Abstract
Aims/Introduction: In order to clarify the enhanced β‐cell dysfunction in type 2 diabetic patients carrying the S20G mutation of the islet amyloid polypeptide gene (S20G‐patients), we first estimated the decline of insulin secretion in Japanese type 2 diabetic patients without the S20G mutation (non‐S20G‐T2D‐patients) by long‐term observation, and then compared it with that of the S20G‐patients.
Materials and Methods: We followed 70 non‐S20G‐T2D‐patients (body mass index <30 kg/m2) for more than 10 years and six S20G‐patients for more than 5 years. We measured fasting C‐peptide (F‐CP) every 1–2 years and carried out a glucagon test at least once during the follow‐up period. F‐CP and a 5‐min value of C‐peptide after glucagon injection (5′‐CP) were used as the indices of insulin secretion. We excluded patients who had renal dysfunction and/or anti‐insulin antibodies in the insulin‐treated patients. The individual annual declines were calculated from the slopes of the regression lines between C‐peptide levels and duration (years after diagnosis).
Results: The mean individual annual declines of both F‐CP and 5′‐CP were significantly greater in the S20G‐patients than the non‐S20G‐T2D‐patients (F‐CP; 0.047 ± 0.026 vs 0.011 ± 0.037 nmol/L/year, P = 0.025, 5′‐CP; 0.139 ± 0.055 vs 0.022 ± 0.012 nmol/L/year, P = 0.008).
Conclusions: We established the annual decline of insulin secretion in the Japanese type 2 diabetic patients by the long‐term observation. The results show that the decline of insulin secretion is more rapid in the S20G‐patients than the non‐S20G‐T2D‐patients. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00102.x, 2011)
Keywords: Islet amyloid polypeptide, Insulin secretion, Type 2 diabetes
Introduction
Type 2 diabetic patients have a considerably high frequency of islet amyloid deposition. Islet amyloid polypeptide (IAPP) is identified as a main constituent of the islet amyloid1,2. This peptide is synthesized and secreted in parallel with insulin by the β‐cell, and is considered to be associated with pathogenesis of human type 2 diabetes as a result of its amyloidogenicity3. However, its pathophysiological role in normal subjects and type 2 diabetic patients is poorly understood4.
We identified the human IAPP cDNA5 and subsequently characterized the human IAPP gene6, and we also found a missense mutation at amino acid 20 (AGCSer to GGCGly), S20G mutation registered as S53G of the precursor protein, in Japanese type 2 diabetic patients7. The nucleotide mutation is registered as rs1800203. The S20G mutation is found only in the Asian population and has a significantly higher frequency (2.6%) than the non‐diabetic population (0.8%)8. Type 2 diabetic patients carrying the S20G mutation appeared to have a relatively early onset and to suffer severe diabetes when there is a strong family history of late‐onset type 2 diabetes8. We reported that the G20‐IAPP variants were more closely related to amyloidogenicity and cytotoxicity than wild‐type IAPP in vitro9. However, there are little clinical data on how impairments of insulin secretion progress in patients carrying the S20G mutation in vivo.
Type 2 diabetes is characterized by the reduction in insulin secretion that results from progressive β‐cell dysfunction and loss of β‐cell mass10,11. However, little is known about the long‐term courses of β‐cell function in type 2 diabetic patients. In the present study, we first evaluated the decline of insulin secretion in Japanese type 2 diabetic patients without the S20G mutation (non‐S20G‐T2D‐patients) over a more than 10‐year observation period, and then compared it with that of type 2 diabetic patients carrying the S20G mutation (S20G‐patients).
