Abstract
Exacerbations of COPD (ECOPD) represent a major burden for patients and health care systems. Innovative sampling techniques have led to the identification of several pulmonary biomarkers. Although some molecules are promising, their usefulness in clinical practice is not yet established. Medline and Highwire databases were used to identify studies evaluating pulmonary sampled biomarkers in ECOPD. We combined 3 terms for ECOPD, 3 for biomarkers and 6 for the sampling method. Seventy-nine studies were considered eligible for inclusion in the review and were analyzed further. Pulmonary biomarkers sampled with non-invasive, semi-invasive and invasive methods were evaluated for their potential to illustrate the disease’s clinical course, to correlate to clinical variables and to predict clinical outcomes, ECOPD etiology and response to treatment. According to published data several pulmonary biomarkers assessed in ECOPD have the potential to illustrate the natural history of disease through the modification of their levels. Among the clinically relevant molecules, those that have been studied the most and appear to be promising are spontaneous and induced sputum biomarkers for reflecting clinical severity and symptomatic recovery, as well as for directing towards an etiological diagnosis. Current evidence on the clinical usefulness of exhaled breath condensate and bronchoalveolar lavage biomarkers in ECOPD is limited. In conclusion, pulmonary biomarkers have the potential to provide information on the mechanisms underlying ECOPD, and several correlate with clinical variables and outcomes. However, on the basis of published evidence, no single molecule is adequately validated for wide clinical use. Clinical trials that incorporate biomarkers in decisional algorithms are required.
Keywords: COPD (chronic obstructive pulmonary disease), Exacerbations of COPD, Biomarkers, Airway inflammation, Cytokines, Spontaneous sputum, Induced sputum, Exhaled breath condensate, Fractional exhaled nitric oxide, Bronchoalveolar lavage
Background
The natural history of chronic obstructive pulmonary disease (COPD) is marked by episodes of deterioration, called exacerbations of COPD (ECOPD) which lead to increased morbidity and mortality [1]. An ERS/ATS Task Force published in 2008 has described ECOPD as one of the clinical outcomes of COPD that should be used for the assessment of patients and for defining the impact of treatment interventions [2]. In addition to the functional and imaging markers, increasing evidence suggests that sampling, either local or systemic, of biological molecules known as biomarkers can provide an insight in the pathophysiological mechanisms of ECOPD [2,3]. Sampling methods elaborated during the last decades offer an innovative basis for the identification of pulmonary biomarkers. These techniques may be totally noninvasive [e.g. exhaled air, exhaled breath condensate (EBC), spontaneous sputum (SS)], semi-invasive (e.g. induced sputum (IS), nasal wash, large airways’ secretions) or invasive [e.g. bronchoalveolar lavage (BAL), lung biopsies]. Although readily implemented in the research laboratory, their wide application has long been hampered by the lack of standardization and the absence of reference values, issues that are being increasingly addressed in the literature [4,5]. The aim of this systematic review is to provide an overview of the pulmonary sampled biomarkers studied in the context of ECOPD and to highlight their associations with clinical variables in an attempt to illustrate the potential clinical implications of current evidence.
Methodology and definitions
A search for articles, published in the English language until April 2013, was conducted using Medline and Highwire databases as well as the reference lists of retrieved publications. Studies were considered pertinent if they evaluated non-systemic, pulmonary sampled biological molecules in the context of ECOPD. Research included – but was not limited to - 3 keywords for ECOPD (COPD exacerbation, COPD deterioration, acute COPD), 3 for biomarkers (biomarkers, cytokines, oxidative stress) and 6 for the sampling methods (exhaled, EBC, BAL, IS, SP, lung biopsies). Abstracts or unpublished reports were not included in the current review, whereas data obtained from mixed populations were excluded from further analysis. Data on cellular, functional or imaging parameters that may be used as markers of disease were not included in the present review. Biomarkers sampled with non-invasive, semi-invasive or invasive methods were evaluated for their potential: 1) to illustrate the natural history of ECOPD through the modification of their levels, 2) to correlate with clinical variables [e.g. symptoms, clinical severity, pulmonary function tests (PFTs) and arterial blood gas (ABG)] and 3) to serve as predictors of clinical outcomes (e.g. recovery, length of hospital stay and ECOPD frequency), ECOPD etiology and response to treatment. The methodology used was in accordance with the PRISMA guidelines [6]. A flow chart diagram of the search strategy and study selection is provided in Figure 1.
Figure 1.
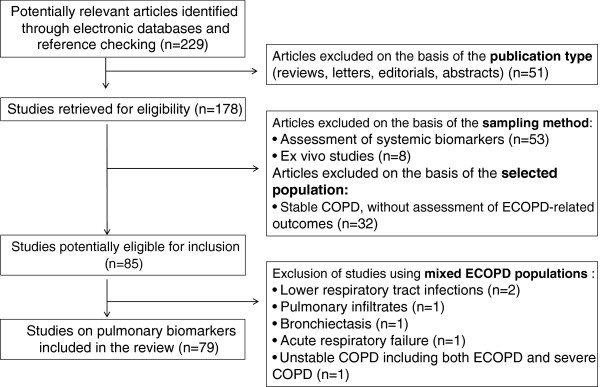
Flow chart diagram of the search strategy and study selection.
As described previously for systemic biomarkers in ECOPD [7] the following terminology was applied invariably:
•‘Baseline’ refers to a time-point of clinical stability, before the development of ECOPD (used for longitudinal studies).
•‘ECOPD onset’ refers to the first time-point at which ECOPD patients were assessed by the investigators.
•The terms ‘stability or recovery’ were avoided and the specific time point of assessment was preferably used.
•‘Stable COPD’ refers to cross-sectional studies comparing ECOPD patients with another group of COPD patients.
Results and discussion
Non-invasive sampling
Exhaled biomarkers
Breath analysis is considered to be a valuable non-invasive technique for sampling volatile biomarkers. With the exception of one study which assessed four volatile biomarkers simultaneously [8], fractional exhaled nitric oxide (FeNO) is the only exhaled biomarker studied in ECOPD (Table 1). Electronic nose, a recently developed omics technique that provides a ‘breathprint’ of exhaled volatile organic compounds, has not been studied yet during ECOPD [9].
Table 1.
Assessment of FeNO in ECOPD
| Ref. | Course: | At ECOPD onset | Course: | Comment | |
|---|---|---|---|---|---|
| |
From Baseline to |
FeNO levels |
Comparisons |
After ECOPD |
|
| |
ECOPD Onset |
(ppb) |
|
onset |
|
| Agusti et al. [10] |
|
€41.0 ± 5.1, † 5.7-76.5 |
ECOPD > controls |
↓ |
GCS. ↓ by M1-2 or by M6-8 |
| Al-Ali et al. [11] |
|
£10.3 (2.7-34) |
↔ECOPD, controls, pneumonia |
|
|
| ↔ECOPD smokers, ex smokers | |||||
| Antus et al. [12] |
|
¥25.3 (21.2–30.1) |
|
↓ |
GCS. ↓ by discharge |
| Antus et al. [13] |
|
¥23.8 (19.4–29.7) |
↔ECOPD smokers, ex smokers |
↓ |
↓ by discharge |
| Bhowmik et al. [14] |
↑* |
#7.40 (4.80–9.60) |
|
↓* |
Exact time points N/R |
| Cosio et al. [15] |
|
N/R |
|
↓ |
GCS. ↓ by M3 |
| Kersul et al. [16] |
|
N/R |
N/R |
↓ |
GCS. ↓ by M3 after discharge |
| Lazar et al. [17] |
|
‡10 (7) |
↔ECOPD, controls (non-smokers, smokers) |
N/R |
Time points: admission, discharge |
| Papi et al. [18] | €15.18 ±1.85 | ↓ | GCS. ↓ by W8-10 | ||
The columns concerning the course of FeNO refer to longitudinal studies (paired samples) and the column concerning comparisons at ECOPD onset refers to cross-sectional studies (unpaired samples).
