Abstract
Aims/Introduction: Endothelial progenitor cells (EPC) play a critical role in adult vasculogenesis and vascular repair. Previous studies have described the dysfunction of EPC in diabetic patients, but the precise mechanism is still unclear. To elucidate the dysfunction of EPC in diabetic patients, we investigated the functions and intracellular signaling of EPC under normal or high glucose conditions. We also examined the number of EPC in the peripheral blood of Japanese type 2 diabetic patients.
Materials and Methods: EPC were cultured with normal or high glucose. Subsequently, the proliferation and the apoptosis of EPC were assessed in the presence or absence of vascular endothelial growth factor (VEGF). The phosphorylation of Akt was assessed by western blot analyses. We compared the number of CD34+CD45low progenitor cells, which is considered as a marker of EPC in non‐diabetic and type 2 diabetic subjects, using flow cytometry.
Results: High glucose decreased the proliferation of EPC and increased the number of apoptotic cells. VEGF significantly increased the proliferation and suppressed the apoptosis of EPC, both of which were abolished by PI 3‐kinase inhibitor, LY294002. High glucose significantly suppressed the basal and VEGF‐stimulated phosphorylation of Akt in EPC. Furthermore, the number of circulating EPC was decreased in type 2 diabetic patients, although there were no significant differences in the serum levels of VEGF between control subjects and diabetic patients.
Conclusions: These findings suggest that high glucose impairs the functions of EPC through the suppression of Akt phosphorylation stimulated by VEGF. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00093.x, 2011)
Keywords: Akt, Endothelial progenitor cells, Vascular endothelial growth factor
Introduction
Endothelial progenitor cells (EPC) play an important role in postnatal neovascularization and vascular repair1,2. Circulating EPC adhere to the sites of vascularization and migrate into the target tissue resulting in the growth of new blood vessels. Previous experimental studies have shown successful results of the transplantation of EPC in targeted ischemic tissues2,3. A number of preclinical studies on the transplantation of EPC have been carried out in the past decade. The safety of EPC transplantation is thought to be established, although the effects are modest4,5. EPC exist in bone marrow, cord blood and in small numbers in peripheral blood and local tissue. Previous studies have provided evidence that decreased numbers and activities of EPC might contribute to impaired vascularization in patients with coronary artery diseases6,7. The EPC levels were negatively correlated with the degree of carotid stenosis, graft vasculopathy and tissue ischemia8,9. Patients with risk factors for coronary artery diseases, such as diabetes, dyslipidemia and hypertension, are also reported to have decreased and dysfunctional EPC10–12. These findings suggest that the dysfunction of EPC might affect not only the efficacy of transplantation therapy with autologous EPC, but also vascular functions.
An essential diabetic complication is the vascular dysfunction of micro‐ and macrovasculatures. From this viewpoint, the dysfunction of EPC might advance the progress of diabetic complications. In fact, impaired functions of EPC were reported in patients with proliferative diabetic retinopathy13. To elucidate the mechanisms of the diabetes‐induced dysfunction of EPC is important for better management of diabetic complications.
Vascular endothelial growth factor (VEGF) increases the proliferation and migration of endothelial cells. The anti‐apoptotic effects of VEGF are also reported to be important in angiogenesis14. VEGF affects not only mature endothelial cells, but also EPC. VEGF induces the mobilization of bone marrow‐derived EPC, resulting in the increase of differentiated EPC and neovascularization15–17. To elucidate the mechanisms by which diabetes interferes with EPC, we investigated the growth and apoptosis of EPC under normal or high glucose conditions and examined the proliferative and anti‐apoptotic effect of VEGF. In addition, we examined the number of EPC in the peripheral blood of Japanese type 2 diabetic patients.
Materials and Methods
Human Umbilical Cord Blood
Human umbilical cord blood was obtained from each donor after the baby’s delivery. Written informed consent was obtained from all mothers before labor and delivery. Protocols for sampling human umbilical cord blood were approved by the Institutional Review Board.
Isolation and Culture of EPC
Isolation and ex vivo expansion of EPC were carried out as previously described18. In brief, cord blood‐derived mononuclear cells from human volunteers were plated on human fibronectin‐coated (Sigma, St. Louis, MO, USA) culture dishes and maintained in Medium 199 (Sigma) supplemented with 20% ES‐qualified fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). After 4 days in culture, non‐adherent cells were removed by washing, new media was applied and the culture was maintained through to day 7. EPC were identified by the surface markers CD34, KDR, CD31 and Tie2, using flow cytometry.