Materials and Methods
Subjects
We studied 70 Japanese non‐S20G‐T2D‐patients and six S20G‐patients as a heterozygote. We followed the non‐S20G‐T2D‐patients for more than 10 years and the S20G‐patients for more than 5 years (Table 1). We clinically observed all patients every 1–2 months during the follow‐up period at the outpatient clinic in Wakayama Medical University Hospital. Type 2 diabetes was diagnosed based on the criteria of the Japan Diabetes Association12. We excluded patients who had an endocrine, hepatic or renal disorder (serum creatinine >97.2 μmol/L) and severe obesity (body mass index ≥30 kg/m2). We also excluded patients who had anti‐insulin antibodies among the insulin treated patients. To assess the presence or absence of the S20G mutation of the IAPP gene, genomic DNA of all patients were analyzed by direct DNA sequencing. Informed consent was obtained from all of the patients and procedures were carried out in accordance with the Declaration of Helsinki. The patients were treated in principle by the anti‐diabetic agents as described here. First, during the lifestyle change to decrease weight and increase activity, the patients were treated with metformin 500–750 mg/day. Next, they were additionally treated with sulfonylureas to obtain A1c level <8.4%. The sulfonylureas were initiated by tolbutamide, gliclazide, glimepiride or glibenclamide. They could be switched to glimepiride or glibenclamide and finally up to glimepiride 6 mg/day or glibenclamide 5 mg/day. When they couldn’t obtain A1c level <8.4% after the treatment, they were initiated by insulin. In principle, they were not treated with thiazolidinediones, glinides, α‐glucosidase inhibitors and dipeptidyl peptidase IV inhibitors. A1c was measured by the high‐performance liquid chromatography method and estimated as a National Glycohemoglobin Standardization Program equivalent value (%) calculated by the formula A1c (%) = HbA1c (Japan Diabetes Society; %) + 0.4%.
Table 1. Clinical features of type 2 diabetes patients with or without the S20G mutation of the islet amyloid polypeptide gene at final analysis.
| Non‐S20G‐T2D‐patients (n = 70) | S20G‐patients (individual) | |||||||
|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | Mean | ||
| Sex | Female; 34 | Female | Male | Female | Male | Male | Male | Female; 2 |
| Age at diagnosis (years) | 43.3 ± 11.7 | 49 | 38 | 13 | 30 | 48 | 22 | 33.3 ± 14.3 |
| Duration of diabetes (years) | 23.7 ± 9.1 | 29 | 36 | 27 | 45 | 18 | 22 | 29.7 ± 9.8 |
| BMI (kg/m2) | 23.2 ± 3.7 | 21.4 | 26.0 | 20.2 | 22.3 | 28.7 | 22.3 | 23.5 ± 3.2 |
| Final A1c (%) | 7.7 ± 0.9 | 8.7 | 8.3 | 7.1 | 8.3 | 12.5 | 7.7 | 8.8 ± 1.9 |
| Follow‐up interval (years) | 18.1 ± 5.2 | 25 | 23 | 26 | 21 | 5 | 5 | 17.5 ± 9.8 |
| Duration from diagnosis to the beginning of insulin therapy (years) | (See Figure 2) | 7 | 14 | 9 | 30 | 12 | 17 | 14.8 ± 3.4 |
| Family history of diabetes (+, positive) | Positive; 42 | + | + | + | + | + | + | Positive; 6 |
Non‐S20G‐T2D‐patients, type 2 diabetes patients without the S20G mutation of the islet amyloid polypeptide gene (IAPP); S20G‐patients, type 2 diabetic patients with the S20G mutation of the IAPP gene. BMI, body mass index.
Assessment of Insulin Secretion
The β‐cell function was evaluated by fasting serum C‐peptide (F‐CP) levels and its responsiveness to intravenous glucagon stimulation, that is a 5‐min value (5′‐CP) after 1 mg glucagon injection as a bolus (glucagon test). We measured F‐CP every 1–2 years and carried out a glucagon test at least once during the follow‐up period. F‐CP in the 169 non‐diabetic subjects (fasting blood glucose <6.1 mmol/L and without a family history of diabetes) was also analyzed as a control (69 male; mean age 62.4 ± 2.6 [from 22 to 85] years; body mass index 22.9 ± 2.7 kg/m2). Serum C‐peptide was measured by immunoenzymometric assay (ST E test TOSOH II C‐peptide; TOSOH, Tokyo, Japan). The within‐run and day‐to‐day precisions (coefficients of variation) were 1.3–2.2% and 3.1–3.8%, respectively. The assay sensitivity was 0.017 nmol/L. We estimated the correlation between either F‐CP or 5′‐CP and duration (years after diagnosis) using total points (n = 527 for F‐CP and n = 169 for 5′‐CP) in the non‐S20G‐T2D‐patients who individually had at least five measurements for F‐CP or two measurements for 5′‐CP. For the estimation of annual decline of endogenous insulin secretion, the individual annual declines (IAD; multiplied [−1] by the annual change) of F‐CP and 5′‐CP were calculated from the slopes of the individual regression lines between either F‐CP or 5′‐CP and duration. All the 70 non‐S20G‐T2D‐patients and all the six S20G‐patients, who had at least five measurements, were evaluated for the IAD of F‐CP. We represented the slopes of the regression lines between F‐CP and duration in a representative non‐S20G‐T2D‐patient (Figure 1a) and the six S20G‐patients (Figure 1b–g). The 42 non‐S20G‐T2D‐patients and the four S20G‐patients who had undergone at least two glucagon tests were also evaluated for the IAD of 5′‐CP.
