Abstract
Introduction
Cigarette smoking is the most commonly encountered risk factor for chronic obstructive pulmonary disease (COPD). However, it is not the only one and there is consistent evidence from epidemiologic studies that nonsmokers may develop chronic airflow limitation. A history of tuberculosis has recently been found to be associated with airflow obstruction in adults older than 40 years. The aim of this study was to evaluate the association between the radiologic changes by tuberculosis and airflow obstruction in a population based sample.
Methods
A nationwide COPD prevalence survey was conducted. We compared the prevalence of airflow obstruction according to the presence of the radiologic change by the tuberculosis.
Results
We analyzed 1,384 subjects who participated in the nationwide Korean COPD survey. All subjects were older than 40 years and took the spirometry and simple chest radiography. We defined the airflow obstruction as FEV1/FVC <0.7. A total of 149 (10.8%) subjects showed airflow obstruction. A total of 167 (12.1%) subjects showed radiologic change by tuberculosis. Among these 167 subjects, 44 (26.3%) had airflow obstruction. For the subjects without radiologic change by tuberculosis, the prevalence of airflow obstruction was only 8.6%. The unadjusted odds ratio for airflow obstruction according to the radiologic change was 3.788 (95% CI: 2.544-5.642).
Conclusions
The radiologic change by tuberculosis was associated with airflow obstruction.
Keywords: Chronic obstructive pulmonary disease (COPD), tuberculosis, risk factor, airflow obstruction
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation which is not fully reversible. Cigarette smoking is the most commonly encountered risk factor for COPD. It is not the only factor, however, and there is consistent evidence from epidemiologic studies that nonsmokers may develop chronic airflow limitation. Thus, the current understanding of risk factors for COPD is in many respects incomplete (1).
A history of tuberculosis has been found to be associated with airflow obstruction in adults older than 40 years (2,3). Tuberculosis can cause chronic lung function impairment and is associated with a mean excess loss in FEV1 and FVC. Lung function loss was greater among those with more severe or later clinical presentation of tuberculosis (4,5). In Korea, the new active pulmonary tuberculosis rate per 100,000 was 59.3 in 2009. This rate increased with the age of the patients (6).
A nationwide COPD prevalence survey in Korea in conjunction with the second South Korean National Health and Nutrition Examination Survey (Korean NHANES II) was conducted from 2001 to 2002. Using Global Initiative for Chronic Obstructive Lung Disease (GOLD) criterion for defining airflow obstruction, 17.2% of Korean adults over the age of 45 years had COPD (7). In Korean NHANES II, chest X-ray films were taken in specially equipped mobile examination cars at the time of spirometry.
The aim of this study was to evaluate the association between the radiologic changes by tuberculosis and airflow obstruction in a population based sample.
Materials and methods
This study was performed as part of a nationwide COPD prevalence survey. It was conducted in conjunction with the second South Korean National Health and Nutrition Examination Survey (Korean NHANES II) from October 15, 2001, to January 20, 2002. Spirometry was performed by specially trained technicians who conformed to the 1994 American Thoracic Society (ATS) recommendations (8). A more detailed description of the nationwide COPD survey methodology and spirometric procedures has been previously published (7). The study protocol was approved by the ethics committee of Hallym University Sacred Heart Hospital, Anyang, Korea.
Study subjects
In 2005, ATS and the European Respiratory Society (ERS) published a new statement on spirometry (9). The acceptability and repeatability criteria of the spirometry in this study were adopted from the 2005 ATS/ERS recommendations. We analyzed the data of subjects older than 40 years with at least three acceptable spirometry performances and available chest X-ray films.
Airway obstruction is defined by the GOLD criteria as FEV1/FVC of less than 70%. The severity of obstruction was also classified according to GOLD criteria.
The radiologic findings were evaluated by two qualified radiologists and categorized as Table 1. The ‘TB scar positive’ case was defined if their chest radiography showed any parenchymal changes by tuberculosis listed in Table 1, irrespective of tuberculosis activity.
Table 1. Radiologic findings used in this study.
| Normal |
| Emphysema |
| Pulmonary tuberculosis, mild, inactive |
| Pulmonary tuberculosis, moderate, inactive |
| Pulmonary tuberculosis, severe, inactive |
| Pulmonary tuberculosis, mild, activity undetermined |
| Pulmonary tuberculosis, moderate, activity undetermined |
| Pulmonary tuberculosis, severe, activity undetermined |
| Pulmonary tuberculosis, mild, active |
| Pulmonary tuberculosis, moderate, active |
| Pulmonary tuberculosis, severe, active |
| Pleural effusion |
| Pleural thickening |
| Others* |
*, including lung mass, solitary pulmonary nodule, bronchiectasis, mediastinal abnormality, diffuse interstitial infiltration, atelectasis, etc.