Proliferation Assay
Seven days after isolation, 2 × 104 EPC were seeded in each six‐well plate for the growth assay. EPC were cultured with Medium 199 containing 5.5 mmol/L glucose (normal glucose conditions) or 20 mmol/L glucose (high glucose conditions) and 2% FBS for 72 h. After the incubation, the numbers of EPC were counted.
DNA synthesis was measured by a Cell Proliferation 5‐bromo‐2‐deoxyuridine (BrdU) ELISA assay (Roche Diagnostics, Mannheim, Germany). Briefly, 1 × 104 cells were plated with Medium 199 containing 5.5 mmol/L glucose (normal glucose conditions), 20 mmol/L glucose (high glucose conditions) or 5.5 mmol/L glucose + 14.5 mmol/L l‐glucose (l‐glu) as an osmotic control and 2% FBS for 72 h in 96‐well plates. In the case of VEGF (human recombinant VEGF; R&D Systems, Minneapolis, MN, USA) stimulation experiments, VEGF was added 24 h before the BrdU assay. PI 3‐kinase inhibitor, LY294002 (2 μmol/L) was pretreated 30 min before VEGF stimulation. The BrdU ELISA was carried out according to the manufacturer’s standard procedure and the absorbance was read at 405 nm on a Wallac 1420 ARVO microplate reader (Aloka, Tokyo, Japan).
Detection of Apoptosis
The numbers of apoptotic EPC induced by the high glucose condition were detected using Hoechst 33342. The adherent EPC were maintained in Medium 199 containing 5.5 mmol/L glucose (normal glucose conditions) or 20 mmol/L glucose (high glucose conditions) and 2% FBS with or without 25 ng/mL of VEGF for 72 h. LY294002 (2 μmol/L) was pretreated before VEGF stimulation. After incubation, cultured EPC were washed with PBS and detached with trypsin‐EDTA. After centrifugation, the cell pellet was suspended at 1–3 × 105 cells/mL in 50 μL of PBS and stained with Hoechst 33342. Apoptotic cells were defined as those with condensed nuclear chromatin. The percentages of apoptotic cells to total live cell counts were calculated from 10 random views.
Western Blot Analysis
The phosphorylation of Akt stimulated by VEGF under normal or high glucose conditions was examined by western blot analysis. Confluently‐grown EPC were starved with Medium 199 containing 0.5% FBS for 24 h, then stimulated with VEGF (25 ng/mL) for the indicated periods. Cells were lysed in 100 μL of lysis buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 10% glycerol, 1% Triton X‐100, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 100 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 50 μg/mL leupeptin, 1 μg/mL pepstatin A and 1 mmol/L Na3VO4). A sample (20 μg) of lysate protein was subjected to SDS‐PAGE under reducing conditions and immunoblotting. The antibodies used for immunoblot analyses were as follows: anti‐phospho‐Akt (Ser473), anti‐Akt (Cell Signaling Technology, Beverly, MA, USA). The primary antibodies were added at a dilution of 1:1000 overnight at 4°C. After washing, the appropriate secondary antibodies were added at a dilution of 1:1000 for 1 h at room temperature. Blots were developed with super signal enhanced chemiluminescence kits and were visualized by chemiluminescence with Image Master.
Quantification of Circulating Endothelial Progenitor Cells
Peripheral blood samples were collected from type 2 diabetics (n = 59) or non‐diabetic control subjects (n = 72) in Nagoya University Hospital and Aichi Gakuin University Dental Hospital. The study protocol and informed consent procedures were approved by the Ethics Committees of Nagoya University Graduate School of Medicine, and School of Dentistry, Aichi Gakuin University. Circulating CD34+CD45low progenitor cells, which are considered as EPC, were counted using flow cytometry analysis as previously described19. Mononuclear cells in peripheral blood were separated with Histopaque 1077 (Sigma‐Aldrich, St. Louis, MO, USA). The buffy coat was washed with PBS containing 10% FBS. Cells were labeled with allophycocyanin (activated protein C)‐conjugated anti‐CD45 monoclonal antibody (Invitrogen) and PC5‐conjugated anti‐CD34 monoclonal antibody (Beckman Coulter, Brea, CA, USA), whereas FITC‐conjugated anti‐mouse F(ab)2 (Invitrogen) was used as a secondary antibody and analyzed by flow cytometry (FACS Calibur; BD Biosciences, Franklin Lakes, NJ, USA). After appropriate gating with low cytoplasmic granularity and with low expression of CD45, the numbers of CD34 positive cells were quantified and expressed as number of cells per 106 total events.