Abbreviations: D day after ECOPD onset, FeNO fraction of exhaled nitric oxide, GCS administration of systemic glucocorticosteroids, M months after ECOPD onset, N/R not reported, ppb parts per billion, W weeks after ECOPD onset.
Symbols: ↔: no difference, ↑: increase, ↓: decrease, € mean ± SEM, † range, £ median (range), ¥ geometric mean (95% CI), # Median (interquartile range), ‡ mean (SD), *: stability samples obtained before and after the ECOPD.
Although, in stable COPD FeNO is derived predominantly from the periphery of the lung [19], in ECOPD it appears to be produced homogeneously in the central and peripheral airways [10]. FeNO at ECOPD onset is characterized by a wide range of concentrations and initially elevated levels as compared to controls have not been invariably documented [10,11]. This lack of data uniformity may be attributed to different study designs and confounding factors, such as smoking or inhaled corticosteroids (ICS), which are known to influence FeNO levels [20]. Despite the wide range of initial concentrations, most studies report a reduction of FeNO during follow-up [10,12-16,18].
FeNO was associated with the presence of a sore throat [14] and during viral ECOPD its levels correlated with sputum eosinophils [18]. Although, a correlation with PFTs has not been invariably demonstrated [10,13], in the study of Antus and coworkers, FeNO on admission correlated positively with post-treatment increases in FEV1, and the decrease of FeNO correlated with the increases in FEV1. In this study, FeNO on admission was a predictor of a significant post-treatment increase in FEV1 with an optimum cut-off point of 26.8 ppb. For this threshold sensitivity was 74%, specificity was 75% and positive and negative predictive values were 60 and 85% respectively [12]. ABG parameters did not correlate significantly with FeNO levels [13].
Concerning clinical outcomes, a weak inverse correlation has been reported between FeNO on admission and the length of hospital stay [12]. Finally, in regard to therapeutic interventions, intravenous glucocorticosteroids (GCS) failed to acutely reduce FeNO levels in ECOPD patients [10] whereas the co-administration of theophylline was not accompanied by an additional reduction of FeNO [15].
Exhaled breath condensate
Exhaled breath is saturated with water vapor which can be condensed by cooling and used to sample a wide range of mediators. EBC samples the entire respiratory tract but newer techniques allow fractionated sampling and provide the ability to collect condensate from different parts of the respiratory tract [21,22]. EBC collection is a promising sampling method but several methodological issues hamper its clinical use [4,5]. In the context of ECOPD, detection rates of some of the sampled biomarkers, notably cytokines, have been particularly low [23,24] whereas for inflammatory mediators of a peptide nature, better detection rates have been achieved with albumin-coated sampling devices [23]. Studies comparing EBC, sputum or BAL in the context of ECOPD are scarce [24-26].
Only a few studies have focused on EBC biomarkers of ECOPD (Table 2) and so far no study has assessed the course of biomarkers between baseline and the onset of ECOPD. EBC pH, one of the most validated EBC biomarkers, has been assessed only by one study which found no correlations with PFTs and ABG parameters [13]. NO-related products have not yet been evaluated in this context, whereas for hydrogen peroxide (H2O2) published data showed correlations with dyspnea but no correlations with the clinical status or the PFTs [27-29].
Table 2.
Assessment of EBC biomarkers at ECOPD
| Biomarker | Ref. | ECOPD onset | Course: | Comment |
|---|---|---|---|---|
| |
|
Comparisons |
After ECOPD |
|
| |
|
|
onset |
|
|
AAT |
[30] |
ECOPD > COPD, controls |
|
|
|
ATP |
[17] |
↔: ECOPD, smokers, non-smokers |
↔ |
Time points: admission, discharge |
|
cysLTs |
[29] |
|
↓ |
ICS with or without systemic GCS. Time points: onset, Visit 1 (D2-4), Visit 2 (2-4D post antibiotics), Visit 3 (21-28D post antibiotics). ↓ by visit 2. |
|
H
2
O
2
|
[27] |
ECOPD > controls (smokers and non-smokers) |
↓ |
GCS. ↓ by D7 |
| |
[28] |
|
↓ |
GCS. ↓ by D3-4 |
| |
[29] |
|
↓ |
ICS with or without GCS. Time points: onset, Visit 1 (D2-4), Visit 2 (2-4D post antibiotics), Visit 3 (21-28D post antibiotics). ↓ by visit 2. |
|
IL-1β |
[24] |
ECOPD > controls, smokers, stable COPD |
|
|
|
IL-6 |
[31] |
ECOPD > controls |
↓ |
No GCS. ↓ by 2 W. Reduced after 6 M of mucolytic therapy |
| |
[24] |
ECOPD > controls, stable COPD, ECOPD In GW > smokers |
|
ICU or GW patients |
|
IL-8 |
[24] |
ECOPD > controls, smokers, stable COPD |
|
ECOPD patients hospitalized either in the ICU or in GW |
| |
[32] |
|
|
Undetectable in most subjects |
| |
[25] |
ECOPD > nonsmokers, asymptomatic smokers, symptomatic smokers |
|
Detected in 14% of healthy smokers, 20% of non-symptomatic smokers, 43% of symptomatic smokers |
| |
[33] |
ECOPD > asthma exacerbations, ↔: ECOPD, controls |
|
GCS. |
|
IL-10 |
[24] |
ECOPD > controls, smokers, stable COPD |
|
ICU or GW patients |
|
IL-12p70 |
[24] |
ECOPD > controls, smokers, stable COPD |
|
ICU or GW patients |
|
IL-17 |
[33] |
↔: ECOPD, asthma exacerbations, controls |
|
GCS. |
|
IFN-γ |
[23] |
ECOPD < controls (p value N/R) |
|
Improved detection when an albumin-coated collector was used |
|
8-isoprostane |
[31] |
ECOPD > controls |
↓ |
No GCS. ↓ by 2 W. Reduced after 6 M of mucolytic therapy |
| [34] |
ECOPD > controls |
↓ |
No GCS. ↓ by 2 W. Reduced further within 2 M |
|
| [25] |
ECOPD > nonsmokers, asymptomatic smokers, Symptomatic smokers |
|
|
|
| |
[29] |
|
↓ |
ICS with or without systemic GCS. Time points: onset, Visit 1 (D2-4), Visit 2 (2-4D post antibiotics), Visit 3 (21-28D post antibiotics). ↓ by visit 2. |
|
LTB4 |
[34] |
ECOPD > controls |
↓ |
No GCS. ↓ by 2 W. Reduced further within 2 M |
| |
[32] |
↔: ECOPD, controls, stable COPD |
↔ |
GCS. Time points: D5,14,30,60 |
| |
[29] |
|
↓ |
ICS with or without systemic GCS. Time points: onset, Visit 1 (D2-4), Visit 2 (2-4D post antibiotics), Visit 3 (21-28D post antibiotics). ↓ by visit 3. |
|
MPO |
[23] |
ECOPD > controls |
|
For samples collected with an albumin-coated apparatus |
|
Ph |
[13] |
↔: ECOPD, controls |
↔ |
Time points: admission, discharge. CO2 standardization method |
|
PGE2 |
[29] |
|
↔ |
ICS with or without systemic GCS. Time points: onset, Visit 1 (D2-4), Visit 2 (2-4D post antibiotics), Visit 3 (21-28D post antibiotics). |
|
Protein |
[24] |
↔: ECOPD, controls, smokers, stable COPD |
|
|
| |
[35] |
|
|
|
|
SLPI |
[23] |
ECOPD > controls |
|
For samples collected with an albumin-coated apparatus |
|
TNF-α |
[24] |
ECOPD > controls, smokers, stable COPD |
|
ICU or GW patients |
| |
[32] |
↔: ECOPD on D5, controls, stable COPD |
↑ |
GCS. Time points: D5, 14, 30, 60. Lowest levels on D5, D14 > D5 and D14 = D30 = D60 |
| [33] | ↔: ECOPD, controls | GCS. |
The column concerning the course of EBC biomarkers refers to longitudinal studies (paired samples) and the column concerning comparisons at ECOPD onset refers to cross-sectional studies (unpaired samples). No study has assessed the course of EBC biomarkers from baseline towards ECOPD onset.