Figure 1.
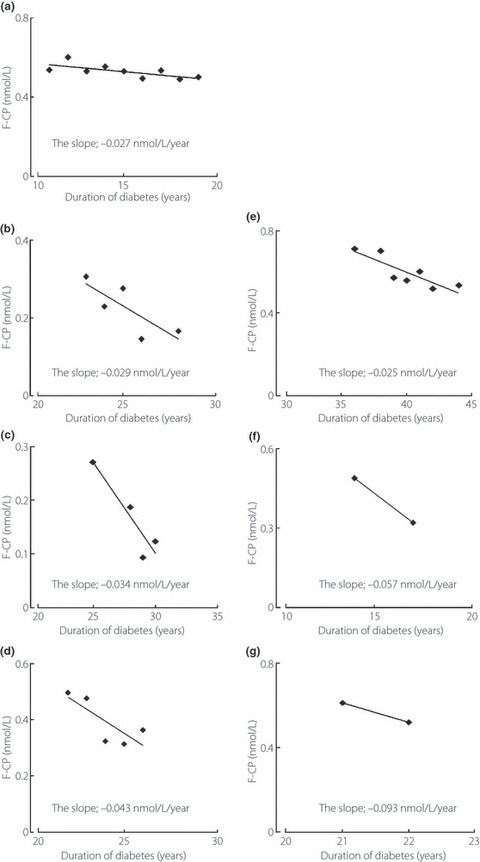
The slopes obtained from the individual regression lines between fasting serum C‐peptide level (F‐CP) and duration in (a) a representative type 2 diabetic patient who lacked the S20G mutation of the islet amyloid polypeptide gene and (a–g) the six type 2 diabetes patients who carried the S20G mutation of the islet amyloid polypeptide gene (the panels from b to g are listed in the patients’ order from 1 to 6).
Statistical Analysis
Data are presented as mean ± SD. Statistical analysis was carried out by a Student’s t‐test between two groups. By using analysis of covariance (ancova), we also adjusted IAD for average A1c level every 3 months during the follow‐up course to compare them between the two groups. The individual regression slope was determined by single linear regression analysis. Multiple regressions were used to estimate the correlation between C‐peptide level and duration of diabetes using total points. We used C‐peptide level as the outcome variable, and duration and the subject as the predictor variables. Subject was treated as a categorical factor using dummy variables. We used the analysis of variance table for the regression. The P‐value from the analysis of variance table was used to determine the probability of the analysis. A P‐value <0.05 was considered as statistically significant.
Results
The clinical data of patients and the shift in the method of the treatment of the 70 non‐S20G‐T2D‐patients at every 5 years are shown in Table 1 and Figure 2, respectively. The prevalence of non‐S20G‐T2D‐patients with insulin therapy gradually increased through the long clinical course.
Figure 2.
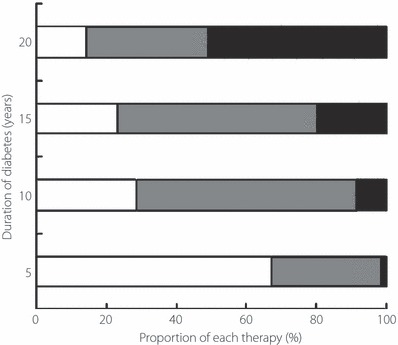
Proportion of each therapy according to duration of diabetes in the type 2 diabetic patients who lacked the S20G mutation of the islet amyloid polypeptide gene. Open bars, gray bars, black bars represent the proportion of the patients (%) in each duration of diabetes who underwent diet therapy, oral hypoglycemic therapy and insulin therapy, respectively.