Measurement of Serum Concentration of VEGF
A fasting venous blood sample was collected. After centrifugation at 4°C, the serum fraction was collected. VEGF165 levels were estimated using the ELISA system according to the manufacturer’s instructions (R&D Systems).
Statistical Analyses
Statistical analyses were carried out using spss for Windows version 12.0 (SPSS, Chicago, IL, USA). All the group values were expressed as means ± SE. Significance was defined as a P‐value <0.05.
Results
Identification of EPC
EPC, isolated from the culture of cord blood‐derived mononuclear cells, expanded from the attached cells. More than 90% of the isolated cells were identified by DiI‐acetylated LDL, which is a marker of vascular endothelial cells (data not shown). Flow cytometric analyses showed positive staining with CD34 (88.6%), CD31 (90.5%), KDR (72.7%) and Tie‐2 (77.2%; Figure 1).
Figure 1.
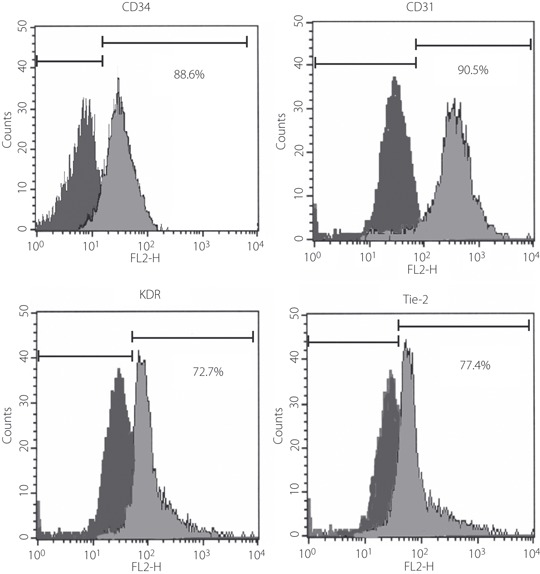
Identification of cultured endothelial progenitor cells (EPC) from cord blood. Flow cytometric analysis showed positive stainings with CD34, CD31, KDR and Tie‐2.
Proliferation and Apoptosis of EPC Under the Normal and High Glucose Conditions
The proliferation of EPC was evaluated by the number of cells and DNA synthesis. EPC were cultured under the normal or high glucose condition for 72 h. The number of EPC was 53% lower under the high glucose condition compared with that under the normal glucose condition (normal glucose, 6.8 × 104 cells/well; high glucose, 3.2 × 104 cells/well, P < 0.05; Figure 2).
Figure 2.
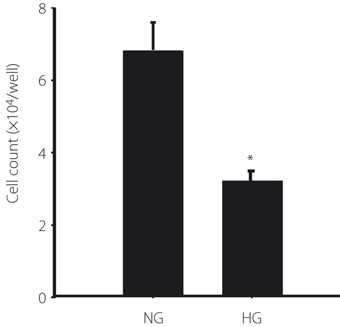
The number of endothelial progenitor cells (EPC) cultured under the normal glucose (NG) or high glucose (HG) condition. After expansion of EPC for 7 days, 2 × 104 cells of EPC were seeded in each six‐well plate and cultured with Medium 199 containing 5.5 mmol/L glucose (NG) or 20 mmol/L glucose (HG) for 72 h. Attached cells on the well were counted. Results are shown as the mean ± SE (n = 3). *P < 0.05 vs normal glucose control.
DNA synthesis of EPC was 38% lower under the high glucose condition compared with that under the normal glucose condition (P < 0.01 vs 5.5 mmol/L glucose; Figure 3). Under the l‐glucose hyperosmotic condition, DNA synthesis was not changed compared with that under the normal glucose condition. VEGF significantly increased DNA synthesis under the normal and l‐glucose hyperosmotic condition (P < 0.05 vs VEGF (−) in each group), which was abolished by PI 3‐kinase inhibitor, LY294002. High glucose suppressed the effect of VEGF on DNA synthesis.