Abbreviations:AAT alpha 1 antitrypsin, ATP adenosine triphosphate, CysLTs cysteinyl-leukotrienes, D day after ECOPD onset, GCS administration of systemic glucocorticosteroids, GW general ward, H2O2 hydrogen peroxide, ICS inhaled corticosteroids, ICU Intensive care unit, IL interleukin, IFN-γ interferon gamma, LTB4 leukotriene B4, M months after ECOPD onset, min: minimum, MPO myeloperoxidase, N/R not reported, PGE2 prostaglandin E2, SLPI secretory leukocyte protease inhibitor, TNF-α tumor necrosis factor alpha, W weeks after ECOPD onset.
Symbols: ↔: no difference, ↑: increase, ↓: decrease.
Isoprostanes and leukotrienes, are arachidonic acid metabolites that have also been measured in EBC samples. So far no double-blind placebo-controlled trial has focused on the effect of treatment interventions on EBC biomarkers. Definite conclusions cannot be drawn based on published evidence, but it has been shown that levels of 8-isoprostane were elevated and fell significantly after treatment, decreasing further in subjects receiving mucolytics for 6-months. Data concerning the effect of GCS and antibiotics on the levels of Leukotriene B4 (LTB4) are controversial [29,34,36].
Despite the abovementioned problems of traceability, pulmonary sampled cytokines have been extensively studied in the context of ECOPD. Of interest is the rather distinct pattern of TNF-α whose levels at day 5 are lower than those at day 14 [32]. This evolution pattern has also been described for systemic TNF-α and could provide evidence of a sustained inflammatory reaction during the late recovery period [37]. Concerning clinical variables, IL-8 at ECOPD onset correlated inversely with PFTs [25] whereas in regard to treatment interventions, clinical interpretation of published data should be done with caution in the absence of double-blind placebo-controlled trials. Small statistically significant differences in the concentrations of IL-6 have been observed after a 2 week antibiotic treatment and were followed by a further reduction after a 6-month course of mucolytics [31]. Interestingly, TNF-α levels at ECOPD onset did not differ according to the use of ICS [24], but at day 60 post hospitalization lower TNF-α levels have been found in patients on ICS therapy [32].
Spontaneous sputum
Most of the SS biomarkers studied during ECOPD (Additional file 1: Table S1 and S2) were evaluated for their associations with the clinical severity and the causal diagnosis. Neutrophil elastase (NE) and some antimicrobial peptides correlated with clinical severity scores [38-40], whereas the magnitude of IL-8 rise from baseline correlated with symptom recovery time [41]. Persistent symptoms were paralleled by persistently elevated levels of IL-8, TNF-α and NE, and clinical resolution was related to their decrease to pre-ECOPD levels [39].
Sputum purulence has long been used for therapeutic decisions [42] but its association with the microbiologic yield has been controversial [43-45]. Published studies report that in ECOPD sputum myeloperoxidase (MPO) and IL-8 are associated with sputum color and purulence [44]. Moreover, MPO has been associated with the sputum leucocyte count [46], IL-8 with sputum bacterial load and the polymorphonuclear macrophages count [46], whereas the log of LTB4 concentration has been associated with the overall chemotactic activity of sputum [47].
As far as specific pathogens are concerned, published data are of particular interest. In a cohort of 50 patients studied during 6 years, acquisition of H. influenza and M.catarrhalis was associated with changes in SS levels of antimicrobial peptides and distinct trends of change were observed at ECOPD as compared to colonization [40]. The study of Dal Negro et al. used a two stage logistic model to identify the cause of an ECOPD. At the first decisional step, TNF-α enabled the recognition of ECOPD associated with the presence of Ps.aeruginosa, whereas at the second decisional step IL-8 and IL-1β discriminated patients with bacterial causes from those with a viral cause and from the non-infected ones [48]. Another study, reported that, sputum NE levels of 350 mU/ml had a sensitivity of 70.6%, a specificity of 84.2%, a positive predictive value of 88.9% and a negative predictive value of 61.5% in distinguishing H. influenza or M.catarrhalis from non bacterial ECOPD [38]. In a prospective study of the same group, TNF-α had the largest area under the curve for the identification of new strain ECOPD whereas combinations of sputum TNF-α, sputum NE and serum CRP performed better than any single biomarker [39]. Finally, following treatment, the decrease of IL-8, MPO, LTB4 and albumin leakage was more substantial after bacterial eradication [49].
Semi-invasive sampling
Induced sputum
Sputum induction is used in clinical practice for microbiological and cell count studies, whereas measurement of inflammatory biomarkers is increasingly implemented in research. Methodological issues that may influence the measurement of biomarkers, such as sample pre-treatment with dithiothreitol, have been an issue of discussion in the literature [50]. Two studies that evaluated the safety profile of sputum induction during ECOPD support the safety of its proper use in this setting [51,52].
Several biomarkers have been measured in the supernatants of induced sputum (Additional file 1: Table S1 and S2) but most of the groups studied cytokine levels, notably IL-6, IL-8 and TNFα. As compared to stable COPD or to baseline, IL-6 [53,54] and TNFa [55] levels were increased at ECOPD onset but this was not invariably observed for IL-8 [53-55]. During ECOPD, high levels of IL-6 have been associated with the presence of common cold symptoms [53] and a higher change from baseline in IL-6 concentration has been associated with a rhinovirus infection [54]. These findings were supported by a study evaluating an experimental virus infection of COPD patients, in which peak viral load correlated positively with peak sputum IL-6, IL-8, NE and TNF-α as well as with peak serum CRP levels [56]. On the other hand, although data are partly controversial [55,57], bacterial exacerbations were associated with increased IL-6, IL-8, TNFα, LTB4 and MPO levels [57], and TNFα was the best predictor of a bacterial ECOPD when compared to CRP and induced sputum MPO [57].
