Abstract
Aims/Introduction: In the present study, whether near‐future glycated hemoglobin (A1C) levels could be predicted by changes in glycated albumin (GA) levels before and after treatment for diabetes was investigated.
Materials and Methods: After starting diabetes treatment, GA and A1C levels are assumed to change exponentially. From this assumption, the equation for predicting near‐future GA and A1C levels was derived. A total of 54 patients with type 2 diabetes mellitus in whom diabetes treatment was initiated or altered were enrolled. By incorporating GA and A1C values before and 2–4 weeks after starting treatment (second visit) into the equation, the predicted GA and A1C levels at the third visit (5–7 weeks after treatment) were obtained.
Results: A strong and positive correlation was observed between predicted GA and measured GA at the third visit (R = 0.669, P < 0.0001). Similarly, a strong and positive correlation was observed between the predicted A1C and the measured A1C at the third visit (R = 0.795, P < 0.0001).
Conclusions: GA and A1C levels 1–3 months after starting diabetes treatment could be predicted using the equation developed. The prediction of near‐future A1C levels using GA levels at two points would be useful for judging the effectiveness of ongoing diabetes treatment at an earlier stage. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00107.x, 2011)
Keywords: Glycated albumin, Glycated hemoglobin, Glycemic control
Introduction
Glycation of various proteins increases in diabetic patients compared with non‐diabetic subjects, and some of these glycated proteins might take part in the onset and progression of chronic diabetic complications1. Of the glycated proteins, glycated hemoglobin (A1C) is used clinically as an indicator for chronic control of plasma glucose levels2,3. It is recommended that A1C should be less than 7.0%, based on the result of the Diabetes Control and Complications Trial (DCCT), in order to prevent the onset and progression of chronic diabetic complications4. As the lifespan of erythrocytes is approximately 120 days, A1C reflects plasma glucose levels for the past 2–3 months. Therefore, approximately 2–3 months are needed for judging whether or not A1C reaches target‐to‐treat levels after starting treatment for diabetes. A1C does not acutely reflect glycemic control state at early‐phase starting treatment for diabetes, because A1C changes slowly. Therefore, according to the American Diabetes Association (ADA) guideline, the therapeutic effect should be judged using A1C 3 months after initiating treatment for diabetes5.
Glycated albumin (GA) is also used as a glycemic control indicator6. As the half‐life of serum albumin is approximately 14 days, GA reflects shorter‐term (approximately 2 weeks) control of plasma glucose levels7. GA is assumed to change approximately three times larger compared with A1C, because albumin is more sensitively glycated than hemoglobin8. Thus, GA is thought to be a useful glycemic control index for the early period after starting treatment for diabetes9. GA has also been shown to be a suitable indicator in diabetic patients on insulin therapy who have large glycemic fluctuations10,11, and in patients with a shortened lifespan of erythrocytes (e.g. anemia and hemodialysis)12,13.
When glycemic control state changes, A1C and GA levels change in accordance with the half‐lives of erythrocytes and serum albumin, respectively, and thus it is possible to predict near‐future A1C and GA values based on A1C and GA levels before and after the start of treatment for diabetes. In addition, because the amount of the decrease in A1C and GA levels differs depending on their half‐lives, comparison of the half‐lives should enable mathematical predictions of the changes in A1C from those in GA. In the present study, we aimed to clarify whether it is possible to predict subsequent A1C values using GA levels at two points – before and after the start of treatment – in patients with type 2 diabetes mellitus. It will be beneficial in clinical practice if future A1C values after the start of treatment for diabetes can be predicted at an earlier stage.