Figure 3.
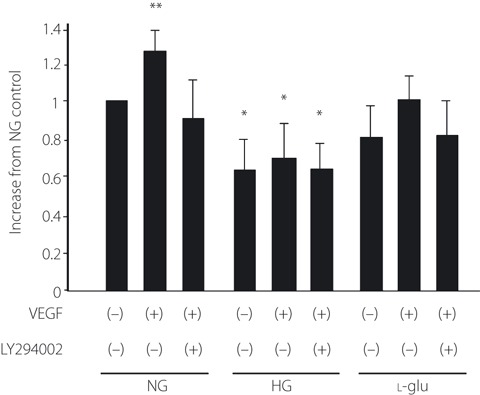
DNA synthesis was measured by a Cell Proliferation BrdU ELISA assay. Cells were incubated with Medium 199 containing 5.5 mmol/L glucose (NG), 20 mmol/L glucose (HG) or 5.5 mmol/L glucose + 14.5 mmol/L l‐glucose as an osmotic control (l‐glu). Vascular endothelial growth factor (VEGF) was added 24 h before the BrdU assay. PI 3‐kinase inhibitor, LY294002, was pretreated 30 min before VEGF stimulation. Results are shown as the mean ± SE (n = 6). *P < 0.01, **P < 0.05 vs normal glucose control.
The apoptotic cells were detected by staining with Hoechist 33342 (Figure 4a). With high glucose, 33.4% of total cells were apoptotic EPC, whereas 7.9% of total cells were apoptotic under the normal glucose condition (Figure 4b). VEGF suppressed the apoptosis of EPC both under the normal and high glucose conditions (normal glucose condition, 32.7% [P < 0.05]; high glucose condition, 58.7% [P < 0.01]). Although VEGF suppressed the high glucose‐induced apoptosis, the percentage of apoptotic cells was still 1.9‐fold higher under the high glucose condition compared with the normal glucose condition (with VEGF under the high glucose condition, 13.7%; with VEGF under normal glucose condition, 7.9%; P < 0.05). In contrast, the inhibitory effect of VEGF on apoptosis was completely eliminated by LY294002 both under the normal and the high glucose conditions.
Figure 4.
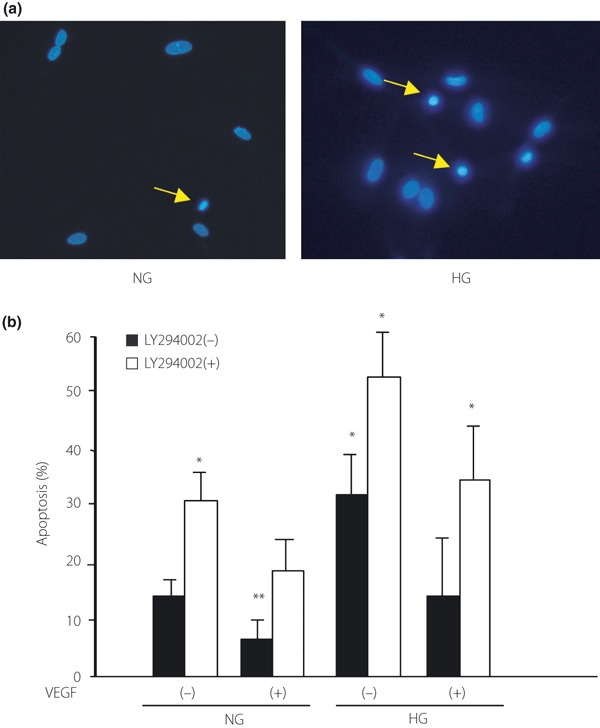
Detection of apoptosis in endothelial progenitor cells (EPC) cultured under the normal or high glucose condition. (a) The apoptotic cells (allows indicate) under 5.5 mmol/L glucose (NG) and 20 mmol/L glucose (HG) conditions were visualized with the staining of the cells with Hoechst 33342. (b) The adherent EPC were maintained in serum starved‐Medium 199 containing NG or HG with or without 25 ng/mL of vascular endothelial growth factor (VEGF) for 72 h. LY294002 was pretreated before VEGF stimulation. Apoptotic cells were detected by staining with Hoechst 33342. Numbers of apoptotic EPC are the mean ± SE percentage of total cells. *P < 0.01, **P < 0.05 vs normal glucose control.