During recovery, some studies described a reduction of the levels of IL-6, IL-8 and TNFα under bronchodilators and systemic GCS with or without antibiotics [15,55], whereas other studies did not demonstrate a reduction of IL-6 and TNFα levels even 3 months after hospital discharge [16]. The adjunction of theophylline was associated with further reductions in IL-8 and TNFα, suggesting that theophylline may potentially enhance the anti-inflammatory action of standard treatment, notably that of systemic GCS [15]. Finally, budesonide and formoterol resulted in a significantly larger decrease in mRNA expression of IL-5 as compared to placebo [58].
Spontaneous and induced sputum data analyzed in conjunction
As illustrated before, sputum analysis has been an area of intensive research. Sputum purulence and sputum cell counts have been particularly promising and were studied for their potential to characterize biological phenotypes of ECOPD and predict response to treatment [59,60]. Differences in the etiology of ECOPD but also in the severity of COPD or in treatment may modify the inflammatory response during ECOPD and notably the presence or not of sputum eosinophilia [53,61]. Concerning the analysis of biological molecules, several studies analyzed in conjunction data obtained from spontaneous and induced sputum (Additional file 1: Table S1 and S2). Although, in ECOPD, both sampling methods resulted in comparable microscopic purulence, salivary contamination and rate of isolation of major pathogens [62], studies comparing biomarkers between the two methods are scarce [38]. As different methodologies may be considered a confounding factor, these data are discussed separately in this section.
At ECOPD onset, the change in sputum IL-8 and IL-6 from baseline is inversely related to the baseline FEV1 indicating that patients with more severe COPD exhibit greater rises in inflammation at ECOPD [63]. Moreover, secretory leukocyte protease inhibitor (SLPI) correlates inversely with sputum polymorphonuclear leukocytes, and within the first 48 h of treatment a fall of the sputum polymorphonuclear count by 70% is accompanied by an increase in sputum SLPI and reductions of IL-8 and TNF-α [64].
In regard to the etiology of ECOPD, increased sputum IL-8 and TNF-α was associated to bacterial infection [64], whereas the rise of sputum IL-8 from baseline correlated with the rise in the airway bacterial load [63]. In the study of Bafadhel and coworkers a panel of biomarkers was evaluated in regard to four ECOPD phenotypes (bacteria-predominant, virus-predominant, sputum eosinophil-predominant and pauci-inflammatory exacerbations). These phenotypes could not be distinguished according to Anthonisen’s criteria and no single biomarker had an area under the curve greater than 0.70 in diagnosing an ECOPD. However, sputum IL-1b and serum CRP performed better in determining bacteria-associated ECOPD. For sputum IL-1b a cutoff point of 125 pg/ml had a sensitivity of 90% and a specificity of 80%, performing better than CRP [59].
Invasive sampling
Bronchoalveolar lavage
BAL obtained through bronchoscopy permits the study of cellular and biochemical components present in the epithelial lining fluid. So far only a few studies assessed simultaneously BAL and other airway sampling techniques in ECOPD [24,65,66]. Based on current evidence, the clinical usefulness of BAL biomarkers in ECOPD is rather limited. BAL biomarkers may illustrate the underlying mechanisms of ECOPD [24,65,67-70], but methodological issues such as low sample numbers or sample manipulation techniques have been considered to be confounding factors limiting the statistical significance of the results [24,65].
Biomarkers of bronchial biopsies obtained during ECOPD
Biomarkers expressed in biopsies obtained during ECOPD (Additional file 1: Table S3) have been evaluated principally for their potential to act as neutrophil [71] or eosinophil [72-74] chemoattractants but associations with clinical variables or outcomes have not been sufficiently assessed. In this regard, in patients suffering from severe ECOPD, increased neutrophilia has been associated with an upregulation of the gene expression of two cytokines (epithelial-derived neutrophil attractant 78 and IL-8) and the expression of receptors CXCR1 and CXCR2. Although no association has been found between the number of neutrophils and the presence of a viral infection, subjects with evidence of viral infection had fewer CXCR1 m-RNA positive cells [71].
Insights from stable COPD sampling
Pulmonary biomarkers sampled during stable COPD have been assessed for their potential to predict an imminent ECOPD and to identify patients at risk for frequent ECOPD (Table 3). Some molecules, such as FeNO [75], exhaled volatile compounds [76], sputum MPO [77] and BAL IL-8 [78], are promising but their clinical usefulness is not yet established due to the lack of large studies. BAL neutrophilia has been associated with an increased frequency of ECOPD [79], whereas sputum eosinophilia appears to be more promising than specific biomarkers in identifying patients at risk for an ECOPD [76,77], those that might benefit from the introduction of ICS [80] and those who could actually stop ICS without increasing the risk for an early exacerbation [77].
Table 3.
Lung biomarkers measured during clinically stability (baseline) as predictors of ECOPD frequency
| Sample | Biomarker | Ref. | Comment |
|---|---|---|---|
|
Exhaled air |
FeNO |
[81] |
↔: frequent (≥3/year), infrequent (≤2/year) ECOPD |
| |
[75] |
Intra-individual FeNO variability is positively associated with the ECOPD frequency |
|
| eNOCoV ≥ 40%: twofold increase in ECOPD rate as compared to COPD with eNOCoV <40%* | |||
| |
VOCs |
[76] |
Several compounds were associate with the number of ECOPD in the previous year |
|
EBC |
pH |
[82] |
No significant correlation with ECOPD frequency over the following 6M |
|
Spontaneous sputum |
Elastase |
[83] |
↔: frequent (≥3/year), infrequent (≤2/year) ECOPD |
|
IL-8 |
[83] |
↔: frequent (≥3/year), infrequent (≤2/year) ECOPD |
|
|
LTB4 |
[83] |
↔: frequent (≥3/year), infrequent (≤2/year) ECOPD |
|
|
MPO |
[83] |
↔: frequent (≥3/year), infrequent (≤2/year) ECOPD |
|
|
Protein leakage |
[83] |
↔: frequent (≥3/year), infrequent (≤2/year) ECOPD |
|
|
Induced sputum |
ECP |
[77] |
Not statistically significant hazard for ECOPD after cessation of ICS |
|
IL-6 |
[53] |
Correlated with the frequency of ECOPD |
|
|
IL-8 |
[84] |
Correlated with the total bacterial count. Bacterial colonization at baseline was associated with ECOPD frequency |
|
| [53] |
Correlated with the frequency of ECOPD |
||
|
LTB4 |
[77] |
Not statistically significant hazard for ECOPD after cessation of ICS |
|
|
MPO |
[77] |
In the monovariate analysis (but not in the multivariate analysis) sputum MPO per neutrophil was a significant hazard for ECOPD after cessation of ICS. MPO level per se were not a significant hazard. |
|
|
SLPI |
[83] |
Negative correlation with ECOPD frequency over the preceding year |
|
| [84] |
Lower levels in samples colonized with a possible pathogen. Bacterial colonization in the stable state was associated with increased frequency of ECOPD. |
||
|
Induced and spontaneous sputum |
ET-1 |
[85] |
ET-1 at stability and the rise of ET-1 during ECOPD did not correlate with the frequency of ECOPD |
|
IL-6 |
[86] |
Patients with frequent ECOPD (≥2.52/y) had a faster rise over time in sputum IL6 |
|
|
IL-8 |
[86] |
No significant relation to exacerbation frequency |
|
|
Small volume lavage of the large airways |
Albumin |
[87] |
↔: patients with ≥3 antibiotic treated ECOPD during the past 2 years, patients without recurrent ECOPD |
|
ECP |
[87] |
↔: patients with ≥3 antibiotic treated ECOPD during the past 2 years, patients without recurrent ECOPD |
|
|
Hyaluronan |
[87] |
Not statistically significant difference in regard to recurrent ECOPD |
|
|
IL-6 |
[87] |
↔: patients with ≥3 antibiotic treated ECOPD during the past 2 years, patients without recurrent ECOPD |
|
|
IL-8 |
[87] |
↔: patients with ≥3 antibiotic treated ECOPD during the past 2 years, patients without recurrent ECOPD |
|
|
MPO |
[87] |
↔: patients with ≥3 antibiotic treated ECOPD during the past 2 years, patients without recurrent ECOPD |
|
|
Tryptase |
[87] |
↔: patients with ≥3 antibiotic treated ECOPD during the past 2 years, patients without recurrent ECOPD |
|
| BAL |
MPO |
[78] |
↔: frequent (≥3/year), infrequent (<3/year) ECOPD |
|
IL-8 |
[78] |
Higher levels in patients with frequent ECOPD, 1 pg/ml increase in IL-8 was associated with 1fold increase in the risk of frequent ECOPD |
|
|
NE |
[78] |
↔: frequent (≥3/year), infrequent (<3/year) ECOPD |
|
| TNF-α | [78] | ↔: frequent (≥3/year), infrequent (<3/year) ECOPD |
Data concerning mRNA expression of biomarkers were not included in this table.
