Abstract
Aims/Introduction: Activation of the renin‐angiotensin system (RAS) in the kidney plays an important role in renal function. The aim of this study was to investigate whether plasma and urinary angiotensinogen levels were associated with renal and cardiovascular prognosis in type 2 diabetic patients.
Materials and Methods: We measured plasma and urinary angiotensinogen levels in the observational follow‐up cohort of 234 Japanese type 2 diabetic patients (144 with normoalbuminuria, 90 with albuminuria) enrolled between 1998 and 1999 and followed them up until the end of 2008. The associations of these markers with the annual decline in the estimated glomerular filtration rate (eGFR) and incidence of renal and cardiovascular composite endpoints (chronic hemodialysis, myocardial infarction, angina pectoris, stroke and cerebral hemorrhage) were evaluated.
Results: At baseline, urinary angiotensinogen levels correlated with urinary albumin‐creatinine ratio, urinary β2‐microglobulin and inversely with eGFR. In contrast, plasma angiotensinogen levels correlated neither with these renal factors nor with urinary angiotensinogen levels. In the follow‐up study (median duration: 9 years), urinary angiotensinogen, but not plasma angiotensinogen, correlated inversely with the annual change in eGFR (r = −0.51, P < 0.001). When patients were divided into four subgroups according to albuminuria and urinary angiotensinogen levels, patients with albuminuria and high urinary angiotensinogen levels showed a progressive decline of eGFR and a higher incidence of renal and cardiovascular composite endpoints.
Conclusions: These results suggest that the higher level of urinary angiotensinogen in type 2 diabetic patients with albuminuria is a high risk factor for worsening renal and cardiovascular complications. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00172.x, 2011)
Keywords: Angiotensinogen, Diabetes mellitus, Glomerular filtration rate
Introduction
Diabetic nephropathy is a representative disorder of chronic kidney disease (CKD) and a leading cause of end‐stage kidney disease (ESKD). This disorder is also associated with high morbidity and mortality of cardiovascular disease (CVD)1–3. Thus, prevention of development and progression of this disorder is of clinical importance to improve prognosis in diabetic patients.
Numerous clinical trials have documented that inhibition of the renin‐angiotensin system (RAS) in diabetic patients can slow the progressive decrease in glomerular filtration rate (GFR) and reduce cardiovascular mortality and morbidity4–6. Based on clinical evidence, inhibition of the RAS is currently the first line treatment for diabetic nephropathy7. These results also support the concept that activation of RAS in diabetic patients is an important pathogenic mechanism of renal and cardiovascular complications7. However, despite the beneficial effects of RAS inhibition, all patients do not always show an improvement in the prognosis of these complications. Therefore, it is important to identify patients at higher risk of poor prognosis and a proper estimation of the status of intrarenal RAS activation may provide crucial information.
The kidney contains all components of the RAS pathway including the production of angiotensinogen8. Thus, the kidney can locally produce angiotensin II (AngII) by a mechanism independent of circulating AngII, known as the classical RAS pathway8. Intrarenally‐produced AngII is reported to play an important role in renal hemodynamics and function as a paracrine factor9.
We recently developed a direct method to quantify human plasma and urinary angiotensinogen levels using enzyme‐linked immunosorbent assays (ELISA)10. Using this new method, we recently reported that urinary angiotensinogen may be a potential biomarker of the severity of CKD and intrarenal RAS status in hypertensive patients in the cross‐sectional studies11,12. However, it is still unclear whether urinary and plasma angiotensinogen levels can be used to predict deterioration of renal function and the incidence of cardiovascular disease in a long longitudinal cohort. In the present study, we measured plasma and urinary angiotensinogen levels using our new ELISA method, in Japanese patients with type 2 diabetes who were enrolled in our observational follow‐up study2. We then investigated whether these markers associate with renal and cardiovascular prognosis.