Materials and Methods
Models and Theoretical Analysis
Calculation of Predicted GA Value
Shi et al.14, assuming that glycated protein synthesis occurs irreversibly, showed that, when plasma glucose levels are improved by treatment for diabetes, GA levels change exponentially and the estimated value using that function is nearly the same as the measured value. Using this function, Tahara and Shima7 reported that changes in GA levels in diabetic patients and theoretically estimated changes in response to ramp and stepwise changes in plasma glucose levels corresponded well. Using this relationship, we attempted to predict the near‐future GA levels based on measuring GA at two points, before (first visit) and after (second visit) the beginning of treatment. Taking GA levels at t0 days before the start of treatment, those at t days after the start of treatment, and when sufficient time passes after the start of treatment (target‐to‐treat levels) to be GA(t0), GA(t) and GA(t∞), respectively, the rate of decrease in the GA value after the start of treatment is shown as [GA(t) − GA(t∞)]/[GA(t0) − GA(t∞)]. Assuming that the decreasing rate of GA changes exponentially with respect to time t, the relationship in Eqn 1 is obtained.
| (1) |
where a and b are constants.
Since 0 = b when t = 0 in Eqn 1, b = 0.
When plasma glucose changes in a stepwise fashion, since the number of days in which the rate of decrease in GA becomes one‐half, is taken into be t(GA1/2), then a = −log2/t(GA1/2) since log 1/2 = a × t(GA1/2).
Taking the proportion between plasma glucose changes in a stepwise fashion and when the change in plasma glucose levels during treatment for diabetes occurs rapidly as k, Eqn 2 is obtained from Eqn 1.
| (2) |
Solving this equation for GA(t), Eqn 3 is obtained.
| (3) |
Calculating t(GA1/2) and t(A1C1/2) When Plasma Glucose Changes Rapidly
Similarly for A1C, the half‐life is defined as t(A1C1/2), and when plasma glucose changes dramatically and A1C is assumed to change similarly to GA, t(GA1/2) and t(A1C1/2) can be calculated using the respective equations below.
| (4) |
| (5) |
Recently, Takahashi et al.9 observed the longitudinal changes in GA and A1C with intensive conventional insulin therapy in diabetic patients with poor glycemic control, but when the half‐lives were calculated using their data, t(GA1/2) and t(A1C1/2) were 17.0 and 44.0 days, respectively. GA(t∞) = 15.0% and A1C(t∞) = 5.4% were used for the GA and A1C values that were the target to treat values for decrease with treatment. These values were set for the following reasons: the median of the reference values of A1C (4.7–6.2%) was 5.4% (5.0% in terms of Japan Diabetes Society [JDS] value) and GA is known to be approximately three times A1C (JDS). From this result, the ratio of the half‐lives of A1C and GA [t(A1C1/2)/t(GA1/2)] was 44.0/17.0 = 2.6.
Calculation of Predicted A1C and GA Levels
Next, a formula to predict the A1C levels using the GA levels was developed using the aforementioned ratio of t(GA1/2) and t(A1C1/2). Here, k during actual treatment can be shown as in Eqn 5 using the GA value at t0 before the start of treatment and t1 after the start of treatment using Eqn 4.
| (6) |
Accordingly, GA levels on day t2 after the start of treatment (third visit) are shown as in Eqn 7 using Eqns 3 and 6.
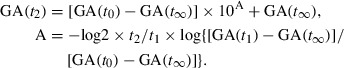 |
(7) |
Similarly, A1C can be shown in Eqns 8 and 9.
| (8) |
[Correction to Eqn (8) first line, after online publication 29 July 2011: “10A” is changed to “10B”.]
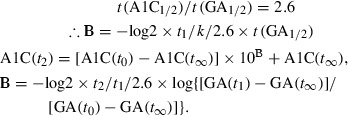 |
(9) |
Patients
The present study included patients with type 2 diabetes mellitus whose A1C levels were 8.4% or more (Table 1). Patients with liver disease, kidney disease or anemia were excluded. The treatment for diabetes was initiated in 46 patients who had not been treated, whereas the treatments were altered for the other eight patients who had been already been given some diabetes treatments. Before (first visit) and 14–28 days (second visit) and 35–49 days (third visit) after the start or the alteration of diabetes treatment, GA and A1C were measured.
