Abstract
Objective
To investigate the prevalence of anti-endothelial cell antibodies (AECA) in connective tissue diseases (CTD) associated with pulmonary arterial hypertension (PAH) and to corroborate the pathologic function of AECA in PAH-associated CTDs.
Methods
AECA were detected by cellular enzyme-linked immunosorbent assay (ELISA) in sera of 19 PAH-associated CTD patients, 22 CTD patients without PAH involvement, and 20 age- and sex-matched healthy individuals as controls. Using IgG purified from the sera of AECA-positive, AECA-negative, and healthy subjects, the effects of AECA on the expression of ICAM-1 and the chemokine regulated upon activation normal T-cell expressed and secreted (RANTES) in cultured endothelial cells were also evaluated.
Results
A total of 12 of the 19 (63.2%) CTD patients with PAH, 9 of the 22 (40.9%) CTD patients without PAH, and 1 of the 20 (5%) healthy controls were positive for AECA, which were calculated as ELISA ratio (ER) values. ER values in PAH-associated CTD patients were significantly higher than those with CTD without PAH (3.68±2.05 versus 1.67±1.07, P<0.001). IgG purified from AECA-positive sera induced a significantly increased level of ICAM-1 expression after 48 h incubation (795.2±32.5 pg/mL) compared with AECA-negative or healthy control IgG (231.5±27.1 and 192.8±33.4 pg/mL, respectively; P<0.001). In addition, RANTES production by cultured human pulmonary arterial endothelial cells (HPAECs) increased in both a time- and concentration-dependent manner in response to incubation with purified AECA-positive IgG.
Conclusions
AECA could be involved in CTD and might participate in the pathogenesis of PAH-associated CTD.
Keywords: Connective tissue diseases (CTD), pulmonary arterial hypertension (PAH), anti-endothelial cell antibodies (AECA)
Introduction
Pulmonary arterial hypertension (PAH) is a rare disease characterized by endothelial dysfunction and cellular proliferation, resulting in progressively elevated pulmonary arterial resistance and, ultimately, right-sided heart failure (1). PAH complicated by connective tissue diseases (CTD) is associated with a poorer prognosis than idiopathic PAH. Nevertheless, the pathophysiologic mechanisms of PAH are currently poorly defined, despite understanding of the underlying inflammatory processes involved (2).
Anti-endothelial cell antibodies (AECA) are a heterogeneous family of antibodies that can specifically recognize endothelial cell proteins and molecules present on the surface of endothelial cells (3). AECA have been detected in patients with various collagen vascular diseases, and their levels fluctuate with disease activities (4,5), indicating that AECA may interfere with pathological processes rather than being an independent existence. Studies using human umbilical vein endothelial cells have suggested that AECA can up-regulate the expression of adhesion molecules and the secretion of cytokines, which may play roles in vascular lesions (6,7). In addition, increased expression of chemokines, such as regulated upon activation normal T-cell expressed and secreted (RANTES), have also been noted in severe cases of PAH (8), highlighting the possible role of AECA-related inflammatory mechanisms in this condition.
Since pulmonary endothelial cell dysfunction appears to play an integral role in the initiation and progression of PAH in humans (9), we hypothesized that production of anti-human pulmonary arterial endothelial cell antibodies (anti-HPAEC) and their association with endothelial dysfunction may be a key step in the PAH pathological process, especially when associated with CTD. We therefore performed the current study to evaluate the prevalence of anti-HPAEC in CTD, in cases associated and not associated with PAH, and examined the relationship between anti-HPAEC and clinical features. The results of this study should help to corroborate the pathologic function of anti-HPAEC in PAH, especially when combined with CTD.