Materials and methods
Study Population and Samples
Japanese patients with type 2 diabetes mellitus were recruited from among participants who were registered in the Shiga Prospective Observational Follow‐up Study between 1998 and 19992. After obtaining written informed consent, each individual provided a spot urine sample and a fasting blood sample at baseline. The plasma and urine samples were kept at −80°C if not analyzed immediately. Based on the level of urinary albumin–creatinine ratio (UACR) at baseline, patients were classified as having normoalbuminuria (UACR < 30 mg/g Cr), microalbuminuria (30 ≤ UACR < 300 mg/g Cr), or overt proteinuria (UACR ≥ 300 mg/g Cr). Finally, 234 patients with normoalbuminuria (n = 144), microalbuminuria (n = 53) and overt proteinuria (n = 37) were enrolled and were followed up until the end of 2008 or the incidence of the renal and cardiovascular composite endpoints. In this study, patients with microalbuminuria and overt proteinuria were combined together into those with albuminuria (diabetic nephropathy). The participants underwent standardized clinical examination and biochemical tests annually, during the follow‐up period. In this study, the values of HbA1c were presented in National Glycohemoglobin Standardization Program values according to the recommendations of the Japanese Diabetes Society13. The study protocol and informed consent procedure were approved by the Ethics Committee of Shiga University of Medical Science.
Measurement of Plasma and Urinary Angiotensinogen Levels
The concentrations of angiotensinogen in plasma and urine samples at baseline were measured with human angiotensinogen ELISA, as reported previously10. The sensitivity of this assay is >0.31 ng/mL. The intra‐ and inter‐assay coefficients of variation were 4.4 and 4.3%, respectively. The urinary concentrations of creatinine were measured simultaneously by the enzymatic method. The urinary level of angiotensinogen was expressed in μg/g Cr.
Follow‐up Evaluation
To evaluate deterioration of renal function, we assessed the annual decline in estimated GFR (eGFR). eGFR was calculated using the simplified prediction equation proposed by the Japanese Society of Nephrology14: eGFR (mL/min/1.73 m2) = 194 × [age (years)]−0.287 × [serum creatinine (mg/dL)]−1.094 × 0.739 (for female). The serum concentration of creatinine was measured using the enzymatic method. The annual decline in eGFR over the course of the study was determined from the slope of the plot of all measurements of eGFR for each individual calculated by linear regression analysis and was expressed in mL/min/1.73 m2/year.
We also investigated the incidence of the renal and cardiovascular composite endpoints, including myocardial infarction, angina pectoris, stroke and cerebral hemorrhage and initiation of chronic hemodialysis. Myocardial infarction was defined as a clinical presentation characterized by typical symptoms, electrocardiographic changes associated with an elevation of cardiac biomarkers and angiographic evidence of coronary thrombosis. Angina pectoris was defined as a history of typical chest pain and electrocardiographic changes compatible with ischemic heart disease or the detection of myocardial perfusion defects with exercise stress tests. Stroke and cerebral hemorrhage were defined as a persistent focal neurological symptom in which onset was sudden and was not due to trauma or a tumor and where the responsible lesion was detected by imaging studies.
Statistical Analysis
Data are expressed as mean ± SD or median (interquartile range). As compared between two groups, unpaired Student’s t‐test for continuous variables and chi‐square test for categorical variables were applied. A comparison among three or more groups was performed by anova with the Tukey‐Kramer HSD test. Due to the skewed distribution, urinary angiotensinogen, UACR and urinary β2‐microglobrin (U‐β2MG) values were log‐transformed before analysis. Pearson regression analysis was applied for analysis of the correlation between two variables, using logarithmic transformed values of non‐normally distributed variables. A multivariate liner regression model was applied to evaluate the independency of factors that showed significant correlation in the univariate model. The cumulative incidences of renal and cardiovascular composite endpoints were estimated using Kaplan–Meier procedure and were compared by the log‐rank test. The follow‐up time was censored if any composite endpoint was observed or if the patient was unavailable for follow‐up. Risk for renal and cardiovascular composite endpoint was evaluated by a Cox hazard regression model. A forward stepwise procedure was used to select explanatory variables with statistically significant effects on the time to the incidence of the endpoint. All analyses were performed by the SPSS software package (version 11; SPSS Inc., Chicago, IL, USA) and JMP for Windows (version 8.0.2; SAS Institute Inc, Cary, NC, USA). A two‐sided P value <0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics
Table 1 lists the clinical characteristics of patients at baseline stratified by the stage of nephropathy. Gender, duration of diabetes, body mass index (BMI), HbA1c, systolic blood pressure (SBP), diastolic blood pressure (DBP), use of RAS inhibitors, triglyceride, UACR, eGFR and U‐β2MG were different between the normoalbuminuria and albuminuria groups.