Analysis
All data are expressed as means and standard deviations or frequencies. We compared the mean spirometry values and the frequency of airflow obstruction according to the status of “TB scar”. We used student t-test and chi-square test for statistical analysis. The logistic regression models for the association between the presence of “TB scar” and airflow obstruction were also used. P value less than 0.05 was considered statistically significant.
Results
Study populations
A total of 1,384 subjects (male 629, female 755) were included for the analysis. The median age of the study population was 51 years. Table 2 describes the demographic characteristics of the study population. Radiologic change by tuberculosis was found in 167 (12.1%) subjects. Most of them showed mild changes. None of them showed active lesion of tuberculosis (Table 3).
Table 2. Demographic characteristics of study population.
| Male (N=629) | Female (N=755) | Total (N=1,384) | |
|---|---|---|---|
| Age group (years), N (%) | |||
| 40-49 | 294 (46.7) | 330 (43.7) | 624 (45.1) |
| 50-59 | 185 (29.4) | 229 (30.3) | 414 (29.9) |
| 60-69 | 117 (18.6) | 156 (20.7) | 273 (19.7) |
| ≥70 | 33 (5.2) | 40 (5.3) | 73 (5.3) |
| Smoking amount (pack-year), N (%) | |||
| 0-10 | 93 (14.8) | 18 (2.4) | 111 (8.0) |
| 10-20 | 120 (19.1) | 7 (0.9) | 127 (9.2) |
| ≥20 | 269 (42.8) | 8 (1.1) | 277 (20.0) |
| Airflow obstruction, N (%) | |||
| Present* | 107 (17.0) | 42 (5.6) | 149 (10.8) |
| Absent | 522 (83.0) | 713 (94.4) | 1,235 (89.2) |
| TB scar, N (%) | |||
| Present† | 107 (17.0) | 60 (7.9) | 167 (12.1) |
| Absent | 522 (83.0) | 695 (92.1) | 1,217 (87.9) |
*, defined as observed FEV1/FVC <0.7; †, presence of any parenchymal change by tuberculosis listed in Table 1.
Table 3. Frequency of radiologic changes by tuberculosis.
| Type of radiology change | N (%) |
|---|---|
| Pulmonary tuberculosis, mild, inactive | 154 (92.2) |
| Pulmonary tuberculosis, moderate, inactive | 1 (0.6) |
| Pulmonary tuberculosis, severe, inactive | 1 (0.6) |
| Pulmonary tuberculosis, mild, activity undetermined | 9 (5.4) |
| Pulmonary tuberculosis, moderate, activity undetermined | 0 (0) |
| Pulmonary tuberculosis, severe, activity undetermined | 2 (1.2) |
| Pulmonary tuberculosis, mild, active | 0 (0) |
| Pulmonary tuberculosis, moderate, active | 0 (0) |
| Pulmonary tuberculosis, severe, active | 0 (0) |
| Total | 167 [100] |
Spirometry results
The mean value of FEV1, FVC and FEV1/FVC of total subjects were 2.87±0.71 (L), 3.68±0.89 (L) and 0.78±0.73, respectively. The percentages of predicted value in FEV1 and FVC were (95.7±14.2)% and (97.3±12.5)%. Table 4 shows the spirometry results of total subjects according to the radiologic changes. Subjects with radiologic changes by tuberculosis showed significantly lower levels of percentage of predicted value for FEV1 and FVC (Table 5).
Table 4. Spirometry results of total subjects by the radiologic change.
| TB scar (+) (N=167) | TB scar (–) (N=1,217) | P | |
|---|---|---|---|
| FEV1 (L) | 2.77±0.78 | 2.88±0.70 | 0.064 |
| FEV1 (% of predicted value) | 90.02±17.05 | 96.51±13.53 | <0.001 |
| FVC (L) | 3.75±0.95 | 3.66±0.88 | 0.236 |
| FVC (% of predicted value) | 95.01±14.10 | 97.65±12.24 | 0.022 |
| FEV1/FVC | 73.81±10.21 | 78.90±6.70 | <0.001 |
TB, tuberculosis; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Table 5. Spirometry results of the subjects with airflow obstruction by the radiologic change.