Materials and methods
Patients and controls
Patients were screened for PAH using Doppler echocardiography based on systolic pulmonary artery pressure >40 mmHg, and confirmed by right-side heart catheterization with mean pulmonary artery pressure at rest >25 mmHg. A total of 41 CTD patients admitted to the Rheumatology Department of China Medical University between 2008 and 2011 were enrolled in the study, including the study group (PAH-associated CTD group, n=19) and disease control group (no-PAH CTD group, n=22). In the 19 PAH-associated CTD patients, the underlying diseases included systemic sclerosis (SSc, n=8), systemic lupus erythematosis (SLE, n=3), mixed connective tissue disease (MCTD, n=4), primary Sjögren syndrome (pSS, n=2), and undifferentiated connective tissue disease (uCTD, n=2), with no significant distribution difference compared to the diseases of the no-PAH CTD group. Sera samples were collected at the time of diagnosis when none of the patients were under systemic steroid or immunosuppressive therapy. Twenty age- and sex-matched healthy volunteers served as the control group. The study protocol was approved by our institutional review board, and each patient gave informed consent.
Cellular enzyme-linked immunosorbent assay (ELISA) for AECA
HPAECs (ScienCell Corp.) at passage 4 were seeded onto 96-microculture plates and allowed to reach confluence. AECA were detected using a cyto-ELISA method as described previously (10). In brief, after being fixed with 0.1% glutaraldehyde, sera samples diluted 1:400 were added to triplicate wells for 1 h at 37 °C. After washing, horseradish peroxidase-conjugated goat anti-human IgG-F(ab')2 diluted 1:1,000 was added for 1 h at 37 °C. Then 3,3', 5,5'-tetramethylbenzidine was added as a substrate and the color reaction was terminated by the addition of 1 mol/L H2SO4. Optical density was measured at 450 nm and the results are expressed as the ELISA ratio (ER) = (S − A)/(B − A), where S is the absorbance of the sample, and A and B are the absorbances of negative and positive reference serum, respectively. Positivity was defined as ER values > mean +3 standard deviations (SD) of the healthy control values. Intra- and inter-assay coefficients of variation were 5% and 12%, respectively. Sera from six AECA-positive patients, six AECA-negative patients, and five AECA-negative healthy subjects were pooled and purified using Protein G Sepharose (GE Hitrap) chromatography.
ICAM-1 and RANTES production by HPAECs
To identify the effect of AECA on cultured HPAECs, we incubated arterial endothelial cells forming a monolayer with IgG purified from AECA-positive, AECA-negative, or healthy control sera. ICAM-1 and RANTES levels were measured using a commercial ELISA kit (USCN Life Science Inc., Wuhan, China), following the manufacturer’s instructions.
Statistical analysis
All data are presented as means ± SD or as percentages. The t-test and χ2 test were used for statistical comparisons. Statistical analysis was performed with the SPSS software version 17.0, and P<0.05 was considered statistically significant.
Results
Characteristics of patients
The clinical characteristics of 41 CTD patients are summarized in Table 1. Analysis of serological factors revealed that PAH-associated CTD patients had significantly higher levels of triglycerides and high-sensitivity C-reactive protein (hsCRP) (P<0.05), but lower platelet numbers (P<0.001) than the no-PAH CTD patients. PAH-associated CTD patients showed an increased tendency to have Raynaud’s syndrome (P<0.05).
Table 1. Clinical characteristic of CTD patients.
| Clinical characteristic | PAH-associated CTD | No-PAH CTD |
|---|---|---|
| No. of patients | 19 | 22 |
| Males/females | 3/16 | 5/17 |
| Age, years | 49±12 | 46±18 |
| Mean disease duration, years | 5.64±6.21 | 6.44±5.16 |
| Systolic blood pressure, mmHg | 126.03±14.9 | 126.32±18.04 |
| Diastolic blood pressure, mmHg | 79.16±4.91 | 74.39±5.18 |
| White blood cells, ×109/L | 5.72±3.37 | 5.35±2.46 |
| Macrophages, % | 7.86±5.79 | 6.54±3.25 |
| Lymphocytes, % | 24.96±9.87 | 27.53±10.58 |
| Platelet, ×109/L | 155±67.47** | 201.57±75.2 |
| Total cholesterol, mmol/L | 4.3±1.28 | 4.22±0.9 |
| Triglycerides, mmol/L | 1.68±0.98* | 0.92±0.44 |
| Fasting plasma glucose, mmol/L | 5.18±1.52 | 5.38±0.78 |
| IgG concentration (mg/mL) | 21.26±8.26 | 16.22±5.92 |
| IgM concentration (mg/mL) | 1.93±1.35 | 1.39±1.24 |
| hsCRP, mg/dL | 16.38±27.06* | 13.02±21.92 |
| ESR, mm/h | 37.33±18.63 | 33±19.33 |
| Raynaud’s syndrome | 13* | 8 |
Data are presented as N or mean ± SD. ESR, erythrocyte sedimentation rate; CTD, connective tissue diseases; PAH, pulmonary arterial hypertension; SD, standard deviations. *, P<0.05, PAH-associated CTD vs. no-PAH CTD; **, P<0.01, PAH-associated CTD vs. no-PAH CTD.