Table 1. Clinical characteristics of the study subjects.
| Normoalbuminuria | Albuminuria | P | |
|---|---|---|---|
| Number | 144 | 90 | |
| Gender (male/female) | 72/72 | 57/33 | <0.05 |
| Age (year) | 60 ± 8 | 59 ± 9 | n.s. |
| Duration of diabetes (year) | 13 ± 8 | 16 ± 8 | <0.01 |
| Body mass index (kg/m2) | 23.1 ± 3.4 | 24.5 ± 3.7 | <0.01 |
| Waist to hip ratio | 0.93 ± 0.08 | 0.95 ± 0.09 | n.s. |
| HbA1c (%) | 7.4 ± 0.8 | 7.9 ± 1.2 | <0.01 |
| Systolic blood pressure (mmHg) | 135 ± 17 | 144 ± 19 | <0.01 |
| Diastolic blood pressure (mmHg) | 77 ± 9 | 81 ± 10 | <0.01 |
| Taking RAS inhibitors (%) | 16 | 31 | <0.01 |
| Past history of CVD (%) | 13 | 20 | n.s. |
| Total cholesterol (mg/dL) | 213 ± 32 | 219 ± 37 | n.s. |
| HDL‐cholesterol (mg/dL) | 60 ± 156 | 56 ± 15 | n.s. |
| Triglycerides (mg/dL) | 111 ± 32 | 135 ± 76 | <0.05 |
| Urinary ACR (mg/g Cr) | 10 (7–15) | 161 (61–672) | <0.05 |
| Estimated GFR (mL/min/1.73 m2) | 81 ± 15 | 69 ± 26 | <0.01 |
| Urinary β2‐microglobulin (μg/g Cr) | 114 (73–172) | 188 (81–907) | <0.01 |
Data are mean ± SD for normally distributed continuous variables or median (25th–75th interquartiles) for skewed continuous variables. Albuminuria represents microalbuminuria and overt proteinuria.
RAS, renin‐angiotensin system; ACR, albumin‐creatinine ratio; Cr, creatinine; GFR, glomerular filtration rate; CVD, cardiovascular disease.
Correlation Between Plasma Angiotensinogen Level and Various Parameters at Baseline
Plasma angiotensinogen levels were not different between two groups (normoalbuminuria: 24.7 ± 5.3, albuminuria: 24.1 ± 5.4 μg/mL). Univariate regression analysis showed weak correlations between plasma angiotensinogen levels and BMI, waist‐hip ratio, SBP, total cholesterol, HDL‐cholesterol and triglyceride, and no correlation with UACR, eGFR and U‐β2MG (Table 2). Plasma angiotensinogen levels were not different between patients treated with RAS inhibitors and those without them (24.4 ± 5.3 vs 24.6 ± 5.6 μg/mL, P = 0.97). Interestingly, plasma angiotensinogen levels were significantly higher in females than males (26.7 ± 5.3 vs 22.6 ± 4.6 μg/mL, P < 0.001). However, plasma angiotensinogen levels did not correlate with UACR, eGFR and U‐β2MG even when patients were analyzed separately according to gender.
Table 2. Factors that correlated with plasma and urinary angiotensinogen levels in univariate analysis.
| Parameter | Plasma angiotensinogen | Urinary angiotensinogen | ||
|---|---|---|---|---|
| r | P | r | P | |
| Age | −0.02 | 0.75 | −0.07 | 0.31 |
| Duration of diabetes | −0.10 | 0.11 | 0.24 | <0.001 |
| Body mass index | 0.17 | 0.009 | 0.13 | 0.04 |
| Waist to hip ratio | 0.36 | <0.001 | 0.10 | 0.15 |
| HbA1c | 0.10 | 0.14 | 0.26 | <0.001 |
| Systolic blood pressure | 0.14 | 0.03 | 0.30 | <0.001 |
| Diastolic blood pressure | 0.10 | 0.13 | 0.23 | <0.001 |
| Total cholesterol | 0.35 | <0.001 | 0.17 | 0.008 |
| HDL‐cholesterol | 0.20 | 0.002 | −0.12 | 0.06 |
| Triglycerides | 0.18 | 0.006 | 0.19 | 0.004 |
| Urinary ACR | 0.01 | 0.88 | 0.77 | <0.001 |
| Estimated GFR | 0.04 | 0.59 | −0.44 | <0.001 |
| Urinary β2‐microglobulin | −0.07 | 0.26 | 0.72 | <0.001 |
Correlation was evaluated with the Pearson’s correlation coefficient.