Table 1. Clinical characteristics of the study patients.
| n | 54 |
| Male/female | 33/21 |
| Age (years) | 60.5 ± 14.2 |
| Inpatients/outpatients | 34/20 |
| A1C (%) | 11.3 ± 2.1 |
| GA (%) | 34.0 ± 9.1 |
| Treatment at first visit | No treatment, 46; Oral hypoglycemic agents, 7; Insulin, 1 |
| First visit‐second visit interval (days) | 19.8 ± 5.7 |
| Second visit‐third visit interval (days) | 27.1 ± 5 |
Data are means ± SD or number. A1C, glycated hemoglobin; GA, glycated albumin.
The study patients were divided into four groups based on diabetes treatments during the course of study (Table 2): group 1, starting diet therapy alone (n = 5); group 2, starting oral hypoglycemic agents (OHA) or increasing the dose of OHA (n = 17); group 3, starting insulin or increasing the dose of insulin (n = 17); and group 4, starting insulin followed by OHA alone (n = 15). Sex and age of the patients for each group are shown in Table 2.
Table 2. Predicted and measured glycated hemoglobin and glycated albumin levels at the first and the third visits in groups divided according to diabetes treatments.
| Diabetes treatment | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Diet | OHA | Insulin | Insulin followed by OHA | |
| n (Male/female) | 5 (3/2) | 17 (5/12) | 17 (8/9) | 15 (12/3) |
| Age (years) | 58.0 ± 15.2 | 64.4 ± 8.7 | 55.8 ± 18.5 | 62.1 ± 12.8 |
| Measured A1C at first visit (%) | 9.8 ± 1.9 | 10.7 ± 1.7 | 11.9 ± 2.0 | 11.8 ± 2.5 |
| Measured GA at first visit (%) | 26.2 ± 4.6 | 30.3 ± 7.1 | 36.6 ± 8.4 | 37.6 ± 10.5 |
| Measured A1C at third visit (%) | 7.9 ± 0.9 | 8.7 ± 0.9 | 8.8 ± 1.0 | 8.4 ± 1.3 |
| Measured GA at third visit (%) | 18.6 ± 1.4 | 20.3 ± 2.4 | 19.6 ± 3.2 | 18.7 ± 3.3 |
| Predicted A1C at third visit (%) | 8.1 ± 1.4 | 8.8 ± 1.1 | 8.6 ± 1.1 | 8.3 ± 1.5 |
| Predicted GA at third visit (%) | 18.2 ± 2.6 | 20.0 ± 2.8 | 18.7 ± 2.3 | 17.9 ± 2.7 |
| Measured/predicted A1C (%)† | 98 ± 8 | 98 ± 8 | 103 ± 10 | 102 ± 9 |
| Measured/predicted GA (%)† | 100 ± 10 | 101 ± 8 | 105 ± 16 | 104 ± 11 |
Data are means ± SD. †Values at the third visit. A1C, glycated hemoglobin; GA, glycated albumin; OHA, oral hypoglycemic agent.
Predicted GA and A1C levels at the third visit were calculated using the aforementioned equations from the respective changes in GA and A1C levels before the start of treatment and 2–4 weeks after the start of treatment (second visit), and the correlations between these predictions and the measured values were investigated. GA(t∞) = 15.0% and A1C(t∞) = 5.4% were used for the target to treat levels.
The institutional committee approved the protocol of the present study, and all participants gave their written informed consent.
Laboratory Methods
Plasma glucose was determined by the glucose oxidase method. A1C was measured with ADAMS‐A1c HA‐8160 (Arkray, Kyoto, Japan) by HPLC with calibration using Japan Diabetes Society lot 315. The value for A1C (%) is estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula A1C (%) = A1C (JDS) (%) + 0.4%, considering the relational expression of A1C (JDS) (%) measured by the previous Japanese standard substance and measurement methods, and A1C (NGSP)16. Serum GA was determined by Hitachi 7600 autoanalyzer (Hitachi Instruments Service, Tokyo, Japan) by the enzymatic method using albumin‐specific proteinase, ketoamine oxidase and albumin assay reagent (Lucica GA‐L; Asahi Kasei Pharma Co., Tokyo, Japan)17,18.