Elevated levels of AECA in patients with PAH-associated CTD
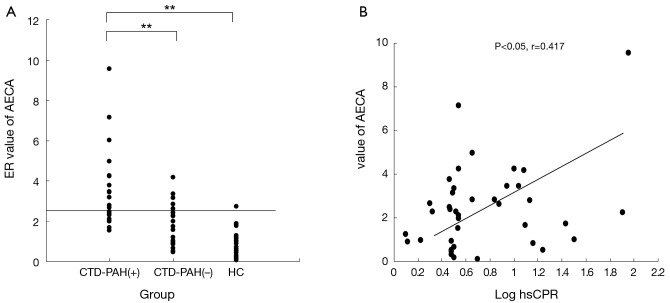
We selected the mean +3 SD of healthy controls (ER =0.93±0.49) as the cut-off AECA value (2.4), and with this criterion, 12 of the 19 (63.2%) PAH-associated CTD patients, 9 of the 22 (40.9%) no-PAH CTD patients, and 1 of the 20 (5%) healthy controls were positive for AECA. AECA levels in PAH-associated CTD patients were significantly higher than those of the no-PAH CTD patients (3.68±2.05 versus 1.67±1.07, P<0.001) or healthy controls (Figure 1A).
Figure 1.
Detection of sera anti-endothelial cell antibodies (AECA). (A) AECA levels in patients with connective tissue disease (CTD) with or without pulmonary arterial hypertension (PAH) and healthy controls (HC). Levels are expressed as the enzyme-linked immunosorbent assay (ELISA) ratio (ER). The horizontal line indicates the cutoff value of the normal serum. **, P<0.001; (B) correlation between sera AECA levels and high-sensitivity C-reactive protein (hsCRP).
Correlation between AECA and clinical features
A positive correlation was observed between ER values of AECA and levels of hsCRP in all CTD patients (P<0.05, r=0.417). The sera levels of AECA were not correlated with other clinical parameters including pulmonary function, blood cell counts, D-dimer, triglycerides, total cholesterol, or erythrocyte sedimentation rate (Figure 1B).
Purified AECA IgG increased the secretion of ICAM-1 and RANTES by HPAECs
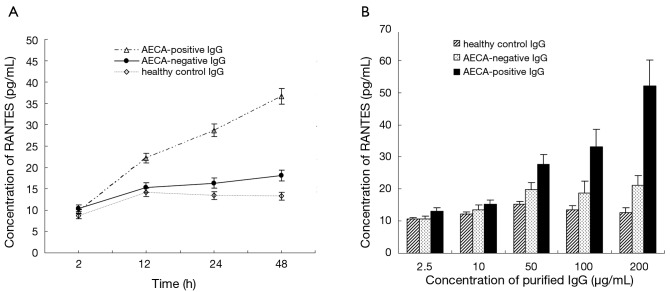
The levels of RANTES secreted by cultured HPAECs increased in response to stimulation from purified AECA-positive IgG in both a time- and concentration-dependent manner, but this effect was not observed in response to purified AECA-negative IgG or healthy control IgG (Figure 2). After 48 h of incubation with IgG purified from AECA-positive sera, the expression level of RANTES was 35.6±1.79 pg/mL, which was significantly higher than that obtained following incubation with AECA-negative or healthy control IgG (17.6±1.04 and 13.8±0.28 pg/mL, respectively; P<0.05; Figure 2). IgG purified from AECA-positive sera (100 µg/mL) also induced a significantly increased level of ICAM-1 expression by HPAECs after 48 h incubation (795.2±32.5 pg/mL), which was significantly higher than that obtained with AECA-negative or healthy control IgG (231.5±27.1 and 192.8±33.4 pg/mL, respectively; P<0.001).