The values of urinary angiotensinogen, urinary ACR and urinary β2‐microglobulin were log‐transformed for the analysis because of their skewed distribution.
ACR, albumin‐creatinine ratio; GFR, glomerular filtration rate.
Correlation Between Urinary Angiotensinogen Level and Various Parameters at Baseline
In contrast to plasma angiotensinogen, urinary angiotensinogen levels were higher in patients with albuminuria (62.0 μg/g Cr [interquartile range: 25.4–146.5]) than in those with normoalbuminuria (17.5 μg/g Cr [11.4–28.2], P < 0.001). Univariate regression analysis showed that urinary angiotensinogen levels correlated positively with UACR and U‐β2MG and inversely with eGFR (Table 2). Interestingly, there was no correlation between urinary angiotensinogen and plasma angiotensinogen (r = 0.08, P = 0.21). Urinary angiotensinogen levels were higher in patients treated with RAS inhibitors (38 μg/g Cr [19–133]) than those without (22 μg/g Cr [13–42], P = 0.001). However, this difference was probably due to the different prescription rate of RAS inhibitors in the two groups (normoalbuminuria: 16%, albuminuria: 32%). When urinary angiotensinogen levels were compared according to the stage of nephropathy, those in each stage were not different between patients treated with RAS inhibitors and those without. Unlike plasma angiotensinogen, the urinary angiotensinogen level in males was similar to that in females. Multiple regression analysis identified UACR and U‐β2MG as the independent and significant factors that correlated with urinary angiotensinogen levels.
Correlation Between Angiotensinogen Level and Annual Decline in eGFR
To explore the predictive role of plasma and urinary angiotensinogen levels for renal dysfunction, we investigated the correlation between each angiotensinogen and the annual change in eGFR during the follow‐up period (median: 9 years, interquartile range: 6–10 years). As shown in Figure 1, urinary angiotensinogen, but not plasma angiotensinogen (r = 0.00, P = 0.99), correlated inversely with the annual change in eGFR (r = −0.51, P < 0.001). As other factors, the annual decline in eGFR correlated strongly with UACR (r = −0.65, P < 0.001) and correlated weakly with triglyceride (r = −0.28, P < 0.001), HDL‐cholesterol (r = 0.15, P = 0.027), HbA1c (r = −0.22, P = 0.001), eGFR at baseline (r = 0.32, P < 0.001), BMI (r = −0.24, P < 0.001), SBP (r = −0.24, P < 0.001) and DBP (r = −0.23, P < 0.001).
Figure 1.
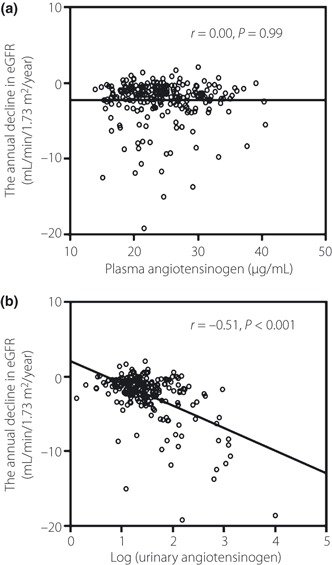
Scatter diagram of the correlation between the annual decline in estimated glomerular filtration rate (eGFR) and (a) plasma angiotensinogen and (b) urinary angiotensinogen. Correlation was evaluated with the Pearson’s correlation coefficient. Data are log‐transformed values of urinary angiotensinogen.