Abbreviations:eNOCoV intra-individual FeNO coefficient of variation, ECP eosinophil cationic protein, ET-1 endothelin 1, FeNO exhaled nitric oxide, ICS inhaled corticosteroids, IL interleukin, LTB4 leukotriene B4, MPO myeloperoxidase, NE neutrophil elastase, SLPI secretory leukoprotease inhibitor, TNFα tumor necrosis factor alpha, VOCs: volatile organic compounds.
Symbols: ↔: no difference.
*The FeNO monthly intra-subject variability was retrospectively assessed by calculating the CoV (mean/SD)x100.
Promising biomarkers and future challenges
As in the case of systemic biomarkers in ECOPD [7], several pulmonary biomarkers are promising but none can be considered validated enough to gain its way into everyday clinical practice. So far no single biomarker can reliably identify an ECOPD at its onset, differentiate different gravities of ECOPD or consistently predict an unfavorable outcome, but most of the studied biomarkers have the potential to illustrate the clinical course of disease through the modification of their levels and to provide information on the underlying mechanisms of disease. Some molecules correlate with clinical variables, whereas a few may predict outcomes, direct toward a causal diagnosis or provide information concerning the response to treatment (Table 4). Pinpointing the most promising biomarkers and highlighting some clinically relevant conclusions, we may mention the following:
Table 4.
Studies assessing biomarkers for their potential to provide clinically relevant information
| Assessed biomarkers | |
|---|---|
|
Clinical variables |
|
| Symptoms at ECOPD onset |
FeNO[14], EBC: H2O2[28], IS: IL-6[53], Nasal wash: IL-6[46], IL-8[46] |
| Clinical severity |
EBC: CysLTs[29], H2O2[29], 8-isoprostane[29], LTB4[29], PGE2[29] |
| SS: lactoferrin[40], LL-37[40], lysozyme[40], NE[31,38], SLPI[40] | |
| Large airway secretions: IL-8[65], TNF-α[65] | |
| PFTs |
FeNO[10,12,13,20] |
| EBC: CysLTs[29], H2O2[29], 8-isoprostane[29], IL-8[25], LTB4[29], MPO[23], PGE2[29], pH[13], SLPI[23] | |
| IS/SS: IL-6[63], IL-8[63] | |
| ABG analysis |
FeNO[13] |
| EBC: pH[13] | |
|
Prediction of clinical outcomes |
|
| Symptomatic recovery |
SS: IL-8[39,41], NE[39], TNF-α[39] |
| Length of hospital stay |
FeNO[12] |
|
Causal diagnosis |
FeNO[18] |
| SS: IL-1β[48], IL-8[38,46,48], LL37[40], lysozyme[40], NE[38,39], SLPI[40], TNFα[38,39,48] | |
| IS: IL-6[54,56,57], IL-8[55-57], LTB4[57], MPO[55,57], NE[56], TNF-α[55-57] | |
| IS/SS: IL-1β[59], IL-8[63,64], TNF-α[64] | |
| NW: IL-6[46] | |
| BAL: IL-6[68], IL-8[68], NE[68] | |
| Response to treatment | FeNO[10,15] |
| EBC: Cys-LTs[29], H2O2[29], IL-6[31], 8-isoprostane[29,31], LTB4[29], PGE2[29], TNFα[24,36] | |
| IS: IL-8[15], TNFα[15,34,36] |
Abbreviations: ABG arterial blood gas, BAL bronchoalveolar lavage, Cys-LTs cysteinyl-leukotrienes, EBC exhaled breath condensate, FeNO: fractional exhaled nitric oxide, H2O2 hydrogen peroxide, IL interleukin, IS/SS induced and spontaneous sputum analyzed in conjunction, LTB4 leukotriene B4, MPO myeloperoxidase, NE neutrophil elastase, PFTs pulmonary function tests, PGE2 prostaglandin E2, SLPI secretory leukoprotease inhibitor, SS spontaneous sputum, TNFα tumor necrosis factor alpha.
■ Although based on a single study, FeNO on admission may predict a significant post-treatment increase in FEV1. An optimum cut-off point of 26.8 ppb had a sensitivity of 74% and a specificity of 75% [12].
■ Among sputum biomarkers, the following ones appear more promising:
o Spontaneous sputum NE, IL-8 and TNF-a may reflect clinical severity and symptomatic recovery [38,39,41].
o Spontaneous sputum antimicrobial peptides (lysozyme, LL37 and SLPI) were associated to the acquisition of H. influenza and M. catarrhalis, and distinct trends of change were observed at ECOPD as compared to colonization [40].
o Spontaneous sputum TNF-α, IL-8, NE and IL-1β as well as induced sputum IL-6, IL-8, TNFα, LTB4 and MPO directed towards an etiological diagnosis [38,48,57,59,64]. Among induced sputum biomarkers TNFα was the best predictor of a bacterial ECOPD [57], whereas sputum IL-1b performed better than CRP in determining bacteria-associated ECOPD [59].
o The decrease of spontaneous sputum IL-8, MPO, LTB4 and albumin leakage was more substantial when bacterial eradication was achieved [49].
■ IL-6 was associated with a rhinovirus infection both in induced sputum [54,56] and in BAL [67].
■ Combinations of different pulmonary and/or systemic biomarkers may perform better than single biomarkers in identifying the causal etiology of ECOPD [39,59].
■ Current evidence on the clinical usefulness of exhaled breath condensate and BAL biomarkers in ECOPD is limited.
■ Several biomarkers sampled during stable COPD have the potential to identify patients at risk for frequent ECOPD, whereas patients with more severe COPD may exhibit greater rises in inflammation at ECOPD [63].