Figure 2.
Time- and concentration-dependent regulated upon activation, normal T-cell expressed and secreted (RANTES) induction by human pulmonary arterial endothelial cells in response to IgG purified from anti-endothelial cell antibodies (AECA)-positive, AECA-negative, and healthy control sera. (A) Endothelial cells were incubated for up to 48 h with IgG (100 µg/mL) purified from AECA-positive, AECA-negative, and healthy control sera; (B) endothelial cells were incubated for 24 h with 2.5, 10, 50, 100, and 200 µg/mL IgG purified from AECA-positive, AECA-negative, and healthy control sera.
Discussion
AECA, a heterogeneous group of antibodies that is distinct from the autoantibodies family, have been detected in a variety of diseases, especially in different types of CTD such as SLE, SSc, and MCTD. In CTD, AECA titers are always found to fluctuate with disease activities, indicating their possible pathologic role rather than a simple phenomenon. Regarding the possible association between AECA and organ manifestations in CTD, Negi (11) reported that AECA were found in 40% of patients with diffuse SSc disease, with a tendency to be associated with digital ischemia and PAH, but no such correlation was reported in other CTD diseases. In this study, we mainly focused on AECA in CTD associated with PAH, and aimed to evaluate the pathogenic value of AECA in pulmonary vascular lesions, as well as to further corroborate previous findings describing the role of AECA in PAH-associated CTD (11). We observed a high prevalence of AECA in CTD patients, and their levels were closely related to levels of the inflammatory marker hsCRP. Moreover, our data showed that AECA levels were significantly higher in PAH-associated CTD patients than in CTD patients without PAH or healthy controls. Therefore, these results were in accordance with previous findings and further support the role of AECA in PAH-associated disease.
Currently, the precise mechanism of the involvement of PAH to CTD remains largely unknown, but there is some convincing evidence showing that endothelial dysfunction is one of the earliest pathogenic events of the process (12). Experimental in vitro and in vivo models showed that AECA might be pathogenic, especially by inducing autoimmune vascular disease (13,14). AECA may bind to endothelial cells’ membrane antigens, and activate endothelial cells by up-regulating the expression of adhesion molecules (e.g., E-selectin, ICAM-1, and VCAM-1), which will in turn cause leukocyte recruitment and adhesion (7). Production of cytokines has also been demonstrated in PAH, especially in PAH combined with CTD, indicating the possible influence of inflammatory mechanisms in this condition.
The chemokine RANTES is known to be an important chemoattractant for T cells and monocytes, facilitating the tight adhesion of circulating leukocytes to the vascular endothelium. RANTES plays a key role in a number of arterial inflammatory processes. Using in situ hybridization and immunohistochemistry methods, Dorfmuller et al. (15) confirmed that RANTES expression was predominant in vascular lesions, and that endothelial cells were the major source of RANTES within the pulmonary artery wall; RANTES expression was associated with CD45+ inflammatory cell infiltrates. Furthermore, RANTES can exert an indirect role in pulmonary hypertension by induction of endothelin-converting enzyme-1 and endothelin-1 system, which serve as potent factors for vasoconstrictive and mitogenic action.
Considering that the endothelial cells of the pulmonary artery wall are the major source of RANTES, and the possibility of differences in phenotype presentation on different endothelial cell surfaces, we selected normal HPAECs as cell substrates in this study to evaluate the effect of AECA on endothelial dysfunction in order to investigate the underlying pathogenic role of AECA in CTD pulmonary vascular lesions. We hypothesized that production of the chemokine RANTES may be associated with AECA, together with up-regulation of the adhesion molecule ICAM-1, a marker of endothelial cell activation, which might be the perpetuating mechanism to amplifying inflammatory responses in PAH-associated CTD. Indeed, we observed increasing levels of RANTES secretion in a time- and dose-dependent manner in response to incubation with purified AECA-positive IgG. These results support a pathologic role of AECA on endothelial cell interactions, and indicate that RANTES is one of the factors involved in the autoimmune and inflammatory multiple step process of PAH in CTD.