Urinary Angiotensinogen and Renal Dysfunction in Patients with Albuminuria
Albuminuria is well known to be a risk factor for renal dysfunction and cardiovascular disease in patients with type 2 diabetes. Based on the strong correlation between urinary angiotensinogen and UACR, it was difficult to determine the specific role of each parameter in renal dysfunction. Therefore, to explore the clinical utility of measuring urinary angiotensiongen, we investigated the predictive effect of the combination of urinary angiotensinogen and albuminuria on deterioration of renal function. For this purpose, patients were divided into four groups according to the median value of urinary angiotensinogen levels (median cut‐off values: 24.7 μg/g Cr) and the presence of albuminuria (>30 mg/g Cr). The eGFR at baseline (mL/min/1.73 m2) was 80 ± 15 in those with low levels of urinary angiotensinogen and normoalbuminuria (L + N, n = 97), 84 ± 14 in those with high levels of urinary angiotensinogen and normoalbuminuria (H + N, n = 47), 82 ± 19 in those with low levels of urinary angiotensinogen and albuminuria (L + A, n = 21) and 66 ± 27 in patients with high levels of urinary angiotensinogen and albuminuria (H + A, n = 69). Among the four subgroups, the annual decline in eGFR during the follow‐up was significantly greater in the H + A subgroup than other subgroups (P < 0.05 vs all other subgroup, Figure 2).
Figure 2.
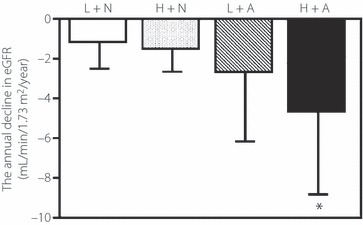
Annual decline in estimated glomerular filtration rate (eGFR) during follow‐up. Patients were divided into four groups using the median value of urinary angiotensinogen level (24.7 μg/g Cr) and the presence of albuminuria (>30 mg/g Cr). Patients with low levels of urinary angiotensinogen and normoalbuminuria (L + N, n = 97); patients with high levels of urinary angiotensinogen and normoalbuminuria (H + N, n = 47); patients with low levels of urinary angiotensinogen and albuminuria (L + A, n = 21) and patients with high levels of urinary angiotensinogen and albuminuria (H + A, n = 69). The respective annual decline in eGFR was: −1.2 ± 1.3, −1.4 ± 1.3, −2.7 ± 3.5 and −4.6 ± 4.2 mL/min/1.73 m2/year. Data are mean ± SD. *P < 0.05 vs each other group (anova with Tukey–Kramer HSD test).
Urinary Angiotensinogen and Renal‐Cardiovascular Outcomes in Patients with Albuminuria
Finally, we evaluated the association between urinary angiotensinogen at baseline and the incidence of renal and cardiovascular composite endpoints. A total of 58 patients experienced any of the composite endpoints (17 for chronic hemodialysis, 10 for myocardial infarction, 18 for angina pectoris, eight for stroke and five for cerebral hemorrhage). The incidence rate of this endpoint was higher in patients with high levels of urinary angiotensinogen than those with low levels of urinary angiotensinogen (36% vs 14%, χ2 = 15.5, P < 0.001). Similarly, the incidence rate of this endpoint was higher in patients with albuminuria than those with normoalbuminuria (47% vs 11%, χ2 = 37.6, P < 0.001). As shown in Figure 3, the cumulative incidence among the four subgroups was the highest in the H + A subgroup (log rank test: P < 0.001 for trend). Multivariate Cox proportional hazard regression model with the forward stepwise procedure identified four predictors of renal and cardiovascular outcomes: the combination of urinary angiotensinogen and albuminuria (adjusted odds ratio 4.5 [95% CI: 2.1–9.5] in H + A subgroup, 3.4 [1.2–9.3] in L + A subgroup and 1.6 [0.6–4.4] in H + N subgroup, 1.0 [reference] in L + N subgroup), age (1.04 [1.00–1.08]), eGFR at baseline (0.97 [0.96–0.98]) and past history of CVD (1.90 [1.06–3.41]).
Figure 3.
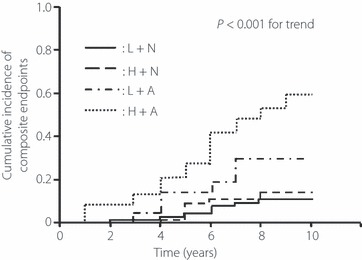
Kaplan–Meier curves for cumulative incidence of renal and cardiovascular composite endpoints. Patients were divided into the four groups using the median value of urinary angiotensinogen level (24.7 μg/g Cr) and the presence of albuminuria (>30 mg/g Cr). Patients with low levels of urinary angiotensinogen and normoalbuminuria (L + N, n = 97); patients with high levels of urinary angiotensinogen and normoalbuminuria (H + N, n = 47); patients with low levels of urinary angiotensinogen and albuminuria (L + A, n = 21) and patients with high levels of urinary angiotensinogen and albuminuria (H + A, n = 69). Difference among the groups was tested by log rank test.