Statistical Analysis
All data are shown as means ± SD. To evaluate relationships between predicted and measured values, single linear univariate regression analyses were carried out. StatView for Windows version 5.0 software (Abacus Concepts, Berkeley, CA, USA) was used for all statistical analyses. Values of P < 0.05 were considered statistically significant.
Results
The A1C and GA levels before the beginning of the treatment (first visit) were 11.3 ± 2.1% and 34.0 ± 9.1% in the study patients, and decreased to 8.6 ± 1.2 and 19.4 ±2.9% at the third visit, respectively (ΔA1C; −2.7 ± 1.5%, ΔGA; −15.6 ± 8.3%; the mean time after beginning treatment was 46.9 days). The predicted GA levels at the third visit calculated by Eqn 7 from GA levels at the first and the second visits (the mean time after beginning treatment was 19.8 days) was 18.9 ± 2.6%. Thus, the predicted GA and the measured GA showed similar levels. A strong and positive correlation (R = 0.669. P < 0.0001) was observed between the predicted GA and the measured GA levels (Figure 1a). Furthermore, the measured A1C levels at the third visit were 8.6 ± 1.1%, and corresponded well with the predicted A1C levels (8.5 ± 1.2%). A strong and positive correlation (R = 0.795. P < 0.0001) was observed between the predicted A1C and the measured A1C levels (Figure 1b). Table 2 shows measured A1C and GA levels at the first and the third visits, as well as their predicted levels at the third visit, in groups divided according to diabetes treatments. In every patient group, both the ratio of GA to the predicted GA and the ratio of A1C to the predicted A1C were approximately 100%, showing that the predicted A1C and GA levels were similar to their measured levels (Table 2).
Figure 1.
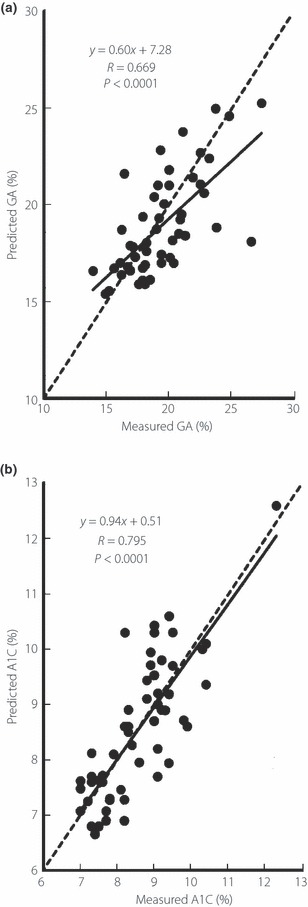
Correlations between (a) predicted and measured glycated albumin (GA), and (b) predicted and measured glycated hemoglobin (A1C) at the third visit. Using the equations with GA and A1C before the start of treatment (first visit), and GA and A1C at the time of the second outpatient visit (second visit), the predicted GA and A1C values at the third visit were calculated, and their correlations with the measured GA and A1C values were investigated. The broken line corresponds to y = x.
A typical case of predicted GA and A1C levels at 14 weeks after diabetes treatment is shown in Figure 2. The patient was a 77‐year‐old man who had not obtained sufficient glycemic control with sulfonylurea (glimepiride 4 mg/day) and he started to be given insulin treatment (biphasic insulin aspart 30/70; final dose 24 U/day). After inpatient treatment for 2 weeks, the treatment was continued on an outpatient basis and a state of good glycemic control was obtained. In this case, both GA and A1C levels predicted by Eqns 7 and 9 using the GA value 15 days after the start of insulin treatment were very close to the measured values 4–14 weeks after the treatment.
Figure 2.