As already illustrated, biomarker discovery has become an area of intense investigation during the recent years. Collaborative efforts to standardize the procedure of biomarker development have identified 6 conceptual steps (discovery, qualification, verification, research assay optimization, biomarker validation and commercialization) [3,88,89]. In this context, the STARD statement and its proposed flowchart are useful resources that may improve the diagnostic accuracy of studies [90]. As a general framework, the aforementioned steps should also be implemented in the study of ECOPD biomarkers, especially when innovating sampling techniques are used. Currently, the majority of pulmonary biomarkers studied during ECOPD are at the initial steps of discovery and qualification, whereas only a limited number of studies have proceeded towards the subsequent steps of biomarker validation. The most advanced evaluation, so far, concerns the use of sputum biomarkers for the etiological diagnosis of ECOPD but the methodological heterogeneity of the conducted studies renders generalized data interpretation challenging. In order to obtain clinically applicable biomarkers, future studies should go beyond the simplistic description of biomarker kinetics and provide clinically oriented data. In parallel to the conduction of meticulous methodological studies, clinical decision studies are needed. Modern sequencing techniques such as metabolomics, proteomics or genomics are particularly promising and may help identify phenotype specific biomarkers. However, in complex diseases where redundant, synergistic or antagonistic mechanisms exist it becomes clear that the identification of clinically relevant biomarkers will depend on our ability to integrate data from disparate sources such as patient medical records and demographics. In this context, data mining strategies and computational models will be increasingly used for the extraction of clinically relevant information.
Conclusions
In the current review, we summarized the published evidence regarding pulmonary biomarkers studied in the context of ECOPD and attempted to highlight their clinical relevance. Several biomarkers hold promise for improving the understanding of the complex mechanisms propagating ECOPD, several molecules correlate with clinical variables and a few are associated with clinical outcomes. Most of the evidence on the effect of treatment is based on a limited number of open-label, single-center studies that are characterized by a small sample size and an undefined statistical power. Despite these promising results, on the basis of the published evidence, no single molecule can be proposed yet for wide use in clinical practice. Further experimental studies and large scale clinical trials that incorporate biomarkers in decisional algorithms are required.
Abbreviations
ABG: Arterial blood gas; BAL: Bronchoalveolar lavage; COPD: Chronic obstructive pulmonary disease; EBC: Exhaled breath condensate; ECOPD: Exacerbations of chronic obstructive pulmonary disease; FeNO: Fractional exhaled nitric oxide; GCS: Glucocorticosteroids; GSH: Glutathione; H2O2: Hydrogen peroxide; ICS: Inhaled corticosteroids; IL: Interleukin; IS: Induced sputum; LTB4: Leukotriene 4; MPO: Myeloperoxidase; NE: Neutrophil elastase; PFTs: Pulmonary function tests; SLPI: Secretory leukocyte protease inhibitor; SS: Spontaneous sputum.
Competing interests
None of the authors has any conflict of interest related to the present manuscript.
Authors’ contributions
AK conceived of the review and drafted the manuscript. KK revised the manuscript for important intellectual content. LPN revised the manuscript for important intellectual content. JWF revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Supplementary Material
Online supplement.
Contributor Information
Angela Koutsokera, Email: angela.koutsokera@chuv.ch.
Konstantinos Kostikas, Email: ktk@otenet.gr.
Laurent P Nicod, Email: laurent.nicod@chuv.ch.
Jean-William Fitting, Email: jean-william.fitting@chuv.ch.
Acknowledgements
The present work was supported by the SysCLAD project (systems prediction of chronic lung allograft dysfunction) a European Union-funded study focusing on the identification of CLAD predicting biomarkers (grant agreement number 305457-2).
References
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;14:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PM, Celli BR. et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;14:416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- Group BDW. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;14:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, Van Beurden WJ, Corradi M, Dekhuijzen R. et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;14:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- Koutsokera A, Loukides S, Gourgoulianis KI, Kostikas K. Biomarkers in the exhaled breath condensate of healthy adults: mapping the path towards reference values. Curr Med Chem. 2008;14:620–630. doi: 10.2174/092986708783769768. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;14:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- Koutsokera A, Stolz D, Loukides S, Kostikas K. Systemic biomarkers in exacerbations of copd: the evolving clinical challenge. Chest. 2012;14:396–405. doi: 10.1378/chest.11-0495. [DOI] [PubMed] [Google Scholar]
- Shorter JH, Nelson DD, McManus JB, Zahniser MS, Sama SR, Milton DK. Clinical study of multiple breath biomarkers of asthma and COPD (NO, CO(2), CO and N(2)O) by infrared laser spectroscopy. J Breath Res. 2011;14:037108. doi: 10.1088/1752-7155/5/3/037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropcke S, Holz O, Lauer G, Muller M, Rittinghausen S, Ernst P, Lahu G, Elmlinger M, Krug N, Hohlfeld JM. Repeatability of and relationship between potential COPD biomarkers in bronchoalveolar lavage, bronchial biopsies, serum, and induced sputum. PLoS One. 2012;14:e46207. doi: 10.1371/journal.pone.0046207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti AG, Villaverde JM, Togores B, Bosch M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 1999;14:523–528. doi: 10.1034/j.1399-3003.1999.14c08.x. [DOI] [PubMed] [Google Scholar]
- Al-Ali MK, Howarth PH. Exhaled nitric oxide levels in exacerbations of asthma, chronic obstructive pulmonary disease and pneumonia. Saudi Med J. 2001;14:249–253. [PubMed] [Google Scholar]
- Antus B, Barta I, Horvath I, Csiszer E. Relationship between exhaled nitric oxide and treatment response in COPD patients with exacerbations. Respirology. 2010;14:472–477. doi: 10.1111/j.1440-1843.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- Antus B, Barta I, Kullmann T, Lazar Z, Valyon M, Horvath I, Csiszer E. Assessment of exhaled breath condensate pH in exacerbations of asthma and chronic obstructive pulmonary disease: a longitudinal study. Am J Respir Crit Care Med. 2010;14:1492–1497. doi: 10.1164/rccm.201003-0451OC. [DOI] [PubMed] [Google Scholar]
- Bhowmik A, Seemungal TA, Donaldson GC, Wedzicha JA. Effects of exacerbations and seasonality on exhaled nitric oxide in COPD. Eur Respir J. 2005;14:1009–1015. doi: 10.1183/09031936.05.00047305. [DOI] [PubMed] [Google Scholar]
- Cosio BG, Iglesias A, Rios A, Noguera A, Sala E, Ito K, Barnes PJ, Agusti A. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax. 2009;14:424–429. doi: 10.1136/thx.2008.103432. [DOI] [PubMed] [Google Scholar]
- Kersul AL, Iglesias A, Rios A, Noguera A, Forteza A, Serra E, Agusti A, Cosio BG. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol. 2011;14:176–183. doi: 10.1016/j.arbres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Lazar Z, Huszar E, Kullmann T, Barta I, Antus B, Bikov A, Kollai M, Horvath I. Adenosine triphosphate in exhaled breath condensate of healthy subjects and patients with chronic obstructive pulmonary disease. Inflamm Res. 2008;14:367–373. doi: 10.1007/s00011-008-8009-6. [DOI] [PubMed] [Google Scholar]
