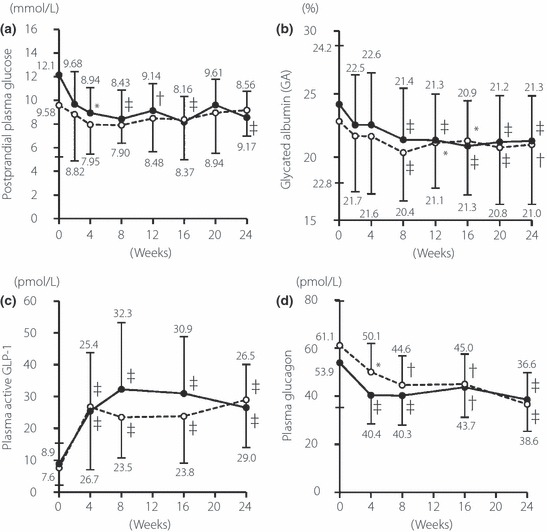
Time‐course of changes in (a) postprandial plasma glucose, (b) glycated albumin, (c) plasma GLP‐1 and (d) plasma glucagon levels. *P < 0.05 vs week 0; †P < 0.01 vs week 0; ‡P < 0.001 vs week 0. Closed circle: drug‐naïve patients. Open circle: anti‐diabetic agent‐pretreated patients.
Discussion
Our study showed that vildagliptin improved glycemic control without serious adverse effects in diabetic patients on hemodialysis, as in diabetic patients without renal dysfunction7–9. Furthermore, switching to vildagliptin from other anti‐diabetic agents did not worsen glycemic control, except in one patient. These results suggest that vildagliptin is an effective anti‐diabetic agent for type 2 diabetic patients on hemodialysis.
Severe hypoglycemia causes serious events in elderly patients on hemodialysis. The participants in this study are considered at high risk because of their advanced age. In fact, the hypoglycemic episodes were observed in two elderly patients treated with mitiglinide before the study, and disappeared after switching to vildagliptin. Thus, vildagliptin‐mediated glycemic control without hypoglycemia would be beneficial for glycemic control in elderly patients on hemodialysis. However, as mentioned above, one patient treated with mitiglinide before the study showed a worsening of glycemic control after switching to vildagliptin that was apparent within 1 week. We also compared all parameters between non‐responders and responders, but found no marked differences in any of the parameters, including GA, GLP‐1 and glucagon levels. Overall, vildagliptin monotherapy was effective in these type 2 diabetic patients on hemodialysis; nevertheless, glucose levels should be carefully monitored, particularly after switching from anti‐diabetic agents.
Increases in GLP‐1 levels and decreases in glucagon levels both contribute to the blood glucose‐lowering effects of DPP‐4 inhibitors4, and we detected similar changes in these hemodialysis patients. Furthermore, the relative change in GLP‐1, but not glucagon, was correlated with that of GA, suggesting that GLP‐1 might contribute more to glucose‐lowering in hemodialysis patients. However, in patients who had been treated with an anti‐diabetic agent before the study, GA decreased significantly without decreases in postprandial PG. These results suggested that vildagliptin‐mediated suppression of glucagon secretion might improve GA by decreasing fasting PG, although we could not measure this in the present study.
Our results suggest that raising plasma GLP‐1 levels could be a better therapeutic target for glycemic control in hemodialysis patients. Interestingly, an α‐glucosidase inhibitor such as voglibose increases plasma GLP‐1 levels with a mechanism different from DPP‐4 inhibitor13, suggesting that some additive or synergistic effects may be expected when these two drugs are used simultaneously in hemodialysis patients.
In conclusion, this study showed that vildagliptin monotherapy is effective in type 2 diabetic patients on hemodialysis. However, limitations of our study included the small number of patients. Further longitudinal and larger randomized studies are needed to confirm the long‐term tolerability and efficacy of vildagliptin on glycemic control and the prevention of other diabetic complications in these high‐risk patients.
Acknowledgements
The authors acknowledge the assistance of Yumiko Omura and Itsuko Miyazawa at Shiga University of Medical Science, and the staff at the Dialysis Center at Rakuwakai Otowa Memorial Hospital. The authors declare no conflict of interest.
References
- 1.Hayashino Y, Fukuhara S, Akiba T, et al. Diabetes, glycaemic control and mortality risk in patients on haemodialysis: the Japan Dialysis Outcomes and Practice Pattern Study. Diabetologia 2007; 50: 1170–1177 [DOI] [PubMed] [Google Scholar]
- 2.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 3.Haviv YS, Sharkia M, Safadi R. Hypoglycemia in patients with renal failure. Ren Fail 2000; 22: 219–223 [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705 [DOI] [PubMed] [Google Scholar]
- 5.Mest HJ, Mentlein R. Dipeptidyl peptidase inhibitors as new drugs for the treatment of type 2 diabetes. Diabetologia 2005; 48: 616–620 [DOI] [PubMed] [Google Scholar]
- 6.Scheen AJ. Pharmacokinetics of dipeptidylpeptidase‐4 inhibitors. Diabetes Obes Metab 2010; 12: 648–658 [DOI] [PubMed] [Google Scholar]
- 7.Pratley RE, Rosenstock J, Pi‐Sunyer FX, et al. Management of type 2 diabetes in treatment‐naive elderly patients: benefits and risks of vildagliptin monotherapy. Diabetes Care 2007; 30: 3017–3022 [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Baron MA, Dejager S, et al. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24‐week, double‐blind, randomized trial. Diabetes Care 2007; 30: 217–223 [DOI] [PubMed] [Google Scholar]
- 9.Rosenstock J, Foley JE, Rendell M, et al. Effects of the dipeptidyl peptidase‐IV inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose tolerance. Diabetes Care 2008; 31: 30–35 [DOI] [PubMed] [Google Scholar]
- 10.The committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1:212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18: 896–903 [DOI] [PubMed] [Google Scholar]
- 12.Bologa RM, Levine DM, Parker TS, et al. Interleukin‐6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis 1998; 32: 107–114 [DOI] [PubMed] [Google Scholar]
- 13.Moritoh Y, Takeuchi K, Hazama M. Chronic administration of voglibose, an alpha‐glucosidase inhibitor, increases active glucagon‐like peptide‐1 levels by increasing its secretion and decreasing dipeptidyl peptidase‐4 activity in ob/ob mice. J Pharmacol Exp Ther 2009; 329: 669–676 [DOI] [PubMed] [Google Scholar]