In conclusion, we reported the presence of AECA in CTD patients associated with PAH, further corroborating the pathogenic role of AECA in this group of conditions, which appears to occur via the induction of RANTES secretion.
Acknowledgements
Funding: This work was supported by Education Department of Liaoning Province Program (200931).
Author contributions: Sheng-Yu Guo, Li-Li Yang and Xiao-Li Zhang designed the study. Wen-Yi Fu collected and analyzed the data. Xiao-Dan Liu performed all the experiments and prepared the manuscript. Xiao-Fei Wang supervised and controlled the quality of this study.
Disclosure: The authors declare no conflict of interest.
References
- 1.Hoeper MM, Ghofrani HA, Gorenflo M, et al. Diagnosis and treatment of pulmonary hypertension: European guidelines 2009. Pneumologie 2010;64:401-14 [DOI] [PubMed] [Google Scholar]
- 2.Kherbeck N, Tamby MC, Bussone G, et al. The role of inflammation and autoimmunity in the pathophysiology of pulmonary arterial hypertension. Clin Rev Allergy Immunol 2013;44:31-8 [DOI] [PubMed] [Google Scholar]
- 3.Ronda N, Leonardi S, Orlandini G, et al. Natural anti-endothelial cell antibodies (AECA). J Autoimmun 1999;13:121-7 [DOI] [PubMed] [Google Scholar]
- 4.Praprotnik S, Rozman B, Blank M, et al. Pathogenic role of anti-endothelial cell antibodies in systemic vasculitis. Wien Klin Wochenschr 2000;112:660-4 [PubMed] [Google Scholar]
- 5.Zeng XJ, Zhu WG, Deng XX, et al. Anti-endothelial cell antibodies in systemic vasculitis: detection and correlation with disease activity. Zhonghua Yi Xue Za Zhi 2004;84:1629-32 [PubMed] [Google Scholar]
- 6.Del Papa N, Guidali L, Sironi M, et al. Anti-endothelial cell IgG antibodies from patients with Wegener’s granulomatosis bind to human endothelial cells in vitro and induce adhesion molecule expression and cytokine secretion. Arthritis Rheum 1996;39:758-66 [DOI] [PubMed] [Google Scholar]
- 7.Carvalho D, Savage CO, Black CM, et al. IgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines. J Clin Invest 1996;97:111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfmüller P, Perros F, Balabanian K, et al. Inflammation in pulmonary arterial hypertension. Eur Respir J 2003;22:358-63 [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Montani D, Perros F, et al. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul Pharmacol 2008;49:113-8 [DOI] [PubMed] [Google Scholar]
- 10.Bloom BJ, Toyoda M, Petrosian A, et al. Anti-endothelial cell antibodies are prevalent in juvenile idiopathic arthritis: implications for clinical disease course and pathogenesis. Rheumatol Int 2007;27:655-60 [DOI] [PubMed] [Google Scholar]
- 11.Negi VS, Tripathy NK, Misra R, et al. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol 1998;25:462-6 [PubMed] [Google Scholar]
- 12.Fleming JN, Schwartz SM. The pathology of scleroderma vascular disease. Rheum Dis Clin North Am 2008;34:41-55; vi. [DOI] [PubMed] [Google Scholar]
- 13.Mihai C, Tervaert JW. Anti-endothelial cell antibodies in systemic sclerosis. Ann Rheum Dis 2010;69:319-24 [DOI] [PubMed] [Google Scholar]
- 14.Worda M, Sgonc R, Dietrich H, et al. In vivo analysis of the apoptosis-inducing effect of anti-endothelial cell antibodies in systemic sclerosis by the chorionallantoic membrane assay. Arthritis Rheum 2003;48:2605-14 [DOI] [PubMed] [Google Scholar]
- 15.Dorfmüller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165:534-9 [DOI] [PubMed] [Google Scholar]