Discussion
In this study, analysis of baseline data showed that urinary angiotensinogen levels correlated with UACR and U‐β2MG and inversely with eGFR. In contrast, plasma angiotensinogen levels did not correlate with these factors or with urinary angiotensinogen levels. Furthermore, follow‐up analysis indicated that patients with albuminuria and high levels of urinary angiotensinogen showed the progressive decline of renal function and the high incidence of renal‐cardiovascular endpoints. These results suggest that the higher level of urinary angiotensinogen in type 2 diabetic patients with nephropathy is a high risk factor for worsening renal and cardiovascular complications.
In the present study, urinary angiotensinogen levels correlated closely with renal factors but did not correlate with plasma angiotensinogen levels. In contrast, plasma angiotensinogen levels correlated with various metabolic factors including BMI, waist‐hip ratio and serum lipids, in agreement with the data of a previous report15, but they did not correlate with renal factors. These results suggest that urinary and plasma angiotensinogen are produced by different sources and play different roles in renal function. Although angiotensinogen is produced and secreted by the liver, it is also produced in the kidney9. Previous studies have investigated whether circulating angiotensinogen is a source of urinary angiotensinogen. In hypertensive and normotensive rats infused human angiotensinogen, the circulating human angiotensinogen was not detectable in the urine, indicating limited glomerular permeability and/or tubular degradation of circulating angiotensinogen16. In the kidney under normal conditions, the expression of angiotensinogen is reported to localize in proximal tubular cells and angiotensinogen produced in proximal tubular cells is considered to be directly released into the renal tubular lumen9. Under diabetic conditions, the expression of angiotensinogen is reported to be enhanced in proximal tubular cells and to be also observed in mesangial cells17,18. Some human studies reported higher levels of urinary angiotensinogen in diabetic patients than in control subjects and patients with non‐diabetic kidney diseases11,19, whereas plasma angiotensinogen levels were similar in diabetic patients and control subjects19. Because the kidney contains all components of the RAS pathway, the enhanced expression of intrarenal angiotensinogen may lead to the intrarenal RAS activation. Thus, these results suggest that urinary angiotensinogen is produced locally in the kidney, but not from plasma, and its levels may associate with intrarenal RAS activation in diabetic patients.
In the present study, patients with high levels of urinary angiotensinogen, not plasma angiotensinogen, showed a greater decline in eGFR during the follow‐up. A similar observation in patients with CKD documented the presence of higher urinary angiotensinogen levels in patients with low eGFR and patients with higher levels of urinary angiotensinogen showed increased risk of renal dysfunction during a mean follow‐up period of 23 months20. Thus, urinary angiotensinogen is considered to be associated with the deterioration of renal function in patients with CKD including diabetic nephropathy.
Albuminuria is well known to be not only a predictor of progression to ESKD but also a risk factor for cardiovascular disease1,2. In this study, urinary angiotensinogen levels correlated closely with UACR as well as previous reports12,21. However, patients with albuminuria and higher levels of urinary angiotensinogen showed a progressive decline in eGFR and the high incidence of renal‐cardiovascular endpoints than those with albuminuria and low levels of angiotensinogen. Thus, the increase of urinary angiotensinogen in patients with albuminuria may predict the patients at risk for worsening renal and cardiovascular complications.
What is the mechanism by which urinary angiotensinogen levels associate with worsening renal and cardiovascular complications? In this study, urinary angiotensinogen levels correlated with UACR and U‐β2MG. Transgenic mice overexpressing angiotensinogen in renal proximal tubular cells were reported to develop albuminuria, hypertension and renal injury22. The induction of diabetes with streptozotocin in these transgenic mice enhanced the aforementioned abnormal changes and induced apoptosis of renal proximal tubular cells23. Although diabetic nephropathy was traditionally considered to cause glomerular damage primarily, it is now widely accepted that deterioration of renal function in diabetic patients correlates with the degree of tubulointerstitial fibrosis24,25. Thus, the enhanced expression of angiotensinogen in proximal tubular cells under diabetic conditions, which may correlate with urinary angiotensiongen levels, may cause the tubulointerstitial injury and, then, result in the decline in eGFR. Also the augmentation of urinary angiotensinogen is considered to lead to increased formation of AngII in the kidney9. Thus, the increase of urinary angiotensinogen may contribute to the development and progression of hypertension, which may associate with renal dysfunction and the incidence of cardiovascular disease. In the present study, urinary angiotenisinogen levels correlated with systolic and diastolic blood pressure as well as a previous report12.