Phosphorylation of Akt in EPC Under the Normal or High Glucose Condition
Because Akt is a key molecule in cell growth and survival, we evaluated the phosphorylation of Akt after 72 h of culture under the normal or high glucose condition. Western blot analysis showed that high glucose significantly decreased the phosphorylation of Akt by 27% compared with that under the normal glucose condition (P < 0.05; Figure 5).
Figure 5.
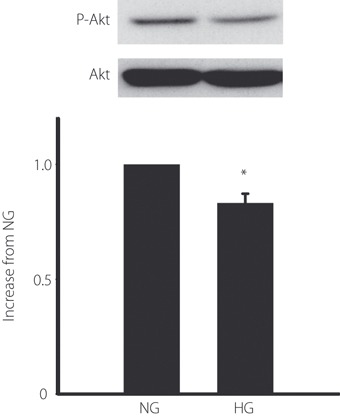
The phosphorylation of Akt under the normal glucose (NG) or high glucose (HG) condition. Confluent‐grown endothelial progenitor cells (EPC) in six‐well multiplates were cultured with Medium 199 containing 5.5 mmol/L glucose (NG) or 20 mmol/L glucose (HG) for 72 h. Phosphorylation of Akt was identified by western blot using anti‐phosphospecific Akt antibody. Results are shown as the mean ± SE (n = 3), *P < 0.05.
VEGF‐Stimulated Akt Signaling Under the High Glucose Condition
To evaluate the intracellular signaling of VEGF, we examined the effect of VEGF on Akt phosphorylation under the normal or high glucose condition. VEGF stimulated the phosphorylation of Akt in a time‐dependent manner, both under the normal and high glucose condition (Figure 6). However, the maximum effect of Akt phosphorylation was significantly lower under the high glucose condition compared with that under the normal glucose condition (normal glucose, 3.1 ± 0.1‐fold; high glucose, 2.0 ± 0.1‐fold, P < 0.05). We also examined the effect of VEGF on Akt phosphorylation under 40 mmol/L high glucose conditions, although this is an over‐physiological condition in vivo. Akt phosphorylation was completely suppressed under the 40 mmol/L high glucose condition (Figure S1).
Figure 6.
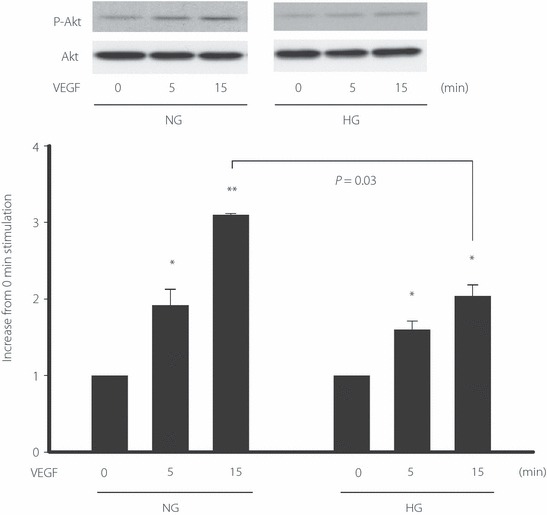
The phosphorylation of Akt stimulated by vascular endothelial growth factor (VEGF) under the normal glucose (NG) or high glucose (HG) condition. Confluently‐grown endothelial progenitor cells (EPC) in six‐well multiplates were cultured with Medium 199 containing 5.5 mmol/L glucose (NG) or 20 mmol/L glucose (HG) for 72 h. After starvation with 0.5% fetal bovine serum for 24 h, EPC were cultured with VEGF (25 ng/mL) for the indicated time. One of three experiments with similar results is shown. *P < 0.05, **P < 0.01 vs 0 min control in each group.
