Abstract
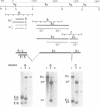
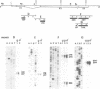
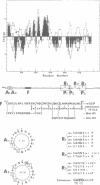
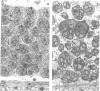
The ref(2)P gene of Drosophila melanogaster is implicated in sigma rhabdovirus multiplication. A permissive allele was cloned and sequenced. The structural gene (3.1 kbp) is divided into three exons. The mRNAs are heterogeneous in size. They differ only in the 5' end of the first exon. The sequence upstream of the short mRNAs contains classical promoter elements. No TATA and CAAT boxes are appropriately positioned upstream of the initiation sites of the long mRNAs, but several repeats, palindromic sequences and inverted CAAT boxes are present. These observations, together with the tissue-dependent distribution of short and long transcripts, support the hypothesis of the existence of at least two classes of genuine initiation sites. The long size of the untranslated leader RNA region suggests a control of gene expression at the translation level. The same translation product of 599 amino acids (76.3 kd) is predicted for all mRNAs, but the in vitro translation product migrates in SDS-PAGE with a higher apparent mol. wt (115-125 kd). The putative ref(2)P protein contains internal repeats, PEST regions which may be signals for protein degradation, and interesting structural motifs such as zinc finger and amphiphilic helices. These later motifs could be mitochondrial pre-sequences. The degeneration of mitochondria is observed in the spermatids of sterile male flies homozygous for the loss-of-function alleles. The amino acid sequence of the ref(2)P product shows no homology with any known protein from the data banks.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. A. Cytodifferentiation of spermatozoa in Drosophila melanogaster: the effect of elevated temperature on spermiogenesis. Mol Gen Genet. 1967;99(3):257–273. doi: 10.1007/BF01797731. [DOI] [PubMed] [Google Scholar]
- Baker A., Schatz G. Sequences from a prokaryotic genome or the mouse dihydrofolate reductase gene can restore the import of a truncated precursor protein into yeast mitochondria. Proc Natl Acad Sci U S A. 1987 May;84(10):3117–3121. doi: 10.1073/pnas.84.10.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Bussereau F., de Kinkelin P., Le Berre M. Infectivity of fish rhabdoviruses for Drosophila melanogaster. Ann Microbiol (Paris) 1975 Apr;126(3):389–395. [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Bricault L. PseqIP: a nonredundant and exhaustive protein sequence data bank generated from 4 major existing collections. Proteins. 1986 Sep;1(1):60–65. doi: 10.1002/prot.340010110. [DOI] [PubMed] [Google Scholar]
- Dente L., Ciliberto G., Cortese R. Structure of the human alpha 1-acid glycoprotein gene: sequence homology with other human acute phase protein genes. Nucleic Acids Res. 1985 Jun 11;13(11):3941–3952. doi: 10.1093/nar/13.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. Reduction of the minimal sequence for initiation of DNA synthesis by qualitative or quantitative changes of an initiator protein. Nature. 1984 Sep 20;311(5983):279–280. doi: 10.1038/311279a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Gay P. Les gènes de la Drosophile qui interviennent dans la multiplication du virus sigma. Mol Gen Genet. 1978 Feb 27;159(3):269–283. doi: 10.1007/BF00268263. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Habliston D. L., Stanley H. P., Bowman J. T. Genetic control of spermiogenesis in Drosophila melanogaster: the effects of abnormal association of centrosome and nucleus in mutant ms(1)6S. J Ultrastruct Res. 1977 Aug;60(2):221–234. doi: 10.1016/s0022-5320(77)80067-1. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Eisenman R. N. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984 Nov;4(11):2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckaby C. S., Conneely O. M., Beattie W. G., Dobson A. D., Tsai M. J., O'Malley B. W. Structure of the chromosomal chicken progesterone receptor gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8380–8384. doi: 10.1073/pnas.84.23.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Gehring W. J. Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell. 1987 Feb 13;48(3):465–478. doi: 10.1016/0092-8674(87)90197-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Gay P., Contamine D. Etude du locus ref(2)P de Drosophila melanogaster. I. Localisation cytogénétique de ref(2)P. Biol Cell. 1986;56(3):227–237. doi: 10.1111/j.1768-322x.1986.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Nakamura N. Influence du dosage de l'allèle non permissif du gène ref(2)P de la Drosophile sur les souches sensibles du virus sigma. Mol Gen Genet. 1978 Feb 27;159(3):285–292. doi: 10.1007/BF00268264. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Persson H., Hennighausen L., Taub R., DeGrado W., Leder P. Antibodies to human c-myc oncogene product: evidence of an evolutionarily conserved protein induced during cell proliferation. Science. 1984 Aug 17;225(4663):687–693. doi: 10.1126/science.6431612. [DOI] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Reardon J. T., Liljestrand-Golden C. A., Dusenbery R. L., Smith P. D. Molecular Analysis of Diepoxybutane-Induced Mutations at the rosy Locus of Drosophila melanogaster. Genetics. 1987 Feb;115(2):323–331. doi: 10.1093/genetics/115.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Stanley H. P., Bowman J. T. Genetic control of spermiogenesis in Drosophila melanogaster: an autosomal mutant (ms(2)3R) demonstrating failure of meiotic cytokinesis. J Ultrastruct Res. 1972 Mar;38(5):563–577. doi: 10.1016/0022-5320(72)90090-1. [DOI] [PubMed] [Google Scholar]
- Rosen L. Carbon dioxide sensitivity in mosquitoes infected with sigma, vesicular stomatitis, and other rhabdoviruses. Science. 1980 Feb 29;207(4434):989–991. doi: 10.1126/science.6101512. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Baumgartner P., Gehring W. J. Structural organization and sequence of the homeotic gene Antennapedia of Drosophila melanogaster. EMBO J. 1986 Apr;5(4):733–739. doi: 10.1002/j.1460-2075.1986.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segretain D., Roussel C. Endocytic origin for periaxonemal vesicles along the flagellum during mouse spermiogenesis. Gamete Res. 1988 Dec;21(4):451–463. doi: 10.1002/mrd.1120210412. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Stanley H. P., Bowman J. T., Romrell L. J., Reed S. C., Wilkinson R. F. Fine structure of normal spermatid differentiation in Drosophila melanogaster. J Ultrastruct Res. 1972 Dec;41(5):433–466. doi: 10.1016/s0022-5320(72)90049-4. [DOI] [PubMed] [Google Scholar]
- Teninges D., Bras-Herreng F. Rhabdovirus sigma, the hereditary CO2 sensitivity agent of Drosophila: nucleotide sequence of a cDNA clone encoding the glycoprotein. J Gen Virol. 1987 Oct;68(Pt 10):2625–2638. doi: 10.1099/0022-1317-68-10-2625. [DOI] [PubMed] [Google Scholar]
- Verma I. M. From c-fos to v-fos. Nature. 1984 Mar 22;308(5957):317–317. doi: 10.1038/308317a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. F., Stanley H. P., Bowman J. The effect of vinblastine on spermiogenesis in Drosophila melanogaster: evidence for two functional classes of cytoplasmic microtubules. J Ultrastruct Res. 1975 Dec;53(3):354–365. doi: 10.1016/s0022-5320(75)80036-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]