In this study, the data of clinical parameters including angiotensinogen were collected only at baseline. Thus, the time‐dependent changes in these parameters during the follow‐up were not evaluated. Also, the information regarding the use of RAS inhibitors during the follow‐up period was not included in this study. Previous studies reported that RAS inhibitors were associated with reduction in urinary angiotensinogen levels12,26. In the present study, the levels of urinary angiotensinogen in patients treated with RAS inhibitors were not different from those without such treatment when data was analyzed separately according to the stage of nephropathy. In Japan, the prescription rate of RAS inhibitors in the past was much lower than that at present. Also, RAS inhibitors tended to be prescribed for patients who showed progression to the advanced stage of nephropathy or those at risk for cardiovascular disease. Thus, the present study does not provide conclusive data on the influence of RAS inhibitors on urinary angiotensinogen levels. Further studies are required to explore whether the reduction of urinary angiotensinogen level by any medication bring about improving renal and cardiovascular prognoses.
In conclusion, the present study demonstrated that urinary angiotensinogen levels correlated with progressive deterioration of renal function and the high incidence of renal‐cardiovascular endpoints in patients with type 2 diabetes mellitus. These results suggest that higher levels of urinary angiotensinogen in patients with diabetic nephropathy are clinically useful to identify patients who are at high risk for worsening renal and cardiovascular complications. Also, the reduction of urinary angiotensinogen levels may be a new therapeutic index to prevent the worsening of renal and cardiovascular complications in diabetic patients with nephropathy.
Acknowledgements
All authors have no conflict of interest to disclose. This study was supported in part by a Grant‐in‐Aid for Diabetic Nephropathy, from the Ministry of Health, Labour and Welfare of Japan.
References
- 1.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 2.Araki S, Haneda M, Koya D, et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 2007; 56: 1727–1730 [DOI] [PubMed] [Google Scholar]
- 3.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009; 20: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Bain RP, et al. The Collaborative Study Group. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. N Engl J Med 1993; 329: 1456–1462 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 7.Koya D, Araki S, Haneda M. Therapeutic management of diabetic kidney disease. J Diabetes Invest 2011; 2: 248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingelfinger JR, Zuo WM, Fon EA, et al. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest 1990; 85: 417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin‐angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287 [DOI] [PubMed] [Google Scholar]
- 10.Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 2007; 293: F956–F960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Ohashi N, Katsurada A, et al. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens 2008; 2: 349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Alper AB Jr, Shenava R, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin‐angiotensin system status in hypertensive patients. Hypertension 2009; 53: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 15.Yasue S, Masuzaki H, Okada S, et al. Adipose tissue‐specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens 2010; 23: 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Nishiyama A, Harrison‐Bernard LM, et al. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension 2003; 41: 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohashi N, Urushihara M, Satou R, et al. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species – ERK/JNK pathways. Hypertens Res 2010; 33: 1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh TJ, Zhang SL, Filep JG, et al. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 2002; 143: 2975–2985 [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Urushihara M, Kotani Y, et al. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci 2009; 338: 478–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto T, Nakagawa T, Suzuki H, et al. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with aeterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 2007; 18: 1558–1565 [DOI] [PubMed] [Google Scholar]
- 21.Urushihara M, Kondo S, Kagami S, et al. Urinary angiotensinogen accurately reflects intrarenal Renin‐Angiotensin system activity. Am J Nephrol 2010; 31: 318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachetelli S, Liu Q, Zhang SL, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int 2006; 69: 1016–1023 [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Brezniceanu ML, Wei CC, et al. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol 2008; 19: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najafian B, Mauer M. Progression of diabetic nephropathy in type 1 diabetic patients. Diabetes Res Clin Pract 2009; 83: 1–8 [DOI] [PubMed] [Google Scholar]
- 25.Araki S, Haneda M, Koya D, et al. Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Diabetes Care 2010; 33: 1805–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa S, Kobori H, Ohashi N, et al. Angiotensin II type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with type 2 diabetic nephropathy. Biomark Insights 2009; 4: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]