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;14:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- Brindicci C, Ito K, Resta O, Pride NB, Barnes PJ, Kharitonov SA. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J. 2005;14:52–59. doi: 10.1183/09031936.04.00125304. [DOI] [PubMed] [Google Scholar]
- Maziak W, Loukides S, Culpitt S, Sullivan P, Kharitonov SA, Barnes PJ. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;14:998–1002. doi: 10.1164/ajrccm.157.3.97-05009. [DOI] [PubMed] [Google Scholar]
- Trischler J, Merkel N, Konitzer S, Muller CM, Unverzagt S, Lex C. Fractionated breath condensate sampling: H2O2 concentrations of the alveolar fraction may be related to asthma control in children. Respir Res. 2012;14:14. doi: 10.1186/1465-9921-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller W, Heimbeck I, Weber N, Khadem Saba G, Korner B, Neiswirth M, Kohlhaufl M. Fractionated exhaled breath condensate collection shows high hydrogen peroxide release in the airways. J Aerosol Med Pulm Drug Deliv. 2010;14:129–135. doi: 10.1089/jamp.2009.0764. [DOI] [PubMed] [Google Scholar]
- Tateosian NL, Costa MJ, Guerrieri D, Barro A, Mazzei JA, Eduardo Chuluyan H. Inflammatory mediators in exhaled breath condensate of healthy donors and exacerbated COPD patients. Cytokine. 2012;14:361–367. doi: 10.1016/j.cyto.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Gessner C, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Engelmann L, Hoheisel G, Gillissen A, Sack U, Wirtz H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir Med. 2005;14:1229–1240. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Mazur W, Stark H, Sovijarvi A, Myllarniemi M, Kinnula VL. Comparison of 8-isoprostane and interleukin-8 in induced sputum and exhaled breath condensate from asymptomatic and symptomatic smokers. Respiration. 2009;14:209–216. doi: 10.1159/000206010. [DOI] [PubMed] [Google Scholar]
- Zakharkina T, Koczulla AR, Mardanova O, Hattesohl A, Bals R. Detection of microorganisms in exhaled breath condensate during acute exacerbations of COPD. Respirology. 2011;14:932–938. doi: 10.1111/j.1440-1843.2011.01977.x. [DOI] [PubMed] [Google Scholar]
- Gerritsen WB, Asin J, Zanen P, van den Bosch JM, Haas FJ. Markers of inflammation and oxidative stress in exacerbated chronic obstructive pulmonary disease patients. Respir Med. 2005;14:84–90. doi: 10.1016/j.rmed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Oudijk EJ, Gerritsen WB, Nijhuis EH, Kanters D, Maesen BL, Lammers JW, Koenderman L. Expression of priming-associated cellular markers on neutrophils during an exacerbation of COPD. Respir Med. 2006;14:1791–1799. doi: 10.1016/j.rmed.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Antczak A, Ciebiada M, Pietras T, Piotrowski WJ, Kurmanowska Z, Gorski P. Exhaled eicosanoids and biomarkers of oxidative stress in exacerbation of chronic obstructive pulmonary disease. Arch Med Sci. 2012;14:277–285. doi: 10.5114/aoms.2012.28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczulla AR, Noeske S, Herr C, Koepke J, Jorres RA, Nell C, Schmid S, Vogelmeier C, Bals R. Alpha-1 antitrypsin is elevated in exhaled breath condensate and serum in exacerbated COPD patients. Respir Med. 2012;14:120–126. doi: 10.1016/j.rmed.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Carpagnano GE, Resta O, Foschino-Barbaro MP, Spanevello A, Stefano A, Di Gioia G, Serviddio G, Gramiccioni E. Exhaled interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: effect of carbocysteine lysine salt monohydrate (SCMC-Lys) Eur J Pharmacol. 2004;14:169–175. doi: 10.1016/j.ejphar.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ko FW, Leung TF, Wong GW, Ngai JC, To KW, Ng S, Hui DS. Measurement of tumor necrosis factor-α, leukotriene B4, and interleukin 8 in the exhaled breath condensate in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J COPD. 2009;14:79–86. [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Wu TC, Hsu JY, Jan MS, Chen CM, MC. Differences in IL-8 in serum and exhaled breath condensate from patients with exacerbated COPD or asthma attacks. J Formos Med Assoc. 2012. http://dx.doi.org/10.1016/j.jfma.2012.09.018. [DOI] [PubMed]
- Biernacki WA, Kharitonov SA, Barnes PJ. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax. 2003;14:294–298. doi: 10.1136/thorax.58.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick G, Thomas PS, Yates DH. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology. 2013. PMID: 11120902. [DOI] [PubMed]
- Ko FW, Leung TF, Wong GW, Ngai J, To KW, Ng S, Hui DS. Measurement of tumor necrosis factor-alpha, leukotriene B4, and interleukin 8 in the exhaled breath condensate in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;14:79–86. [PMC free article] [PubMed] [Google Scholar]
- Koutsokera A, Kiropoulos TS, Nikoulis DJ, Daniil ZD, Tsolaki V, Tanou K, Papaioannou AI, Germenis A, Gourgoulianis KI, Kostikas K. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med. 2009;14:919–926. doi: 10.1016/j.rmed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Sethi S, Muscarella K, Evans N, Klingman KL, Grant BJ, Murphy TF. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest. 2000;14:1557–1565. doi: 10.1378/chest.118.6.1557. [DOI] [PubMed] [Google Scholar]
- Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;14:491–497. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- Parameswaran GI, Sethi S, Murphy TF. Effects of bacterial infection on airway antimicrobial peptides and proteins in COPD. Chest. 2011;14:611–617. doi: 10.1378/chest.10-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Mullerova H, Donaldson GC, Wedzicha JA. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;14:527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;14:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- Tsimogianni AM, Papiris SA, Kanavaki S, Stathopoulos GT, Sotiropoulou C, Manali ED, Michalopoulou P, Roussos C, Kotanidou A. Predictors of positive sputum cultures in exacerbations of chronic obstructive pulmonary disease. Respirology. 2009;14:1114–1120. doi: 10.1111/j.1440-1843.2009.01615.x. [DOI] [PubMed] [Google Scholar]
- Brusse-Keizer MG, Grotenhuis AJ, Kerstjens HA, Telgen MC, van der Palen J, Hendrix MG, van der Valk PD. Relation of sputum colour to bacterial load in acute exacerbations of COPD. Respir Med. 2009;14:601–606. doi: 10.1016/j.rmed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, Kruesmann F, Haverstock D, Perroncel R, Choudhri SH, Arvis P. Sputum colour and bacteria in chronic bronchitis exacerbations: a pooled analysis. Eur Respir J. 2012;14:1354–1360. doi: 10.1183/09031936.00042111. [DOI] [PubMed] [Google Scholar]
- Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;14:71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- Crooks SW, Bayley DL, Hill SL, Stockley RA. Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur Respir J. 2000;14:274–280. doi: 10.1034/j.1399-3003.2000.15b09.x. [DOI] [PubMed] [Google Scholar]
- Dal Negro RW, Micheletto C, Tognella S, Visconti M, Guerriero M, Sandri MF. A two-stage logistic model based on the measurement of pro-inflammatory cytokines in bronchial secretions for assessing bacterial, viral, and non-infectious origin of COPD exacerbations. COPD. 2005;14:7–16. doi: 10.1081/COPD-200050680. [DOI] [PubMed] [Google Scholar]
- White AJ, Gompertz S, Bayley DL, Hill SL, O’Brien C, Unsal I, Stockley RA. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax. 2003;14:680–685. doi: 10.1136/thorax.58.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Burbure C, Pignatti P, Corradi M, Malerba M, Clippe A, Dumont X, Moscato G, Mutti A, Bernard A. Uteroglobin-related protein 1 and clara cell protein in induced sputum of patients with asthma and rhinitis. Chest. 2007;14:172–179. doi: 10.1378/chest.06-0835. [DOI] [PubMed] [Google Scholar]