In the non‐S20G‐T2D‐patients, F‐CP was negatively correlated with duration of diabetes (r = −0.841, P < 0.001), using 527 samples obtained from all of the 70 non‐S20G‐T2D‐patients, who individually had at least five measurements (Figure 3a). 5′‐CP during glucagon tests were also negatively correlated with duration (r = −0.585, P < 0.01), using 154 samples obtained from the 42 non‐S20G‐T2D‐patients who individually had at least two measurements (Figure 3b). F‐CP in the non‐diabetic subjects was 0.558 ± 0.241 nmol/L (Figure 3a, the arrow) and F‐CP was not correlated with age (r = 0.0005, P = 0.996, n = 169).
Figure 3.
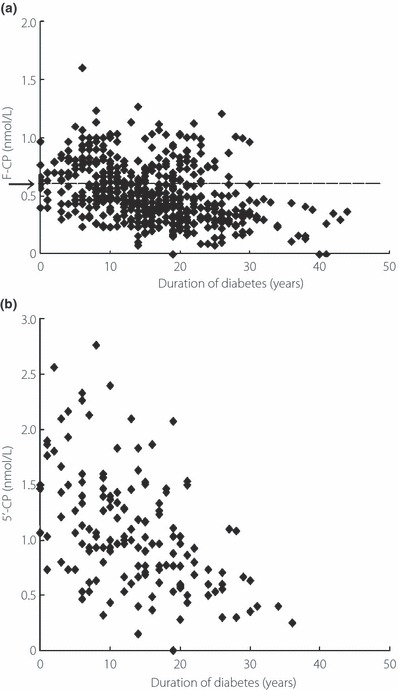
Correlations between (a) fasting serum C‐peptide level (F‐CP) and (b) 5‐min level of serum C‐peptide level after the intravenous injection of 1 mg glucagon (5′‐CP) and duration of diabetes in the type 2 diabetic patients who lacked the S20G mutation of the islet amyloid polypeptide gene. (a) F‐CP in the non‐diabetic subjects are indicated as the arrow (0.558 ± 0.241 nmol/L; mean vales ± SD), and the dashed line indicates the virtual line extended the value horizontally.
The mean IAD of F‐CP in the 70 non‐S20G‐T2D‐patients was calculated as 0.011 ± 0.037 nmol/L/year (Figure 4a). The mean IAD of F‐CP in the six type 2 diabetic patients carrying the S20G mutation was calculated as 0.047 ± 0.026 nmol/L/year, which was significantly greater than that of the 70 non‐S20G‐T2D‐patients (P = 0.025, Figure 4a). The significant difference was preserved, even after adjusted by A1c (P = 0.047). The mean IAD of 5′‐CP in the four patients with the S20G mutation was also significantly greater than that in the 42 non‐S20G‐T2D‐patients (0.138 ± 0.055 nmol/L/year vs 0.022 ± 0.012 nmol/L/year, P = 0.008, Figure 4b).
Figure 4.
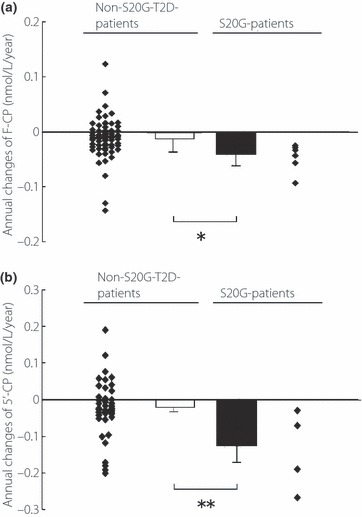
(a) Annual changes of fasting serum C‐peptide level (F‐CP) in the type 2 diabetic patients without (non‐S20G‐T2D‐patients, n = 70) and those with the S20G mutation of the IAPP gene (S20G‐patients, n = 6). (b) Those who had 5‐min level of serum C‐peptide level after the intravenous injection of 1 mg glucagon (5′‐CP) in the 42 non‐S20G‐T2D‐patients and the four S20G‐patients. Individual values are shown as scatter plots and mean vales ± SD as columns, with statistical significance of the difference below columns. *P = 0.025, **P = 0.008 for the difference between the indicated groups. Individual annual decline was multiplied (−1) by the annual change.