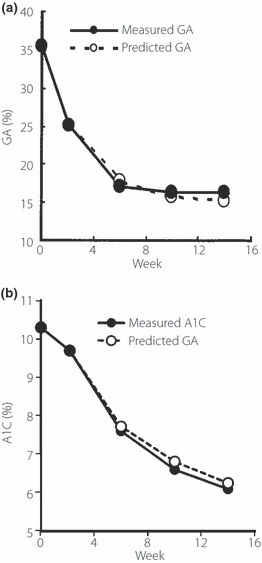
Relationships between predicted and measured (a) glycated albumin (GA) and (b) glycated hemoglobin (A1C) values (a patient in whom glycemic control worsened during sulfonylurea treatment and insulin was started in the hospital).
Discussion
We attempted to predict near‐future GA and A1C levels using GA levels before and after the beginning of diabetes treatment. The predicted GA and A1C levels calculated by the equation developed and the measured values showed excellent positive correlations. Therefore, it was shown that GA and A1C levels in the near future could be predicted using GA levels before and after the beginning of treatment.
There have been numerous studies on the changes in A1C and GA after glycemic control has improved7,14,19–24, but there have been no attempts to accurately predict A1C based on changes in GA after the start of treatment. In the present study, based on the report of Shi et al. that when A1C or GA changes, the change occurs with an exponential function14, we devised an understanding of various changes as the rate of change, with the initial value taken as 1 and the treatment target value as 0. We then made modifications so that various cases could be handled with the same formula. In addition, A1C levels could be predicted from the changes of GA levels at an earlier stage, because the exponential changes of A1C and GA are dependent on their half‐lives. The prediction of near‐future A1C by GA levels before and at an early stage after starting diabetes treatment in the present study is the first trial.
Using this formula, near‐future GA and A1C levels can be predicted. As a result, the efficacy of treatment can be understood early, and treatment plans can be developed at an early stage. In particular, it will be possible to change or add treatments at an early stage for patients in whom the treatment effect is insufficient. A1C shows the mean glycemic control status in the preceding 2–3 months, but using the prediction formula developed in the present study, the treatment effect after 2–4 weeks can make a prediction of A1C levels 1–2 months later. The prediction of A1C levels is suggested to be useful, especially when lowering plasma glucose levels quickly is needed, preoperative diabetes treatment is needed or outpatient insulin treatment is started.
The premise of the present study was that, with predictions of near‐future GA and A1C levels, the same glycemic control condition could be maintained after treatment. When glycemic control status worsens in cases, for example, when diet therapy cannot be maintained, the patient decides to stop taking diabetes treatment drugs or complications with infection occur, the measured levels will obviously become higher than the predicted levels. In other words, when there is divergence between the measured and predicted levels, it can be assumed that a situation affecting glycemic control status, such as those aforementioned, exists. In addition, when gastrointestinal tract bleeding or other problems occur, the predicted A1C levels will diverge higher from the measured levels.
Considering all of the aforementioned, the formula for predicted A1C and GA levels using GA levels before and after the beginning of treatment is thought to be highly effective for determining the treatment effect at an early stage in clinical practice. The present study comprised patients with type 2 diabetes mellitus whose glycemic control were improved after initiating diabetes treatment. Thus, it should be investigated in future whether this formula is also applicable for patients without improvement of glycemic control.
Recently, the value of self‐monitored blood glucose (SMBG) is increasingly being emphasized according to the widespread usage of various insulin regimens. The evaluation of glycemic control by SMBG, instead of A1C and GA, might be widely distributed in future.
When t is made infinite (∞) in the prediction formula proposed in the present study, A1C and GA converge to 5.4 and 15.0%, respectively. Therefore, the error becomes larger for estimates for a time fairly far in the future, and might result in divergence from the measured value. High accuracy can be obtained for the predicted GA and A1C levels until approximately 3–4 months after the start of treatment. In addition, the extent of maximal decrease of A1C differs among diabetes drugs used. Therefore, as it is thought that A1C(∞) and GA(∞) differ with each drug, in future studies it will be necessary to establish A1C(∞) and GA(∞) for each drug and develop individual prediction equations.