| TB scar (+) (N=44) | TB scar (–) (N=105) | P | |
|---|---|---|---|
| FEV1 (L) | 2.25±0.73 | 2.48±0.75 | 0.083 |
| FEV1 (% of predicted value) | 74.36±18.03 | 82.60±16.59 | 0.008 |
| FVC (L) | 3.69±0.97 | 3.84±1.06 | 0.401 |
| FVC (% of predicted value) | 91.71±14.51 | 97.60±15.86 | 0.036 |
| FEV1/FVC | 60.57±9.04 | 64.27±6.10 | 0.015 |
TB, tuberculosis; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Prevalence of airflow obstruction
Among the 1,384 subjects, 149 (10.8%) showed airflow obstruction on spirometry. The presence of airflow obstruction was more frequent in subjects with radiologic change of tuberculosis. Among 167 subjects with radiologic changes by tuberculosis, 44 (26.3%) subjects showed airflow obstruction. Only 8.6% of subjects without radiologic changes showed airflow obstruction. The difference of prevalence of airflow obstruction was statistically significant (P<0.001, chi-square test) (Table 6). The unadjusted odds ratio (OR) for airflow obstruction according to the presence of radiologic changes by tuberculosis was 3.8 (95% CI: 2.54-5.64). After adjustment for smoking status, the OR was 3.12 (95% CI: 2.01-4.67).
Table 6. Presence of airflow obstruction according to radiologic change.
| TB scar (+) [%] | TB scar (–) [%] | Total [%] | |
|---|---|---|---|
| Airflow obstruction (+) | 44 [26.3] | 105 [8.6] | 149 [10.8] |
| Airflow obstruction (–) | 123 [73.7] | 1,112 [91.4] | 1,235 [89.2] |
| Total | 167 [100] | 1,217 [100] | 1,384 [100] |
P<0.001, chi-square test. TB, tuberculosis.
The distribution of airflow obstruction severity, according to the GOLD spirometry classification, was also evaluated. More subjects were classified into a more severe stage when they showed radiologic changes by tuberculosis. 11.3% of the subjects with radiologic changes were classified into GOLD 3 or 4, but only 4.8% of subjects without radiologic changes were in GOLD 3 or 4 (Figure 1).
Figure 1.
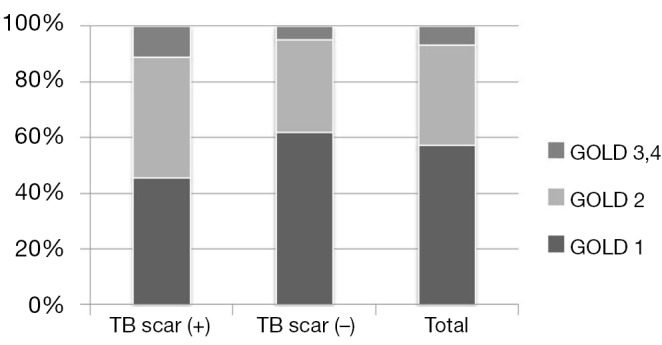
Distribution of GOLD spirometry classification according to radiologic change. GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Discussion
Pulmonary tuberculosis is associated with chronic airflow obstruction at diagnosis, during treatment, and several years after treatment ended (10). Tuberculosis can cause chronic lung function impairment in gold miners which increases incrementally with the number of episodes of tuberculosis, affecting approximately 18% of subjects with one episode, 27% of subjects with two episodes, and 35% of subjects with three episodes of tuberculosis (4). Prevalence of airflow obstruction varied from 28% to 68% (11,12) of patient with pulmonary tuberculosis. A history of tuberculosis was also revealed to be associated with airflow obstruction in a large population-based study (2,3). However, little is known about the effect of radiologic changes by tuberculosis on airflow obstruction. We think that this is the first study that evaluated the association between the radiologic changes by tuberculosis and airflow obstruction in the general population.
In this population-based study, 12.1% of the study subjects showed radiologic changes by tuberculosis. Most of them showed mild change. Among the subjects with radiologic changes by tuberculosis, 26.3% of them had airflow obstruction. This figure was much higher than that of subjects without radiologic changes and statistically significant. The smoking-adjusted odds ratio for airflow obstruction according to the presence of radiologic changes by tuberculosis was over 3.12, which was statistically significant. Also, subjects with radiologic changes were categorized into more severe stages in GOLD spirometry classification. These findings suggest that tuberculosis can be not only a risk factor but also a prognostic factor for COPD.
Extensive lesions by tuberculosis may produce restrictive changes but obstructive alterations were also identified (13,14). Patients with more extensive disease at presentation had lower FEV1 at follow-up (11). The degree of airflow obstruction was correlated with the extent of disease assessed by radiography (11), despite of the radiologic improvement after six months of antimicrobial chemotherapy (12). However, as shown in Table 3, almost all (97.6%) of radiologic changes by tuberculosis detected in this study were minimal, so the possibility of airflow obstruction caused by extensive lesion can be ruled out. There might be another mechanism for airflow obstruction such as chronic airway inflammation rather than airway fibrosis especially in the case of minimal radiologic changes by tuberculosis.
We also found that subjects with radiologic changes by tuberculosis also showed lower percentages of predicted values in FEV1 and FVC than subjects without radiologic changes irrespective of the presence of airflow obstruction (Tables 4,5). This confirms the previous finding by Ross et al. that pulmonary tuberculosis is associated with excess loss of lung function (5).