- Bathoorn E, Liesker J, Postma D, Koeter G, Van Oosterhout AJ, Kerstjens HA. Safety of sputum induction during exacerbations of COPD. Chest. 2007;14:432–438. doi: 10.1378/chest.06-2216. [DOI] [PubMed] [Google Scholar]
- Gao P, Gibson PG, Zhang J, He X, Hao Y, Li P, Liu H. The Safety of sputum induction in adults with acute exacerbation of COPD. Clin Respir J. 2012. PMID: 17136956. [DOI] [PubMed]
- Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;14:114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;14:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, Dales RE. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;14:349–355. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L. et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;14:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathoorn E, Liesker JJ, Postma DS, Koeter GH, van der Toorn M, van der Heide S, Ross HA, Van Oosterhout AJ, Kerstjens HA. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis. 2009;14:101–109. doi: 10.2147/copd.s4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathoorn E, Liesker JJ, Postma DS, Boorsma M, Bondesson E, Koeter GH, Kauffman HF, Van Oosterhout AJ, Kerstjens HA. Anti-inflammatory effects of combined budesonide/formoterol in COPD exacerbations. COPD. 2008;14:282–290. doi: 10.1080/15412550802363360. [DOI] [PubMed] [Google Scholar]
- Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A. et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;14:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- He ZY, Ou LM, Zhang JQ, Bai J, Liu GN, Li MH, Deng JM, MacNee W, Zhong XN. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;14:445–452. doi: 10.1159/000321374. [DOI] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Maestrelli P, Turato G, Ruggieri MP, Roggeri A, Calcagni P, Mapp CE, Ciaccia A, Fabbri LM. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;14:1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest. 2000;14:380S–385S. doi: 10.1378/chest.117.5_suppl_2.380S. [DOI] [PubMed] [Google Scholar]
- Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;14:317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S, Walters EH, Griffiths A, Wood-Baker R, Johns DP, Reid DW. Airway inflammation and anti-protease defences rapidly improve during treatment of an acute exacerbation of COPD. Respirology. 2009;14:495–503. doi: 10.1111/j.1440-1843.2009.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;14:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrelli P, Saetta M, Di Stefano A, Calcagni PG, Turato G, Ruggieri MP, Roggeri A, Mapp CE, Fabbri LM. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med. 1995;14:1926–1931. doi: 10.1164/ajrccm.152.6.8520757. [DOI] [PubMed] [Google Scholar]
- Singh M, Lee SH, Porter P, Xu C, Ohno A, Atmar RL, Greenberg SB, Bandi V, Gern J, Amineva S. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;14:1369–1378. doi: 10.1016/j.jaci.2010.02.035. e1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;14:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, Anderson GP. Serum amyloid A opposes lipoxin A to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2012;14:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi B, Bason C, Balleari E, Fiasella F, Pesci A, Ghio R, Fabiano F. Increased bronchoalveolar granulocytes and granulocyte/macrophage colony-stimulating factor during exacerbations of chronic bronchitis. Eur Respir J. 1997;14:846–850. [PubMed] [Google Scholar]
- Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;14:968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Maestrelli P, Turato G, Mapp CE, Pieno M, Zanguochi G, Del Prete G, Fabbri LM. Airway eosinophilia and expression of interleukin-5 protein in asthma and in exacerbations of chronic bronchitis. Clin Exp Allergy. 1996;14:766–774. doi: 10.1111/j.1365-2222.1996.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Qiu YS, Majumdar S, Gamble E, Matin D, Turato G, Fabbri LM, Barnes N, Saetta M, Jeffery PK. Exacerbations of Bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am J Respir Crit Care Med. 2001;14:109–116. doi: 10.1164/ajrccm.164.1.2007050. [DOI] [PubMed] [Google Scholar]
- Bocchino V, Bertorelli G, Bertrand CP, Ponath PD, Newman W, Franco C, Marruchella A, Merlini S, Del Donno M, Zhuo X, Olivieri D. Eotaxin and CCR3 are up-regulated in exacerbations of chronic bronchitis. Allergy. 2002;14:17–22. [PubMed] [Google Scholar]
- De Laurentiis G, Maniscalco M, Cianciulli F, Stanziola A, Marsico S, Lundberg JO, Weitzberg E, Sofia M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm Pharmacol Ther. 2008;14:689–693. doi: 10.1016/j.pupt.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Basanta M, Ibrahim B, Dockry R, Douce D, Morris M, Singh D, Woodcock A, Fowler SJ. Exhaled volatile organic compounds for phenotyping chronic obstructive pulmonary disease: a cross-sectional study. Respir Res. 2012;14:72. doi: 10.1186/1465-9921-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesker JJ, Bathoorn E, Postma DS, Vonk JM, Timens W, Kerstjens HA. Sputum inflammation predicts exacerbations after cessation of inhaled corticosteroids in COPD. Respir Med. 2011;14:1853–1860. doi: 10.1016/j.rmed.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Tumkaya M, Atis S, Ozge C, Delialioglu N, Polat G, Kanik A. Relationship between airway colonization, inflammation and exacerbation frequency in COPD. Respir Med. 2007;14:729–737. doi: 10.1016/j.rmed.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Sukkar MB, Wood LG, Tooze M, Simpson JL, McDonald VM, Gibson PG, Wark PA. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur Respir J. 2012;14:721–729. doi: 10.1183/09031936.00022011. [DOI] [PubMed] [Google Scholar]
- Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, Monteiro W, Berry M, Parker D, Wardlaw AJ, Pavord ID. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;14:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- Bazeghi N, Gerds TA, Budtz-Jorgensen E, Hove J, Vestbo J. Exhaled nitric oxide measure using multiple flows in clinically relevant subgroups of COPD. Respir Med. 2011;14:1338–1344. doi: 10.1016/j.rmed.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Terada K, Muro S, Sato S, Ohara T, Haruna A, Marumo S, Kinose D, Ogawa E, Hoshino Y, Niimi A. et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;14:951–955. doi: 10.1136/thx.2007.092858. [DOI] [PubMed] [Google Scholar]
- Gompertz S, Bayley DL, Hill SL, Stockley RA. Relationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPD. Thorax. 2001;14:36–41. doi: 10.1136/thorax.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;14:759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland M, Bhowmik A, Sapsford RJ, Seemungal TA, Jeffries DJ, Warner TD, Wedzicha JA. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax. 2001;14:30–35. doi: 10.1136/thorax.56.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, Maccallum PK, Wedzicha JA. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;14:1995–2004. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riise GC, Ahlstedt S, Larsson S, Enander I, Jones I, Larsson P, Andersson B. Bronchial inflammation in chronic bronchitis assessed by measurement of cell products in bronchial lavage fluid. Thorax. 1995;14:360–365. doi: 10.1136/thx.50.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol. 2006;14:971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- Koulman A, Lane GA, Harrison SJ, Volmer DA. From differentiating metabolites to biomarkers. Anal Bioanal Chem. 2009;14:663–670. doi: 10.1007/s00216-009-2690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, De Vet HC, Lijmer JG. Standards for Reporting of Diagnostic A. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;14:W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplement.