Circulating CD34+CD45low Progenitor Cells in Age‐Matched Non‐Diabetic Subjects and Type 2 Diabetic Patients
Circulating EPC were counted as CD34+CD45low progenitor cells by surface markers using flow cytometry analysis in age‐matched non‐diabetic subjects and type 2 diabetic patients (Figure 7). The clinical characteristics are shown in Table 1. Bodyweight, body mass index, fasting blood glucose and HbA1c were significantly higher in type 2 diabetic patients than in non‐diabetic control subjects. As shown in Figure 8, the number of CD34+CD45low progenitor cells in peripheral blood was significantly decreased in type 2 diabetic patients by 14.2% (P < 0.05). No significant differences in the serum concentrations of VEGF were observed between control subjects and type 2 diabetic patients.
Figure 7.
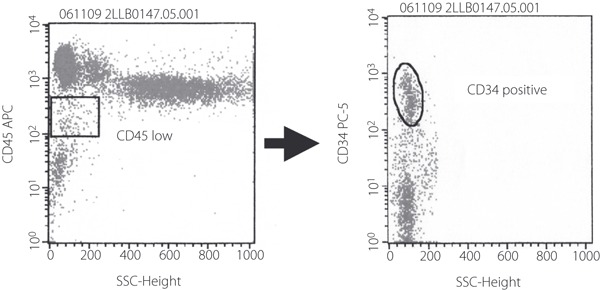
Quantification of endothelial progenitor cells (EPC) by flow cytometry. Circulating EPC were identified by flow cytometry with low cytoplasmic granularity and with the expression of cell surface antigens, such as CD45lowCD34+. Representative flow cytometry analysis is shown.
Table 1. Baseline clinical characteristics of non‐diabetic and type 2 diabetes subjects.
| Non‐diabetic | Type 2 diabetes | P‐value | |
|---|---|---|---|
| Age | 54.5 ± 1.4 | 56.1 ± 1.4 | 0.426 |
| Male/female | 25/29 | 27/21 | 0.441 |
| Bodyweight (kg) | 58.5 ± 1.2 | 66.2 ± 2.1 | 0.002* |
| Body mass index | 22.5 ± 0.3 | 25.2 ± 0.7 | 0.001* |
| Systolic blood pressure (mmHg) | 124.8 ± 2.4 | 132.1 ± 3.0 | 0.063 |
| Diastolic pressure (mmHg) | 75.7 ± 1.4 | 75.7 ± 1.3 | 0.983 |
| Fasting blood glucose (mg/dL) | 91.8 ± 1.5 | 147.7 ± 8.1 | <0.001* |
| HbA1c (%) | 5.4 ± 0.0 | 7.4 ± 0.2 | <0.001* |
| VEGF (pg/mL) | 240.0 ± 31.0 | 229.0 ± 21.3 | 0.787 |
VEGF, vascular endothelial growth factor. *P < 0.05.
Figure 8.
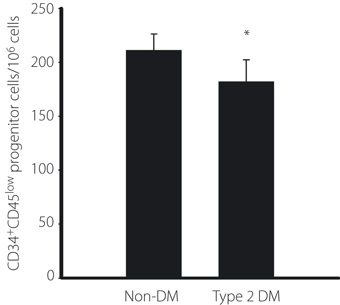
Quantification of circulating CD34+CD45low progenitor cells. CD34+CD45low progenitor cells were counted by surface markers using flow cytometry analysis in age‐matched non‐diabetic subjects (non‐DM) and type 2 diabetic patients (type 2 DM). CD34+CD45low progenitor cells were quantified and expressed as number of cells per 106 total events or number of cells per milliliter of blood. *P < 0.05 vs non‐DM.
Next, we examined the correlation coefficients of clinical data with the number of CD34+CD45low progenitor cells (Table 2). Data were normally distributed and suitable for parametric analysis. Pearson’s correlation coefficients were calculated using the spss statistics package. In the present study, HbA1c and the number of CD34+CD45low progenitor cells were significantly and negatively correlated (r = −0.265, P = 0.004).