Discussion
The present study has shown that endogenous insulin secretion in non‐obese Japanese type 2 diabetic patients gradually deteriorates and that the IAD of F‐CP in the patients without the S20G mutation of the IAPP gene is calculated as 0.011 ± 0.037 nmol/L/year by the longitudinal follow‐up study. There are no reports of such a long‐term follow‐up study of more than 10 years measuring circulating C‐peptide in type 2 diabetic patients. These results make it possible to evaluate the decline of the F‐CP in patients carrying S20G and show that it is four times greater than that in patients who do not carry it.
In type 2 diabetic patients, it is clear that progressive reductions in both β‐cell mass and β‐cell function are present10,13. A longitudinal study that examined the course of islet dysfunction in patients with type 2 diabetes, the United Kingdom Prospective Diabetes Study14, showed that the β‐cell function estimated by the homeostasis model assessment index deteriorated during the first 6 years of observation in the type 2 diabetic patients without insulin therapy. In the present study, we also indirectly showed the reduction of β‐cell function by the gradually increasing prevalence of insulin requiring‐patients throughout the long clinical course (Figure 2). Furthermore, there are two reports of the histological examination of the β‐cell that provide information on the relationship between β‐cell mass and duration15,16. Both are cross‐sectional studies using autopsy samples. In one study using European type 2 diabetic patients (n = 47), the annual decline ratio of the β‐cell mass was estimated as 1.7% per year to initial β‐cell mass or 1.35% to the average β‐cell mass in non‐diabetic subjects by estimation of the simple linear regression line15. The other report concerned β‐cell mass of autopsy samples in lean Japanese type 2 diabetic patients (n = 14) and showed an approximately 2% mass reduction ratio to non‐diabetic control subjects per year16. In the present study, by using the mean IAD of F‐CP, the annual reduction ratio of F‐CP to the mean F‐CP of non‐diabetic subjects was calculated as 1.97% (0.011/0.558). In contrast, the annual reduction ratio of F‐CP to the initial F‐CP (F‐CP crosses to the regression line at duration 0 year) in Japanese type 2 diabetic patients was also reported to be approximately 1% by the cross‐sectional analysis17. Interestingly, through the accumulation of these data, although the number and the design of these studies are limited, it can be shown that the annual decline ratio of F‐CP is similar to that of the β‐cell mass.
We already reported that the G20‐IAPP variant had greater amyloidogenicity and cytotoxicity than wild‐type IAPP in vitro9, but little is known about its behavior in vivo or in humans. In the present study, although the number of the affected patients was limited, we clarified the faster progressive deterioration of insulin secretion in the affected type 2 diabetic patients than in that of the non‐S20G‐T2D‐patients by using F‐CP. Furthermore, glucagon test can be used as an easy assessment for the capacity of residual insulin secretion and it is informative for the choice of insulin therapy used in type 2 diabetic patients18–20. As the results of the glucagon tests, it was confirmed that the decline of endogenous insulin secretion in the S20G‐patients was also greater than that in the non‐S20G‐T2D‐patients, even in the residual state. Gathering together, these data of both F‐CP and 5′‐CP provide new evidence for the stronger effect of the S20G mutation on the β‐cell dysfunction in human diabetes and can support the position that there is a stronger association of cytotoxicity with the G20‐IAPP variant than wild‐type IAPP in vitro. More detailed study concerning the G20‐IAPP variant in vivo or in humans might shed light on the mechanism of IAPP‐mediated β‐cell loss and/or dysfunction in human type 2 diabetes.
Although we have suggested that there is a more rapid deterioration of insulin secretion in the S20G‐patients than the non‐S20G‐T2D‐patients, the result should also be interpreted carefully, because it has been studied in a limited number of patients. Because of the limited number of patients, especially in the S20G‐patients, the clinical features and underlying pathogenesis might be heterogeneous. However, these data depend on the long‐term follow‐up data of each patient, and that at least could partially compensate for the limitation of the small number of patients. It is hoped that there will be a study that analyzes a larger number of patients to elucidate the role of IAPP in mutated and non‐mutated type 2 diabetic patients.