The present findings are relevant for public health. COPD is projected to be the third leading cause of death by 2020. Unfortunately, current pharmacological treatment does not reduce the mortality rate or modify the natural disease course, so, prevention is an important strategy for managing COPD. COPD can be prevented in three levels. Primary preventions could be achieved by modification or reduction of the disease’s main causal factor (e.g., cigarette smoking). Secondary prevention could focus on screening or early detection of COPD. Tertiary prevention might include management of identified COPD patients to augment health status, reduce or slow disease progression or diminish exacerbations and other adverse outcomes (15). Several issues relate to early diagnosis in terms of cost and benefits of identification of groups with increasing risk and severity. The current findings suggest that subjects with radiologic change by tuberculosis could be candidate for screening COPD. Therefore, early diagnosis of COPD might be possible cost-effectively.
This study has some limitations. The spirometric definitions of COPD generally required a post-bronchodilator measurement (1,16), the spirometry examination in this nationwide survey did not include a test of reversibility of obstruction. While some subset of the study population had a probability of having pure asthma, we believe the presence of airflow obstruction using pre-bronchodilator FEV1/FVC is reasonable as a criterion of airflow obstruction after the age of 40 years in this large epidemiologic survey. As the current views of reversibility misclassify large segments of the population (17,18), the degree of reversibility of airflow limitation is no longer recommended for differential diagnosis with asthma (1). Safety issue was another consideration in this large epidemiologic survey. Fatal or near-fatal paradoxical bronchospasms after administration of a bronchodilator are described elsewhere (19). FEV1 and other respiratory indices obtained without bronchodilation are good markers of overall health (20).
In conclusion, a presence of radiologic change even mild, by tuberculosis was associated with airflow obstruction. The present findings would reduce the burden of airflow obstruction by the contribution of early detection of airflow obstruction.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevent of chronic obstructive pulmonary disease (updated 2014; cited 05 February 2014). Available online: http://www.goldcopd.org
- 2.Menezes AM, Hallal PC, Perez-Padilla R, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study. Eur Respir J 2007:30:1180-5 [DOI] [PubMed] [Google Scholar]
- 3.Hooper R, Burney P, Vollmer WM, et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J 2012;39:1343-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hnizdo E, Singh T, Churchyard G, et al. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000;55:32-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross J, Ehrlich RI, Hnizdo E, et al. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010;65:1010-5 [DOI] [PubMed] [Google Scholar]
- 6.Korea Centers for Disease Control and Prevention. Annual Report on the Notified Tuberculosis Patients in Korea 2009. Available online: http://www.stoptbk.org/eng/board/data/view.asp?bidx=757&page=1&pctgIdx=&stype=&skey=
- 7.Kim DS, Kim YS, Jung KS, et al. Prevalence of chronic obstructive pulmonary disease in Korea. Am J Respir Crit Care Med 2005;172:842-7 [DOI] [PubMed] [Google Scholar]
- 8.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995;152:S77-121 [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38 [DOI] [PubMed] [Google Scholar]
- 10.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009;374:733-43 [DOI] [PubMed] [Google Scholar]
- 11.Willcox PA, Ferguson AD. Chronic obstructive airway disease following treated pulmonary tuberculosis. Respir Med 1989;83:195-8 [DOI] [PubMed] [Google Scholar]
- 12.Plit ML, Anderson R, Van Rensburg CE, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J 1998;12:351-6 [DOI] [PubMed] [Google Scholar]
- 13.Hallett WY, Martin CJ. The diffuse obstructive pulmonary syndrome in a tuberculosis sanatorium. I. Etiologic factors. Ann Intern Med 1961;54:1146-55 [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculosis destroyed lung. Respir Med 2003;97:1237-42 [DOI] [PubMed] [Google Scholar]
- 15.Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721-32 [DOI] [PubMed] [Google Scholar]
- 16.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of ATS/ERS position paper. Eur Respir J 2004;23:932-46 [DOI] [PubMed] [Google Scholar]
- 17.Soriano JB, Davis KJ, Coleman B, et al. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003;124:474-81 [DOI] [PubMed] [Google Scholar]
- 18.Soriano JB, Mannino DM. Reversing concepts on COPD irreversibility. Eur Respir J 2008;31:695-6 [DOI] [PubMed] [Google Scholar]
- 19.Cocchetto DM, Sykes RS, Spector S. Paradoxical bronchospasm after use of inhalation aerosols: a review of the literature. J Asthma 1991;28:49-53 [DOI] [PubMed] [Google Scholar]
- 20.Ashley F, Kannel WB, Sorlie PD, et al. Pulmonary function: relation to aging, cigarette habit, and mortality. Ann Intern Med 1975;82:739-45 [DOI] [PubMed] [Google Scholar]