Table 2. Correlation of clinical data with the number of CD34+CD45low progenitor cells.
| R | P‐value | |
|---|---|---|
| Bodyweight (kg) | −0.028 | 0.749 |
| Body mass index | 0.009 | 0.918 |
| Fasting blood glucose (mg/dL) | −0.146 | 0.114 |
| HbA1c (%) | −0.265 | 0.004 |
| Insulin (IU/mL) | 0.243 | 0.068 |
Discussion
Clinical trials of transplantation using mononuclear cells from bone marrow or peripheral blood that included EPC have been carried out for the treatment of ischemic diseases20,21. The investigation on EPC functions showed that EPC play an important role not only in vasculogenesis, but also in vascular repair1. In the present study, we showed that high glucose suppressed Akt phosphorylation of EPC, which might result in increased apoptosis and decreased growth in EPC. VEGF‐stimulated Akt phosphorylation was also inhibited under the high glucose condition. The number of EPC was decreased in type 2 diabetic patients, although there were no significant differences in the serum levels of VEGF between control subjects and diabetic patients. These results suggest that high glucose impairs EPC functions through the inhibition of Akt.
There is increasing evidence that a decrease in the number and functional activities of EPC is closely associated with cardiovascular deficits6,7. The decreased number of EPC might predict death from cardiovascular causes22. The number and functions of EPC are also decreased in patients with cardiovascular risk factors including diabetes8,10,11,23. We have shown a negative correlation between HbA1c and the number of EPC in Japanese type 2 diabetic patients, which is consistent with previous studies in type 1 and type 2 diabetic patients of different races10,11. These results suggest that high glucose directly affects the number of EPC.
VEGF prevents the apoptotic death of endothelial cells during angiogenesis or experimental hypoxia22–24. VEGF also mediates the survival of immature vessels through KDR via the PI3‐K/Akt pathway24. Akt, which is located downstream of PI3‐kinase, is an essential regulator of various cellular processes, including glucose metabolism and cell survival25,26. Several studies have shown that VEGF promotes cell growth and inhibits apoptosis through Akt27,28. We have shown the anti‐apoptotic and proliferative effects of VEGF in EPC through Akt. Our observations are consistent with a previous study showing that VEGF gene transfer to EPC increases vasculogenesis in EPC transplantation to ischemic tissues29. High glucose impaired the anti‐apoptotic and proliferative effects of VEGF in EPC. We have shown that human EPC isolated from cord blood have impaired VEGF‐stimulated phosphorylation of Akt and increased apoptosis by high glucose, which are consistent with the results of EPC in diabetic miniswine by another group30. We have not examined other VEGF signaling pathways, such as ERK, nor p38 MAP kinase. Further studies are required to clarify the impacts of each signaling pathways of VEGF in EPC. However, the present results suggest that the suppression of Akt signaling under the high glucose condition might be one of the causes of EPC dysfunction in diabetic patients.
In summary, the present study showed that high glucose impairs Akt signaling in EPC, followed by their decreased proliferation and increased apoptosis. The anti‐apoptotic functions of VEGF in EPC are important for preventing atherosclerosis. However, VEGF‐Akt signaling is also impaired under the high glucose condition. These results suggest that hyperglycemia in diabetic patients might not only reduce the efficacy of transplantation therapy with autologous EPC for ischemic tissues, but also cause vascular dysfunction resulting in the progression of diabetic complications.
Supplementary Material
Figure S1 The phosphorylation of Akt stimulated by vascular endothelial growth factor (VEGF) under 40 mmol/L high glucose condition (HG).
Supporting info item
Acknowledgements
We thank Miss Yuko Maehata and Ms Keiko Shimamoto for excellent technical assistance; the cord blood donors and Dr Osamu Narita, Dr Sentoyo Kato, Dr Chikako Ito and the staff of Narita hospital and Chubu Rosai Hospital (Nagoya, Japan) for help in collecting the cord blood. This research was supported in part by the ‘Strategic Research AGU‐Platform Formation (2008–2012)’ Project for Private Universities: matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology(MEXT) of Japan and in part by a Grant‐in‐Aid for Scientific Research on the Priority Areas ‘System cell engineering by multi‐scale manipulation’ from MEXT. We have no conflict of interest in this work.