In conclusion, we established the annual decline of endogenous insulin secretion in non‐obese Japanese type 2 diabetic patients by long‐term observation. The results clearly show that the decline of endogenous insulin secretion is more rapid in type 2 diabetic patients with the S20G mutation than those without it. These results are not only clinically useful for the appropriate selection of therapy for type 2 diabetic patients, but also have the pathophysiologically important potential to reveal the mechanisms of β‐cell dysfunction or loss associated with IAPP.
Acknowledgement
No potential conflicts of interest relevant to this article were reported. The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Cooper GJ, Willis AC, Clark A, et al. Purification and characterization of a peptide from amyloid‐rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA 1987; 84: 8628–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermark P, Wernstedt C, Wilander E, et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide‐like protein also present in normal islet cells. Proc Natl Acad Sci USA 1987; 84: 3881–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull RL, Westermark GT, Westermark P, et al. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab 2004; 89: 3629–3643 [DOI] [PubMed] [Google Scholar]
- 4.Zraika S, Hull RL, Verchere CB, et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia 2010; 53: 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanke T, Bell GI, Sample C, et al. An islet amyloid peptide is derived from an 89‐amino acid precursor by proteolytic processing. J Biol Chem 1988; 263: 17243–17246 [PubMed] [Google Scholar]
- 6.Nishi M, Sanke T, Seino S, et al. Human islet amyloid polypeptide gene: complete nucleotide sequence, chromosomal localization, and evolutionary history. Mol Endocrinol 1989; 3: 1775–1781 [DOI] [PubMed] [Google Scholar]
- 7.Sakagashira S, Sanke T, Hanabusa T, et al. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes 1996; 45: 1279–1281 [DOI] [PubMed] [Google Scholar]
- 8.Seino S. S20G mutation of the amylin gene is associated with Type II diabetes in Japanese. Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus. Diabetologia 2001; 44: 906–909 [DOI] [PubMed] [Google Scholar]
- 9.Sakagashira S, Hiddinga HJ, Tateishi K, et al. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild‐type amylin. Am J Pathol 2000; 157: 2101–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn SE, Zraika S, Utzschneider KM, et al. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009; 52: 1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes CJ. Type 2 diabetes‐a matter of beta‐cell life and death? Science 2005; 307: 380–384 [DOI] [PubMed] [Google Scholar]
- 12.Kuzuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002; 55: 65–85 [DOI] [PubMed] [Google Scholar]
- 13.Butler AE, Janson J, Bonner‐Weir S, et al. Beta‐cell deficit and increased beta‐cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102–110 [DOI] [PubMed] [Google Scholar]
- 14.United Kingdom Prospective Diabetes Study Group . U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249–1258 [PubMed] [Google Scholar]
- 15.Rahier J, Guiot Y, Goebbels RM, et al. Pancreatic beta‐cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008; 10(Suppl 4): 32–42 [DOI] [PubMed] [Google Scholar]
- 16.Sakuraba H, Mizukami H, Yagihashi N, et al. Reduced beta‐cell mass and expression of oxidative stress‐related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002; 45: 85–96 [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi S, Fujimoto S, Hamasaki A, et al. Analysis of factors influencing pancreatic beta‐cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract 2008; 82: 353–358 [DOI] [PubMed] [Google Scholar]
- 18.Faber OK, Binder C. C‐peptide response to glucagon. A test for the residual beta‐cell function in diabetes mellitus. Diabetes 1977; 26: 605–610 [DOI] [PubMed] [Google Scholar]
- 19.Madsbad S, Krarup T, McNair P, et al. Practical clinical value of the C‐peptide response to glucagon stimulation in the choice of treatment in diabetes mellitus. Acta Med Scand 1981; 210: 153–156 [DOI] [PubMed] [Google Scholar]
- 20.Scheen AJ, Castillo MJ, Lefebvre PJ. Assessment of residual insulin secretion in diabetic patients using the intravenous glucagon stimulatory test: methodological aspects and clinical applications. Diabetes Metab 1996; 22: 397–406 [PubMed] [Google Scholar]