Acknowledgement
None of the authors have conflicts of interest to declare.
References
- 1.Cohen MP. Nonenzymatic glycation: a central mechanism in diabetic microvasculopathy? J Diabet Complications 1998; 2: 214–217 [DOI] [PubMed] [Google Scholar]
- 2.Koenig RJ, Peterson CM, Jones RL, et al. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med 1976; 295: 417–420 [DOI] [PubMed] [Google Scholar]
- 3.Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science 1978; 20: 21–27 [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol 2008; 2: 1114–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995; 18: 440–447 [DOI] [PubMed] [Google Scholar]
- 8.Iberg N, Fluckiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem 1986; 261: 13542–13545 [PubMed] [Google Scholar]
- 9.Takahashi S, Uchino H, Shimizu T, et al. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short‐term changes in glycemic control. Endocr J 2007; 54: 139–144 [DOI] [PubMed] [Google Scholar]
- 10.Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008; 55: 503–507 [DOI] [PubMed] [Google Scholar]
- 11.Koga M, Murai J, Saito H, et al. Glycated albumin and glycated hemoglobin are differently influenced by endogenous insulin secretion in patients with type 2 diabetes mellitus. Diabetes Care 2010; 33: 270–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panzer S, Kronik G, Lechner K, et al. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood 1982; 59: 1348–1350 [PubMed] [Google Scholar]
- 13.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18: 896–903 [DOI] [PubMed] [Google Scholar]
- 14.Shi K, Tahara Y, Noma Y, et al. The response of glycated albumin to blood glucose change in the circulation in streptozotocin‐diabetic rats – comparison of theoretical values with experimental data. Diabetes Res Clin Pract 1992; 17: 153–160 [DOI] [PubMed] [Google Scholar]
- 15.Schnedl WJ, Lahousen T, Wallner SJ, et al. Silent hemoglobin variants and determination of HbA1c with the high‐resolution program of the HPLC HA‐8160 hemoglobin analyzer. Clin Biochem 2005; 38: 88–91 [DOI] [PubMed] [Google Scholar]
- 16.The Committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzuma T, Usami T, Yamakoshi M, et al. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 2002; 324: 61–71 [DOI] [PubMed] [Google Scholar]
- 18.Kouzuma T, Uemastu Y, Usami T, et al. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta 2004; 346: 135–143 [DOI] [PubMed] [Google Scholar]
- 19.Bunn HF, Handy DH, Kamin S, et al. The biosynthesis of human hemoglobin A1c. Slow glycisylation of hemoglobin in vivo. J Clin Invest 1976; 57: 1652–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day JF, Thornburg RW, Thorpe SR, et al. Nonenzymatic glucosylation of rat albumin. Studies in vitro and in vivo. J Biochem 1979; 254: 9394–9400 [PubMed] [Google Scholar]
- 21.Higgins PJ, Bunn HF. Kinetic analysis of the nonenzymatic glycosylation of hemoglobin. J Biol Chem 1979; 256: 5204–5208 [PubMed] [Google Scholar]
- 22.Day JF, Ingebretsen CG, Ingebretsen WR, et al. Nonenzymatic glycosylation of serum proteins and hemoglobin: response to changes in blood glucose levels in diabetic rats. Diabetes 1980; 29: 524–527 [DOI] [PubMed] [Google Scholar]
- 23.Beach KW. A theoretical model to predict the behaviour of glycosylated haemoglobin levels. J Theor Biol 1979; 81: 547–561 [DOI] [PubMed] [Google Scholar]
- 24.Mortensen HB, Volund A, Christophersen C. Glycosylation of human haemoglobin A. Dynamic variation in A1C described by a model. Clin Chim Acta 1982; 136: 75–81 [DOI] [PubMed] [Google Scholar]