References
- 1.Cubbon RM, Kahn MB, Wheatcroft SB. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci (Lond) 2009; 117: 173–190 [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003; 107: 461–468 [DOI] [PubMed] [Google Scholar]
- 3.Murohara T. Therapeutic vasculogenesis using human cord blood‐derived endothelial progenitors. Trends Cardiovasc Med 2001; 11: 303–307 [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G‐CSF‐mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single‐blinded, dose‐escalation clinical trial. Stem Cells 2009; 27: 2857–2864 [DOI] [PubMed] [Google Scholar]
- 5.Mund JA, Ingram DA, Yoder MC, et al. Endothelial progenitor cells and cardiovascular cell‐based therapies. Cytotherapy 2009; 11: 103–113 [DOI] [PubMed] [Google Scholar]
- 6.Hill JM, Zalos G, Halcox JPJ, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600 [DOI] [PubMed] [Google Scholar]
- 7.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001; 89: E1–E7 [DOI] [PubMed] [Google Scholar]
- 8.Fadini GP, Schiavon M, Cantini M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 2006; 24: 1806–1813 [DOI] [PubMed] [Google Scholar]
- 9.Simper D, Wang S, Deb A, et al. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation 2003; 108: 143–149 [DOI] [PubMed] [Google Scholar]
- 10.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004; 53: 195–199 [DOI] [PubMed] [Google Scholar]
- 11.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002; 106: 2781–2786 [DOI] [PubMed] [Google Scholar]
- 12.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG‐CoA reductase inhibitors. Kidney Int 2005; 67: 1672–1676 [DOI] [PubMed] [Google Scholar]
- 13.Tan K, Lessieur E, Cutler A, et al. Impaired function of circulating CD34(+) CD45(−) cells in patients with proliferative diabetic retinopathy. Exp Eye Res 2010; 91: 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999; 13: 9–22 [PubMed] [Google Scholar]
- 15.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85: 221–228 [DOI] [PubMed] [Google Scholar]
- 16.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 2002; 105: 732–738 [DOI] [PubMed] [Google Scholar]
- 17.Ackah E, Yu J, Zoellner S, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF‐mediated angiogenesis. J Clin Invest 2005; 115: 2119–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naruse K, Hamada Y, Nakashima E, et al. Therapeutic neovascularization using cord blood‐derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005; 54: 1823–1828 [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, Hayashi M, Takeshita K, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004; 24: 1442–1447 [DOI] [PubMed] [Google Scholar]
- 20.Tateishi‐Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone‐marrow cells: a pilot study and a randomised controlled trial. Lancet 2002; 360: 427–435 [DOI] [PubMed] [Google Scholar]
- 21.Kajiguchi M, Kondo T, Izawa H, et al. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J 2007; 71: 196–201 [DOI] [PubMed] [Google Scholar]
- 22.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353: 999–1007 [DOI] [PubMed] [Google Scholar]
- 23.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004; 109: 1615–1622 [DOI] [PubMed] [Google Scholar]
- 24.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995; 1: 1024–1028 [DOI] [PubMed] [Google Scholar]
- 25.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′‐kinase/Akt signal transduction pathway. Requirement for Flk‐1/KDR activation. J Biol Chem 1998; 273: 30336–30343 [DOI] [PubMed] [Google Scholar]
- 26.Naruse K, Rask‐Madsen C, Takahara N, et al. Activation of vascular protein kinase C‐beta inhibits Akt‐dependent endothelial nitric oxide synthase function in obesity‐associated insulin resistance. Diabetes 2006; 55: 691–698 [DOI] [PubMed] [Google Scholar]
- 27.Kumar P, Miller AI, Polverini PJ. p38 MAPK mediates gamma‐irradiation‐induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3‐kinase‐Akt‐Bcl‐2 pathway. J Biol Chem 2004; 279: 43352–43360 [DOI] [PubMed] [Google Scholar]
- 28.Park CW, Kim HW, Lim JH, et al. Vascular endothelial growth factor inhibition by dRK6 causes endothelial apoptosis, fibrosis, and inflammation in the heart via the Akt/eNOS axis in db/db mice. Diabetes 2009; 58: 2666–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shintani S, Kusano K, Ii M, et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med 2006; 3(Suppl 1): S123–S128 [DOI] [PubMed] [Google Scholar]
- 30.Mieno S, Boodhwani M, Robich MP, et al. Effects of diabetes mellitus on VEGF‐induced proliferation response in bone marrow derived endothelial progenitor cells. J Card Surg 2010; 25: 618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The phosphorylation of Akt stimulated by vascular endothelial growth factor (VEGF) under 40 mmol/L high glucose condition (HG).
Supporting info item


